Development of N,N-Dimethylglycine-Amantadine for Adjunctive Dopaminergic Application: Synthesis, Structure and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DMG-Am
2.3. Powder X-ray Diffraction (PXRD)
2.4. Single Crystal X-ray Diffraction (SCXRD)
2.5. Thermal Analysis (DSC)
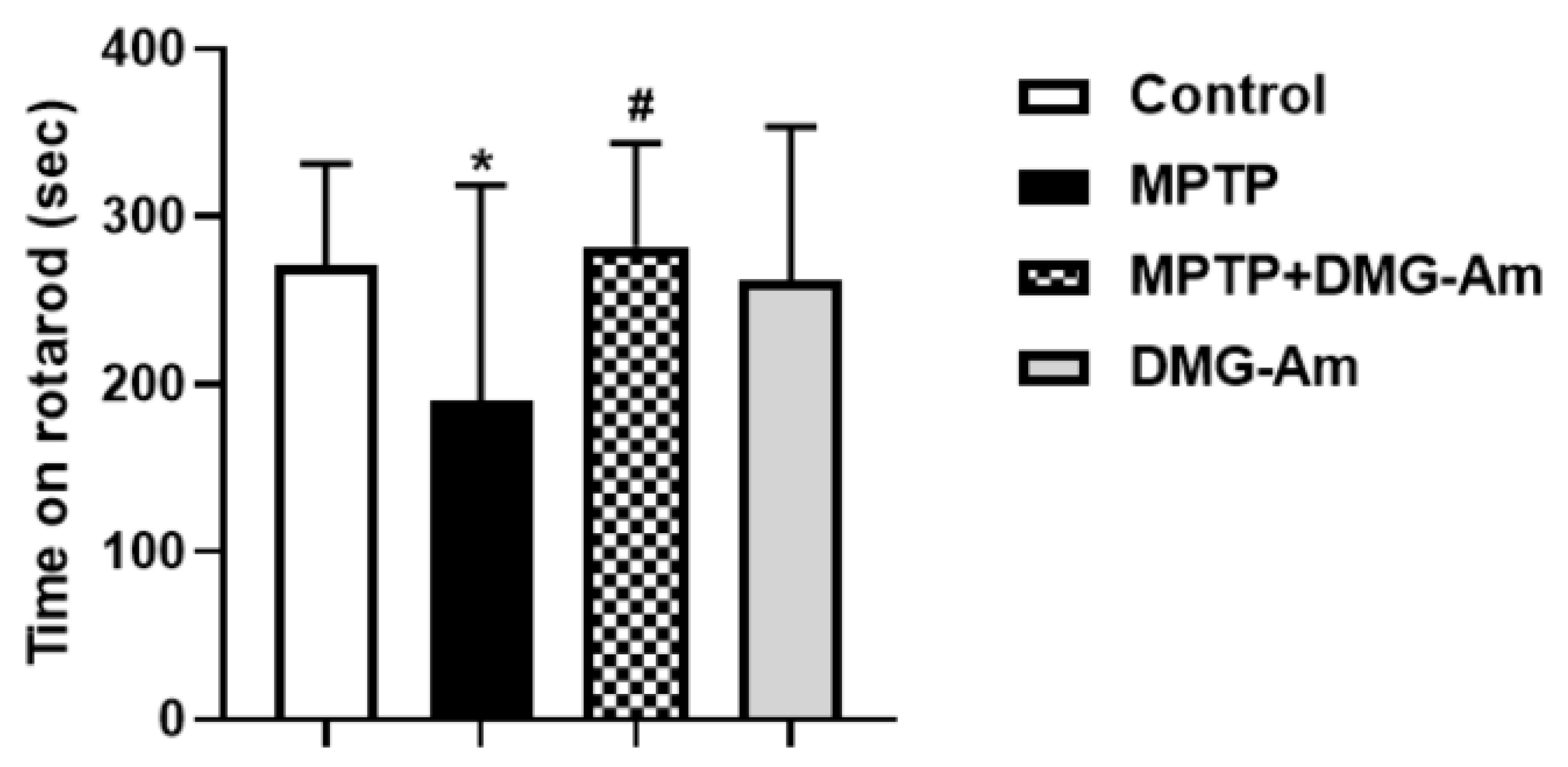
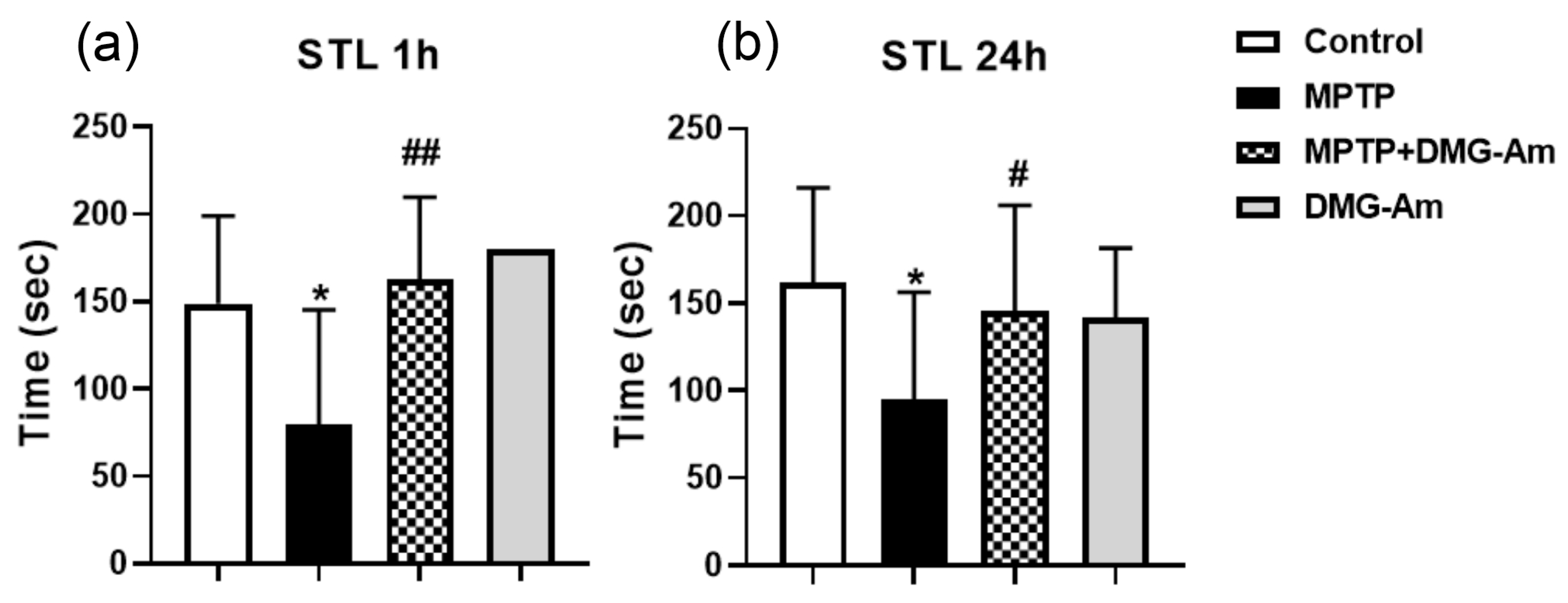
2.6. Neurobehavioral Studies
2.6.1. Rotarod Test
2.6.2. Passive Avoidance Test
2.6.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauser, R.A.; Walsh, R.R.; Pahwa, R.; Chernick, D.; Formella, A.E. Amantadine ER (Gocovri®) Significantly Increases on Time without Any Dyskinesia: Pooled Analyses from Pivotal Trials in Parkinson’s Disease. Front. Neurol. 2021, 12, 645706. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Ba, M.; Ren, C.; Yu, L.; Dong, S.; Yu, G.; Liang, H. An updated meta-analysis of amantadine for treating dyskinesia in Parkinson’s disease. Oncotarget 2017, 8, 57316. