Improving Thermostability of GH11 Xylanase XynASP by the Design of Loop Region
Abstract
:1. Introduction
2. Results
2.1. Identification of The Thermal Stability Key Residues
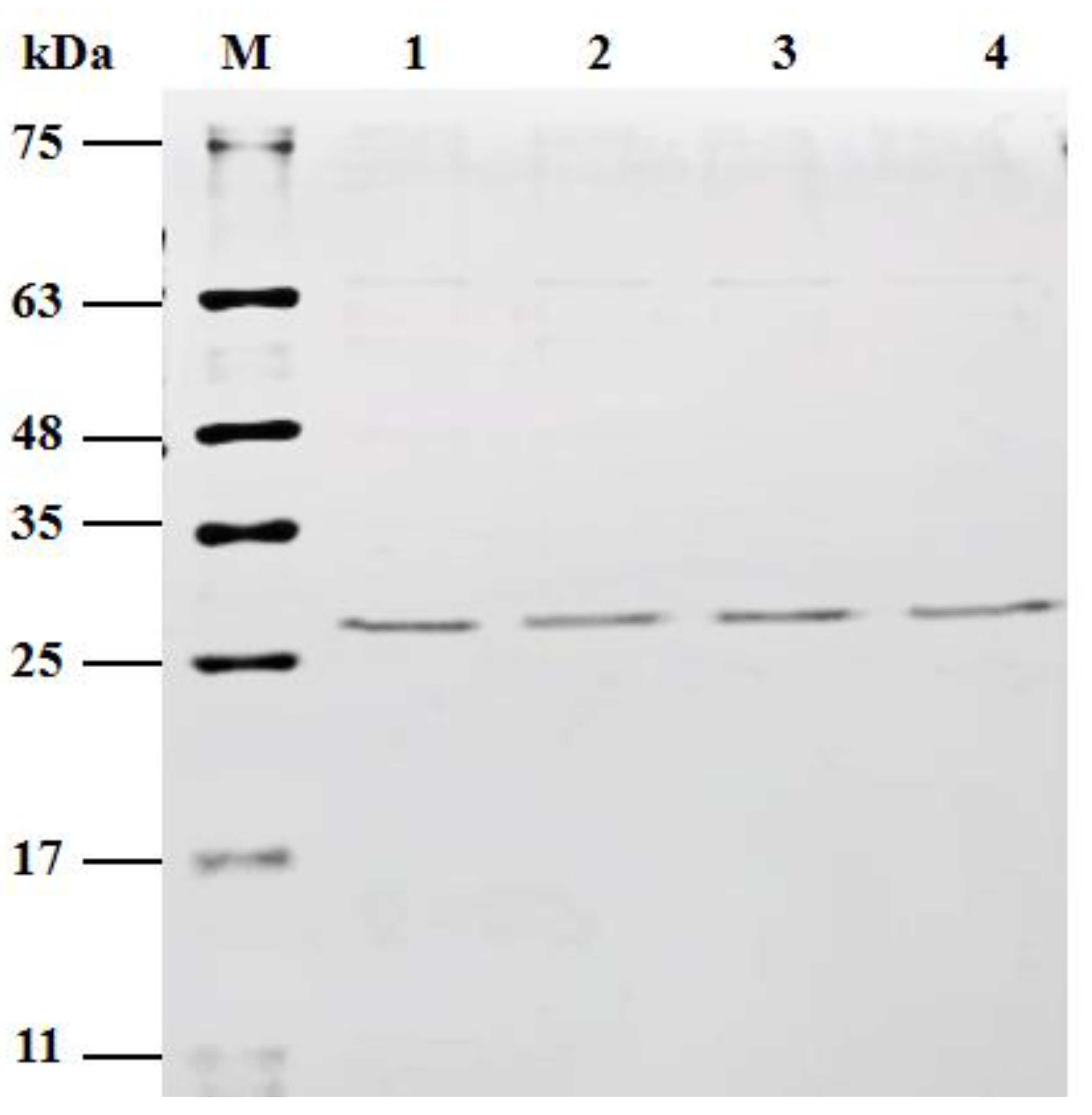
2.2. Construction and Characterization of Three Single Mutants
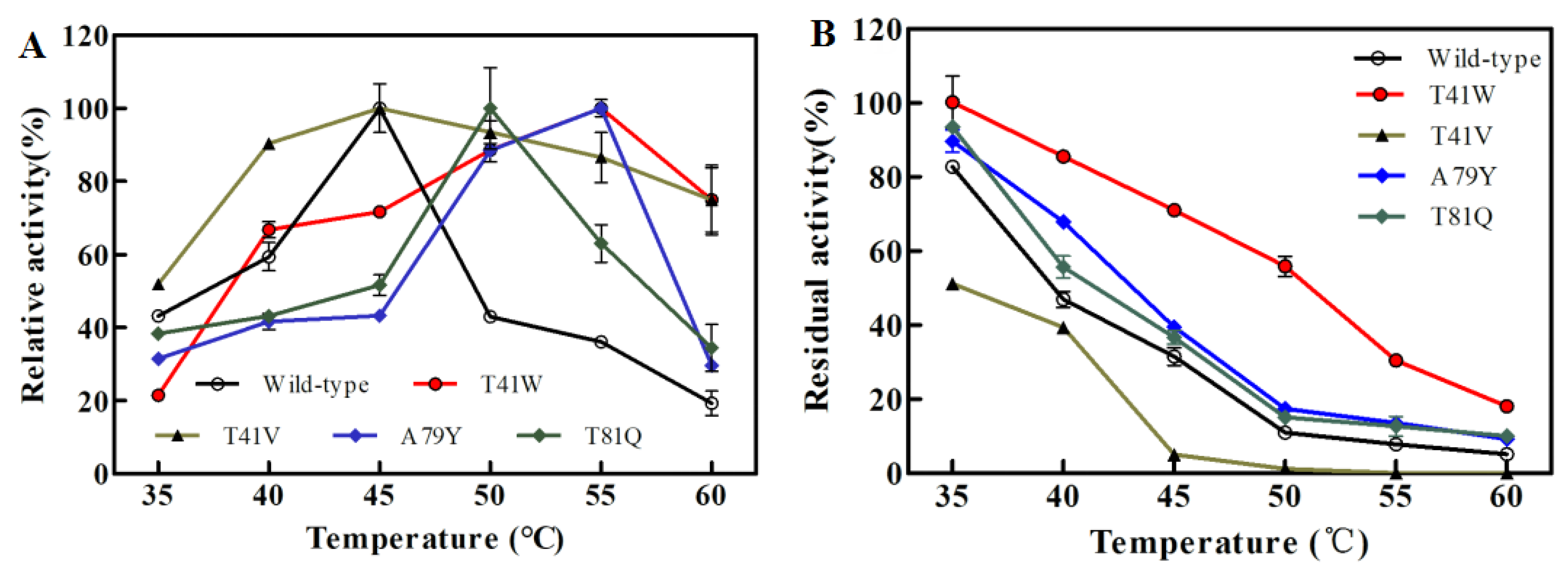
2.3. Effects of Temperature on the Single Mutants
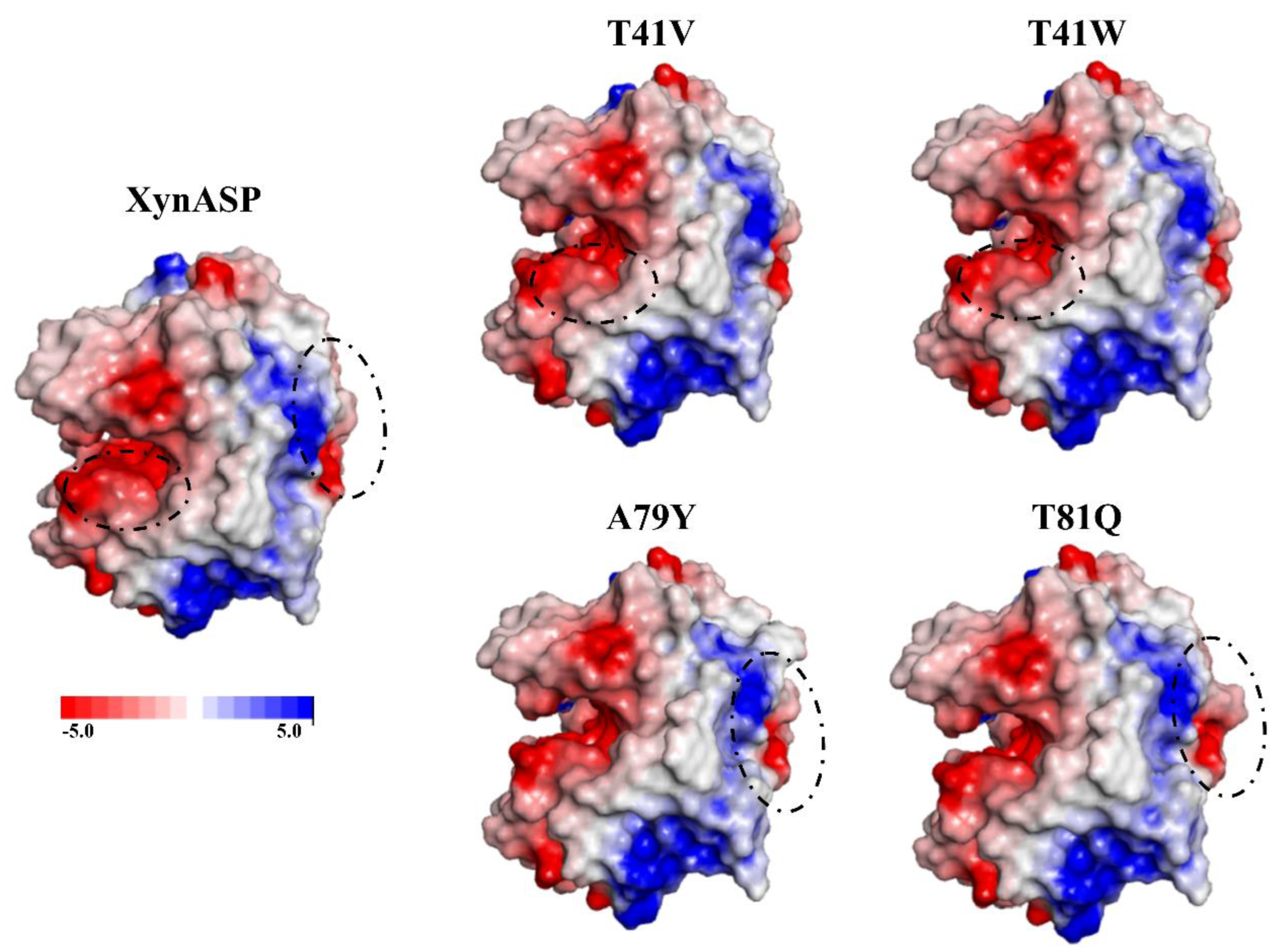
2.4. Enhanced Thermal Stability of the Single Mutants Explored by Structural Simulations
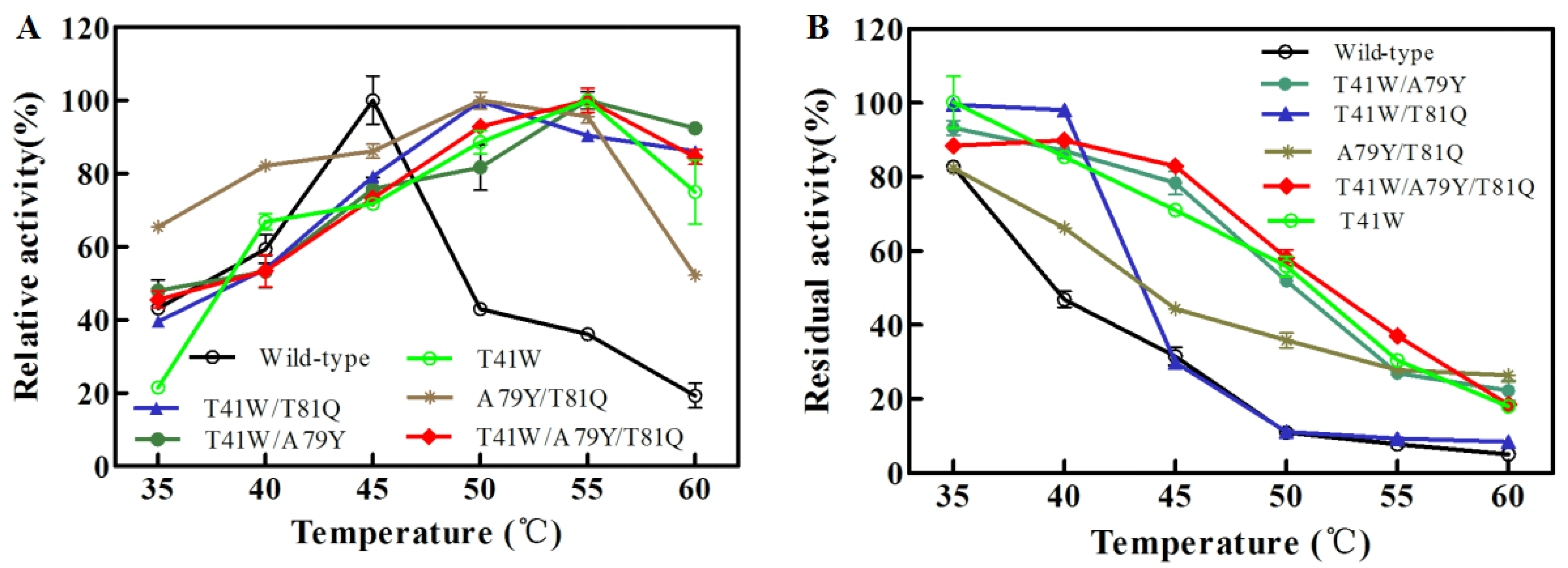
2.5. Temperature Characterization of Combined Multiple-Site Mutants
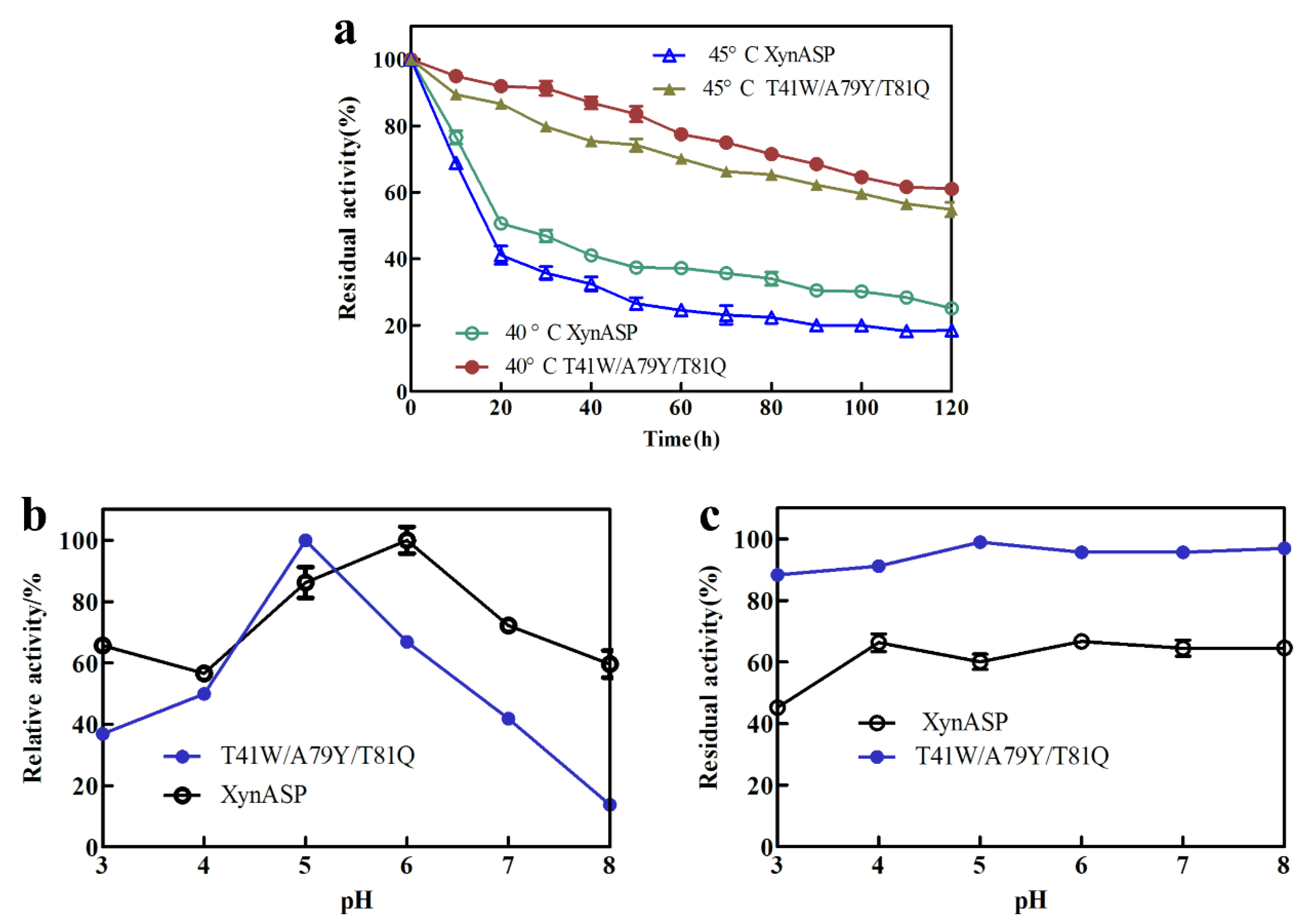
2.6. Thermostability and pH Stability of the Triple mutant T41W/A79Y/T81Q
3. Materials and Methods
3.1. Materials
3.2. Homology Modeling and Prediction of Thermal Stability Sites
3.3. Site-Directed Mutagenesis
3.4. Protein Expression and Purification
3.5. Enzyme Assays
3.6. Measurements of Temperature and pH Characteristics in the Wild-Type and Mutants
3.7. Electrostatic Property Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mobarec, H.; Villagomez, R.; Karlsson, E.N.; Linares-Pastén, J.A. Microwave-assisted xylanase reaction: Impact in the production of prebiotic xylooligosaccharides. RSC Adv. 2021, 11, 11882–11888. [Google Scholar] [CrossRef]
- Basit, A.; Liu, J.; Rahim, K.; Jiang, W.; Lou, H. Thermophilic xylanases: From bench to bottle. Crit. Rev. Biotechnol. 2018, 38, 989–1002. [Google Scholar] [CrossRef]