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Mahlknecht, P. Pharmacologic treatment of motor symptoms associated with Parkinson disease. Neurol. Clin. 2020, 38, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.; Eggert, K.; Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Trenkwalder, C.; Ehret, R.; Azulay, J.P.; Isaacson, S.; Felt, L. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov. Disord. 2017, 32, 1701–1709. [Google Scholar] [CrossRef]
- Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Isaacson, S.H.; Nausieda, P.A.; Truong, D.D.; Agarwal, P.; Hull, K.L.; Lyons, K.E.; Johnson, R. ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID study): A randomized clinical trial. JAMA Neurol. 2017, 74, 941–949. [Google Scholar] [CrossRef]
- Rezaei, M.; Alirezaei, M. Protective effects of Althaea officinalis L. extract in 6-hydroxydopamine-induced hemi-Parkinsonism model: Behavioral, biochemical and histochemical evidence. J. Physiol. Sci. 2014, 64, 171–176. [Google Scholar] [CrossRef]
- Fahn, S. Levodopa-induced neurotoxicity. CNS Drugs 1997, 8, 376–393. [Google Scholar] [CrossRef]
- Shulman, L.M. Levodopa toxicity in Parkinson disease: Reality or myth?: Reality—practice patterns should change. Arch. Neurol. 2000, 57, 406–408. [Google Scholar] [CrossRef]