- Vucinic, J.; Novikov, G.; Montanier, C.Y.; Dumon, C.; Schiex, T.; Barbe, S. A Comparative Study to Decipher the Structural and Dynamics Determinants Underlying the Activity and Thermal Stability of GH-11 Xylanases. Int. J. Mol. Sci. 2021, 22, 5961. [Google Scholar] [CrossRef]
- De Amo, G.S.; Bezerra-Bussoli, C.; da Silva, R.R.; Kishi, L.T.; Ferreira, H.; Mariutti, R.B.; Arni, R.K.; Gomes, E.; Bonilla-Rodriguez, G.O. Heterologous expression, purification and biochemical characterization of a new xylanase from Myceliophthora heterothallica F.2.1.4. Int. J. Biol. Macromol. 2019, 131, 798–805. [Google Scholar] [CrossRef]
- Mhiri, S.; Bouanane-Darenfed, A.; Jemli, S.; Neifar, S.; Bejar, S. A thermophilic and thermostable xylanase from Caldicoprobacter algeriensis: Recombinant expression, characterization and application in paper biobleaching. Int. J. Biol. Macromol. 2020, 164, 808–817. [Google Scholar] [CrossRef]
- Qiu, J.; Han, H.; Sun, B.; Chen, L.; Yu, C.; Peng, R.; Yao, Q. Residue mutations of xylanase in Aspergillus kawachii alter its optimum pH. Microbiol. Res. 2016, 182, 1–7. [Google Scholar] [CrossRef]
- Xiong, K.; Hou, J.; Jiang, Y.; Li, X.; Teng, C.; Li, Q.; Fan, G.; Yang, R.; Zhang, C. Mutagenesis of N-terminal residues confer thermostability on a Penicillium janthinellum MA21601 xylanase. BMC Biotechnol. 2019, 19, 51. [Google Scholar] [CrossRef]
- Li, G.; Chen, X.; Zhou, X.; Huang, R.; Zhang, R. Improvement of GH10 family xylanase thermostability by introducing of an extra α-helix at the C-terminal. Biochem. Biophys. Res. Commun. 2019, 515, 417–422. [Google Scholar] [CrossRef]
- Kota, N.; Yuta, K.; Kenji, K.; Eisuke, T.T.; Rie, Y.; Satoshi, N.; Kiyoshi, Y. Increase in the thermostability of Bacillus sp. strain TAR-1 xylanase using a site saturation mutagenesis library. Biosci. Biotechnol. Biochem. 2018, 82, 1715–1723. [Google Scholar]
- Teng, C.; Jiang, Y.; Xu, Y.; Li, Q.; Li, X.; Fan, G.; Xiong, K.; Yang, R.; Zhang, C.; Ma, R.; et al. Improving the thermostability and catalytic efficiency of GH11 xylanase PjxA by adding disulfide bridges. Int. J. Biol. Macromol. 2019, 128, 354–362. [Google Scholar] [CrossRef]
- Paës, G.; Berrin, J.-G.; Beaugrand, J. GH11 xylanases: Structure/function/properties relationships and applications. Biotechnol. Adv. 2012, 30, 564–592. [Google Scholar] [CrossRef]
- Wang, X.; Luo, H.; Yu, W.; Ma, R.; You, S.; Liu, W.; Hou, L.; Zheng, F.; Xie, X.; Yao, B. A thermostable Gloeophyllum trabeum xylanase with potential for the brewing industry. Food Chem. 2016, 199, 516–523. [Google Scholar] [CrossRef]
- Kumar, V.; Dangi, A.K.; Shukla, P. Engineering Thermostable Microbial Xylanases Toward its Industrial Applications. Mol. Biotechnol. 2018, 60, 226–235. [Google Scholar] [CrossRef]