- Weiner, W.J. Is levodopa toxic? Arch. Neurol. 2000, 57, 408–410. [Google Scholar] [CrossRef]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978, 14, 633–643. [Google Scholar]
- Tse, D.C.; McCreery, R.L.; Adams, R.N. Potential oxidative pathways of brain catecholamines. J. Med. Chem. 1976, 19, 37–40. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography–tandem mass spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef]
- Friesen, R.W.; Novak, E.M.; Hasman, D.; Innis, S.M. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J. Nutr. 2007, 137, 2641–2646. [Google Scholar] [CrossRef]
- Luka, Z.; Pakhomova, S.; Loukachevitch, L.V.; Newcomer, M.E.; Wagner, C. Folate in demethylation: The crystal structure of the rat dimethylglycine dehydrogenase complexed with tetrahydrofolate. Biochem. Biophys. Res. Commun. 2014, 449, 392–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bai, K.; Xu, W.; Zhang, J.; Kou, T.; Niu, Y.; Wan, X.; Zhang, L.; Wang, C.; Wang, T. Assessment of free radical scavenging activity of dimethylglycine sodium salt and its role in providing protection against lipopolysaccharide-induced oxidative stress in mice. PLoS ONE 2016, 11, e0155393. [Google Scholar] [CrossRef] [PubMed]