- Han, N.; Miao, H.; Ding, J.; Li, J.; Huang, Z. Improving the thermostability of a fungal GH11 xylanase via site-directed mutagenesis guided by sequence and structural analysis. Biotechnol. Biofuels 2017, 10, 133. [Google Scholar] [CrossRef]
- Koen, B.; Stnislav, M.; Antonin, K.; Marques, S.M.; Niels, H.; Milos, M.; Radka, C.; Jitka, W.; Jan, B.; David, B. Evolutionary Analysis as a Powerful Complement to Energy Calculations for Protein Stabilization. ACS Catal. 2018, 8, b01677. [Google Scholar]
- Xu, L.H.; Du, Y.L. Rational and semi-rational engineering of cytochrome P450s for biotechnological applications. Syn. Syst. Biotechnol. 2018, 3, 283–290. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Qu, G.; Feng, Y.; Reetz, M.T. Utility of B-Factors in Protein Science: Interpreting Rigidity, Flexibility, and Internal Motion and Engineering Thermostability. Chem. Rev. 2019, 119, 1626–1665. [Google Scholar] [CrossRef]
- Paës, G.; Cortés, J.; Siméon, T.; O’Donohue, M.; Tran, V. Thumb-loops up for catalysis: A structure/function investigation of a functional loop movement in a gh11 xylanase. Comput. Struct. Biotechnol. J. 2012, 1, e201207001. [Google Scholar]
- Li, C.; Li, J.; Wang, R.; Li, X.; Wu, M. Substituting Both the N-Terminal and “Cord” Regions of a Xylanase from Aspergillus oryzae to Improve Its Temperature Characteristics. Appl. Biochem. Biotechnol. 2018, 185, 1044–1059. [Google Scholar] [CrossRef]
- Hakulinen, N.; Turunen, O.; Jimg, J. Three-dimensional structures of thermophilic β-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa: Comparison of twelve xylanases in relation to their thermal stability. Eur. J. Biochem. 2003, 270, 1399–1412. [Google Scholar] [CrossRef]
- Joo, J.C.; Pack, S.P.; Yong, H.K.; Yoo, Y.J. Thermostabilization of Bacillus circulans xylanase: Computational optimization of unstable residues based on thermal fluctuation analysis. J. Biotechnol. 2011, 151, 56–65. [Google Scholar] [CrossRef]
- Siddique, M.; Faiz, M.; Mahjabeen, R.; Amber, A.; Muhammad, A. Characterization of recombinant endo-1,4-β-xylanase of Bacillus halodurans C-125 and rational identification of hot spot amino acid residues responsible for enhancing thermostability by an in-silico approach. Mol. Biol. Rep. 2019, 46, 3651–3662. [Google Scholar]
- Musil, M.; Stourac, J.; Bendl, J.; Brezovsky, J.; Prokop, Z.; Zendulka, J.; Martinek, T.; Bednar, D.; Damborsky, J. FireProt: Web server for automated design of thermostable proteins. Nucleic Acids Res. 2017, 45, 393–399. [Google Scholar] [CrossRef]
- Pongpamorn, P.; Watthaisong, P.; Pimviriyakul, P.; Jaruwat, A.; Lawan, N.; Chitnumsub, P.; Chaiyen, P. Identification of a Hotspot Residue for Improving the Thermostability of a Flavin-Dependent Monooxygenase. ChemBioChem 2019, 20, 3020–3031. [Google Scholar] [CrossRef]
- Ashraf, N.M.; Krishnagopal, A.; Hussain, A.; Kastner, D.; Sayed, A.M.A.; Mok, Y.-K.; Swaminathan, K.; Zeeshan, N. Engineering of serine protease for improved thermostability and catalytic activity using rational design—ScienceDirect. Int. J. Biol. Macromol. 2019, 126, 229–237. [Google Scholar] [CrossRef]