- Palanimuthu, D.; Wu, Z.; Jansson, P.J.; Braidy, N.; Bernhardt, P.V.; Richardson, D.R.; Kalinowski, D.S. Novel chelators based on adamantane-derived semicarbazones and hydrazones that target multiple hallmarks of Alzheimer’s disease. Dalton Trans. 2018, 47, 7190–7205. [Google Scholar] [CrossRef] [PubMed]
- Lees, A. Alternatives to levodopa in the initial treatment of early Parkinson’s disease. Drugs Aging 2005, 22, 731–740. [Google Scholar] [CrossRef]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1198–1204. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlis pro. 2022. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 26 August 2022).
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXL-97. Program for Crystal-Structure Refinement; ScienceOpen, Inc.: Burlington, MA, USA, 1997. [Google Scholar]
- Shin, K.S.; Zhao, T.T.; Choi, H.S.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Effects of gypenosides on anxiety disorders in MPTP-lesioned mouse model of Parkinson׳ s disease. Brain Res. 2014, 1567, 57–65. [Google Scholar] [CrossRef]
- Manna, S.; Bhattacharyya, D.; Mandal, T.; Dey, S. Neuropharmacological effects of deltamethrin in rats. J. Vet. Sci. 2006, 7, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Jarvik, M.; Kopp, R. An improved one-trial passive avoidance learning situation. Psychol. Rep. 1967, 21, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Basarić, N.; Molčanov, K.; Matković, M.; Kojić-Prodić, B.; Mlinarić-Majerski, K. Adamantane-retropeptides, new building blocks for molecular channels. Tetrahedron 2007, 63, 7985–7996. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Sanz, D.; Foces-Foces, M.C.; Cano, F.H.; Fayet, J.P.; Vertut, M.C.; Elguero, J. Adamantylazoles. 5. The molecular structure of 1-(1-adamantyl)pyrazoles. J. Heterocycl. Chem. 1986, 23, 1045–1050. [Google Scholar] [CrossRef]
- Villhauer, E.B.; Brinkman, J.A.; Naderi, G.B.; Burkey, B.F.; Dunning, B.E.; Prasad, K.; Mangold, B.L.; Russell, M.E.; Hughes, T.E. 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A Potent, Selective, and Orally Bioavailable Dipeptidyl Peptidase IV Inhibitor with Antihyperglycemic Properties. J. Med. Chem. 2003, 46, 2774–2789. [Google Scholar] [CrossRef]