- Cheng, Z.; Lan, Y.; Guo, J.; Ma, D.; Peplowski, L. Computational Design of Nitrile Hydratase from Pseudonocardia thermophila JCM3095 for Improved Thermostability. Molecules 2020, 25, 4806. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.Y.; Guo, C.; Cui, C.; Wu, Z.L. Enhancing the thermal stability of ketoreductase ChKRED12 using the FireProt web server. Process Biochem. 2020, 101, 207–212. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, 252–258. [Google Scholar] [CrossRef]
- Guerois, R.; Nielse-n, J.E.; Serrano, L. Predicting Changes in the Stability of Proteins and Protein Complexes: A Study of More Than 1000 Mutations. J. Mol. Biol. 2002, 320, 369–387. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.F.; Wang, J.Q.; Tang, C.D.; Wu, M.C. Enhanced thermostability of a mesophilic xylanase by N-terminal replacement designed by molecular dynamics simulation. J. Sci. Food Agr. 2013, 93, 3016–3023. [Google Scholar] [CrossRef]
- Li, T.B.; Zhu, X.L.; Ye, M.Y.; Wang, M.C.; Zhu, J.J.; Shen, F.J.; Li, E.Z. Rapid improvement in thermostability of GH11 xylanase (XynASP) from Aspergillus saccharolyticus JOP1030–1 by tryptophan residue substitution. J. Biotech Res. 2022, 13, 26–39. [Google Scholar]
- Jaenicke, R.; Schurig, H.; Beaucamp, N.; Ostendorp, R. Advances in Protein Chemistry Enzymes and Proteins from Hyperthermophilic Microorganisms Structure and Stability of Hyperstable Proteins: Glycolytic Enzymes from Hyperthermophilic Bacterium Thermotoga Maritima. Adv. Protein Chem. 1996, 48, 181–269. [Google Scholar] [PubMed]
- Rodrigues, C.H.; Pires, D.E.; Ascher, D.B. DynaMut: Predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, 174–181. [Google Scholar] [CrossRef]
- Rao, S.N.; Head, M.S.; Kulkarni, A.; Lalonde, J.M. Validation studies of the site-directed docking program LibDock. J. Chem. Inf. Model 2007, 47, 2159–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Zhou, J.; You, C.; Huang, Q.; Lu, H. Amino acid substitutions in the N-terminus, cord and α-helix domains improved the thermostability of a family 11 xylanase XynR8. J. Ind. Biotechnol. 2012, 39, 1279–1288. [Google Scholar] [CrossRef]
- You, C.; Huang, Q.; Xue, H.; Yang, X.; Lu, H. Potential hydrophobic interaction between two cysteines in interior hydrophobic region improves thermostability of a family 11 xylanase from Neocallimastix Patriciarum. Biotechnol. Bioeng. 2010, 105, 861–870. [Google Scholar]
- Konecny, R.; Baker, N.A.; Mccammon, J.A. iAPBS: A programming interface to Adaptive Poisson-Boltzmann Solver (APBS). Comput. Sci. Discov. 2012, 5, 015005. [Google Scholar] [CrossRef]
- Purmonen, M.; Valjakka, J.; Takkinen, K.; Laitinen, T.; Rouvinen, J. Molecular dynamics studies on the thermostability of family II xylanases. Protein Eng. Des. Sel. 2007, 20, 551–559. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Reetz, M.T.; Carballeira, J.D.; Vogel, A. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew. Chem. Int. Ed. Engl. 2010, 118, 7909–7915. [Google Scholar] [CrossRef]
- Zhou, Y.; Bianca, P.; Hao, W.; Lv, J.; Gao, R.; Zheng, G. The additive mutational effects from surface charge engineering: A compromise between enzyme activity, thermostability and ionic liquid tolerance. Biochem. Eng. J. 2018, 148, 195–204. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yang, S.; Wang, X.; Cai, H.; Wang, Y.; Li, C.; Li, E. Improving Thermostability of GH11 Xylanase XynASP by the Design of Loop Region. Crystals 2022, 12, 1228. https://doi.org/10.3390/cryst12091228
Li T, Yang S, Wang X, Cai H, Wang Y, Li C, Li E. Improving Thermostability of GH11 Xylanase XynASP by the Design of Loop Region. Crystals. 2022; 12(9):1228. https://doi.org/10.3390/cryst12091228
Chicago/Turabian StyleLi, Tongbiao, Siwen Yang, Xiaoxiao Wang, Hongxuan Cai, Ye Wang, Chao Li, and Enzhong Li. 2022. "Improving Thermostability of GH11 Xylanase XynASP by the Design of Loop Region" Crystals 12, no. 9: 1228. https://doi.org/10.3390/cryst12091228
APA StyleLi, T., Yang, S., Wang, X., Cai, H., Wang, Y., Li, C., & Li, E. (2022). Improving Thermostability of GH11 Xylanase XynASP by the Design of Loop Region. Crystals, 12(9), 1228. https://doi.org/10.3390/cryst12091228