- Kelly, R.P.; Falcone, M.; Lamsfus, C.A.; Scopelliti, R.; Maron, L.; Meyer, K.; Mazzanti, M. Metathesis of a UV imido complex: A route to a terminal UV sulfide. Chem. Sci. 2017, 8, 5319–5328. [Google Scholar] [CrossRef]
- Minkov, V.S.; Boldyreva, E.V. The effect of partial methylation of the glycine amino group on crystal structure in N, N-dimethylglycine and its hemihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, o283–o287. [Google Scholar] [CrossRef]
- Bélanger-Gariépy, F.; Brisse, F.; Harvey, P.; Butler, I.; Gilson, D. Structure of adamantanamine hydrochloride at 143 K. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 756–759. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Tatton, N.; Kish, S. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 1997, 77, 1037–1048. [Google Scholar] [CrossRef]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bazyleva, A.B.; Blokhin, A.V.; Kabo, A.G.; Kabo, G.J.; Emel’yanenko, V.N.; Verevkin, S.P. Thermodynamic properties of 1-aminoadamantane. J. Chem. Thermodyn. 2008, 40, 509–522. [Google Scholar] [CrossRef]
- Smeyne, R.J.; Jackson-Lewis, V. The MPTP model of Parkinson’s disease. Mol. Brain Res. 2005, 134, 57–66. [Google Scholar] [CrossRef]
- Schapira, A.H.; Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011, 26, 1049–1055. [Google Scholar] [CrossRef]
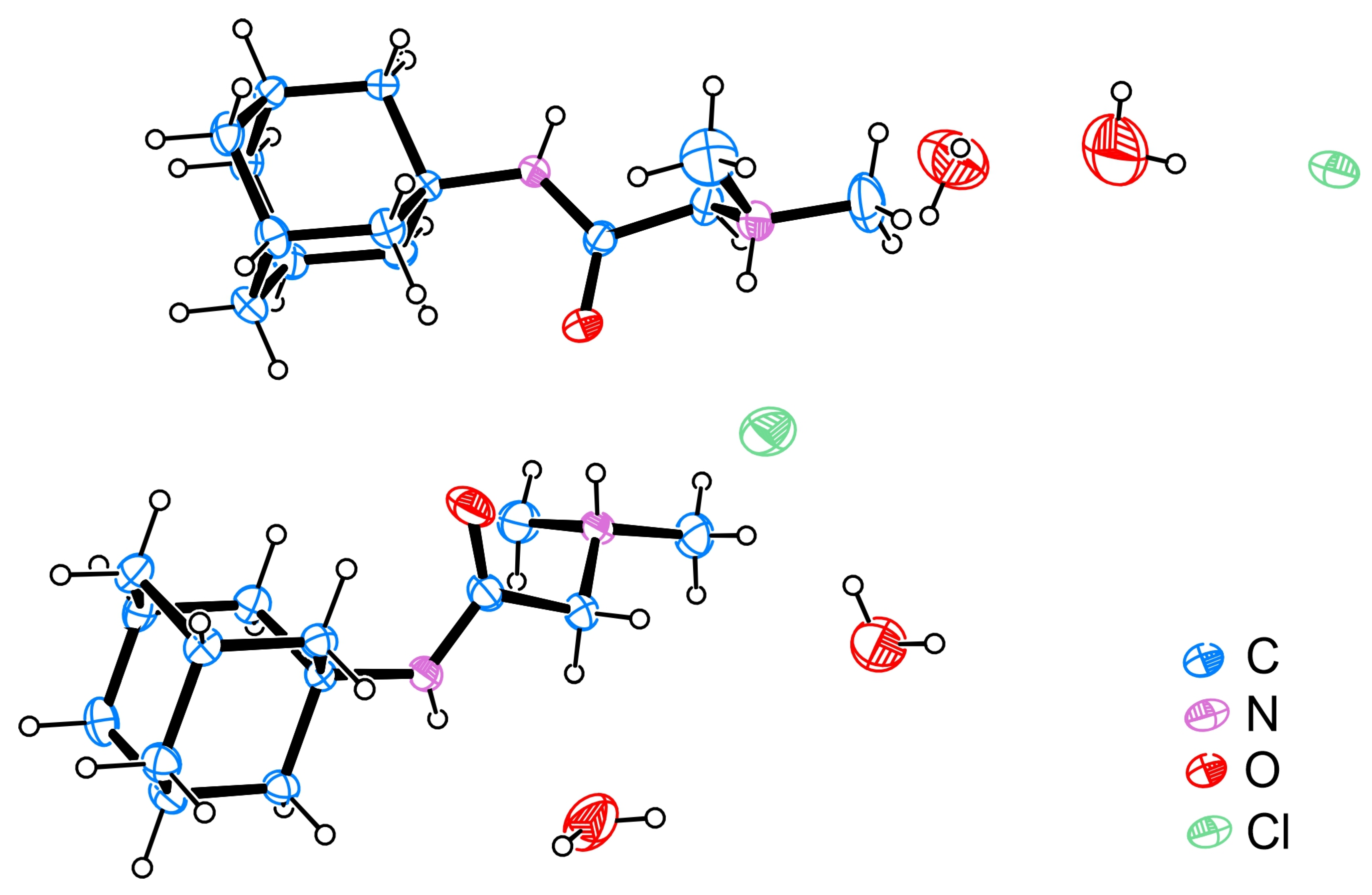
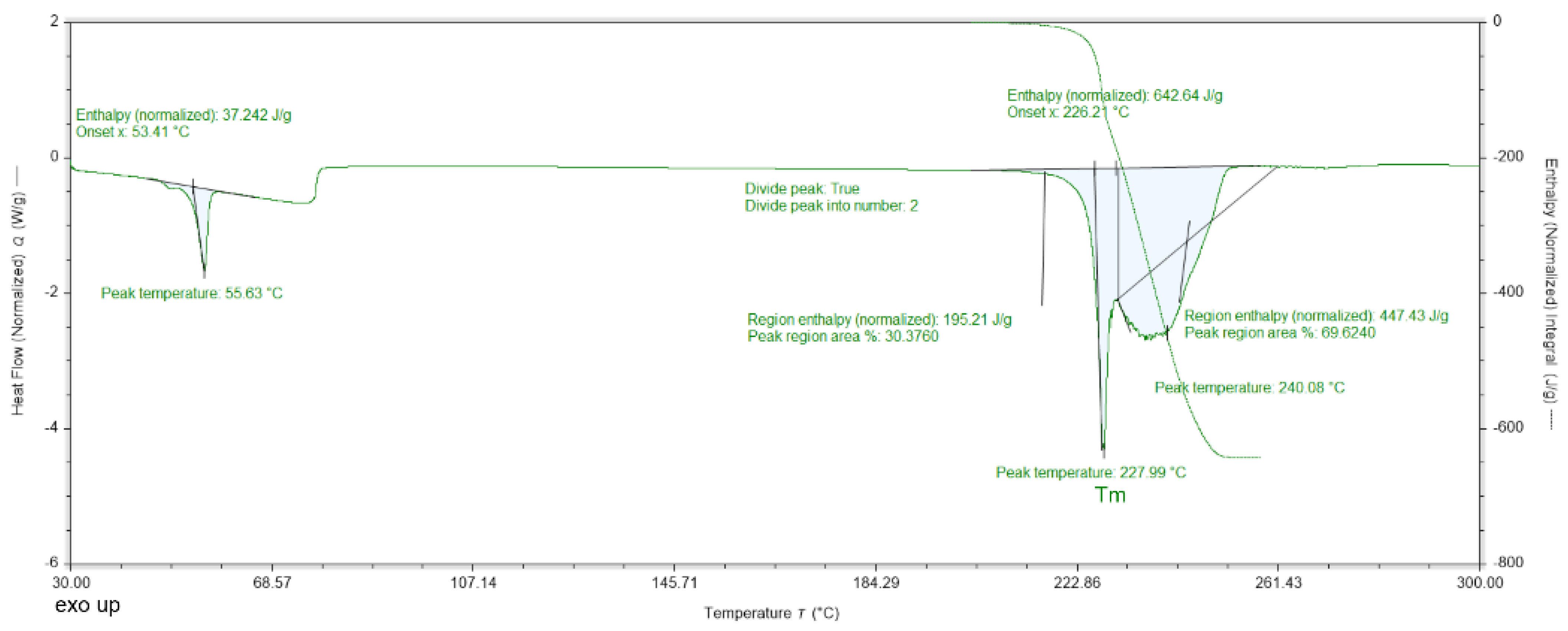



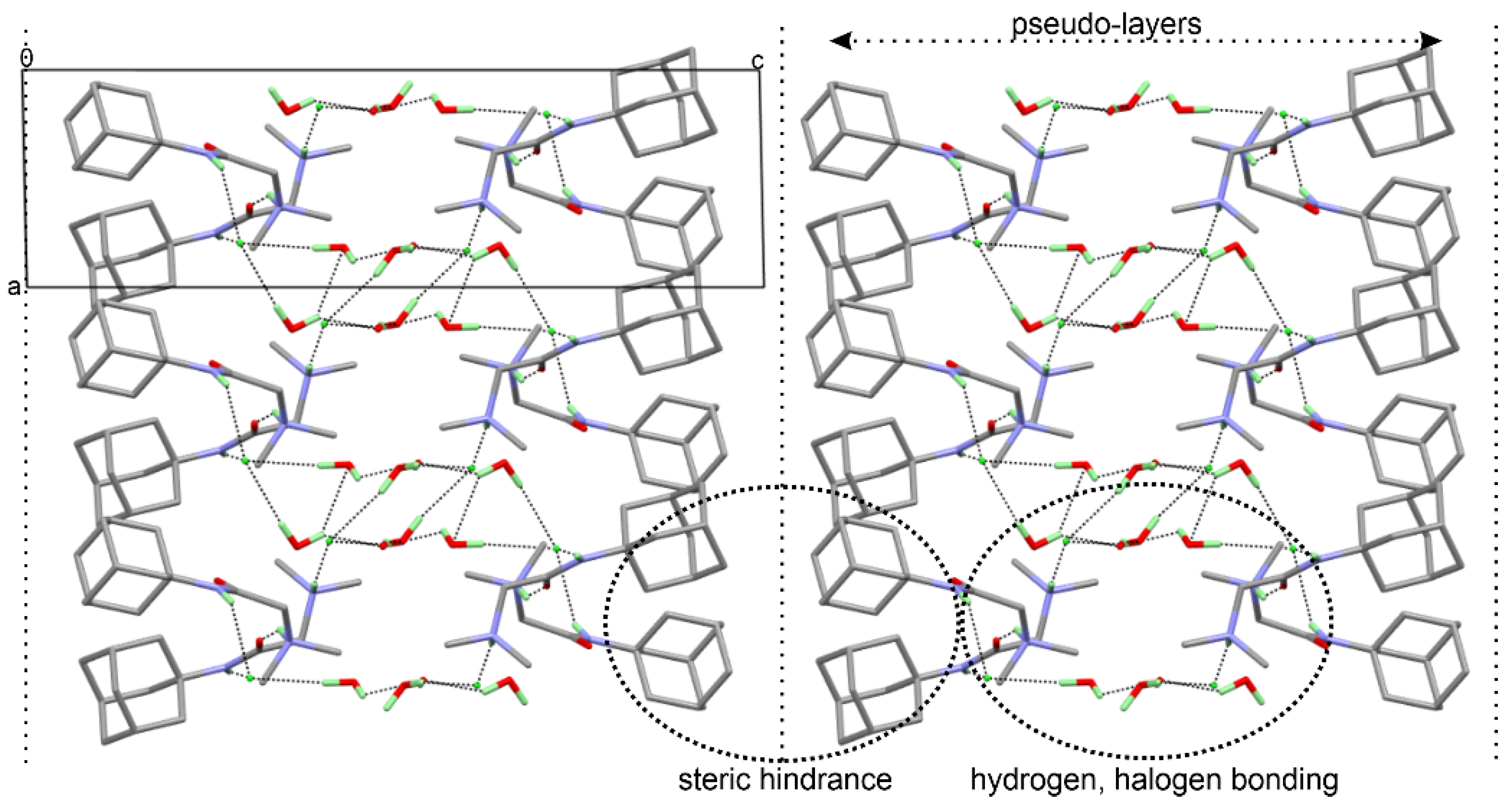
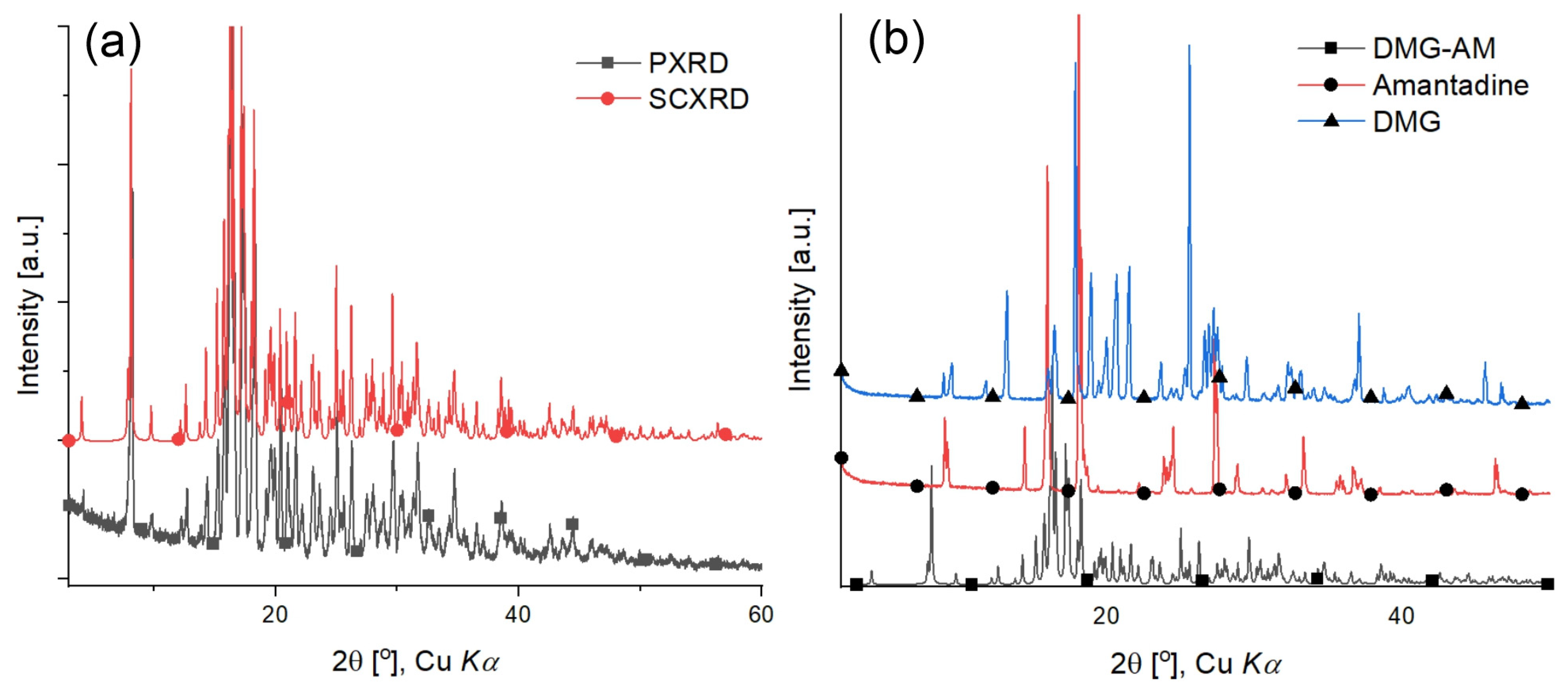
| DMG-Am | DMG [30] | DMG.H2O [30] | Am [31] | |
|---|---|---|---|---|
| Empirical formula | C28H56.88Cl2N4O5.13 | C4H9NO2 | C4H9NO2.0.5H2O | C10H18CN+Cl- |
| Formula weight | 602.72 | 103.12 | 112.13 | 187.71 |
| Temperature/K | 289.15 | 295(2) | 295(2) | 143 |
| Crystal system | triclinic | orthorhombic | monoclinic | monoclinic |
| Space group | P-1 | Pbca | C2/c | C2/c |
| a/Å | 6.4635(7) | 11.2228(3) | 20.0403(8) | 20.549(10) |
| b/Å | 11.7170(18) | 10.0097(3) | 10.7329(4) | 11.138(5) |
| c/Å | 22.528(3) | 18.7285(4) | 11.1120(5) | 9.658(6) |
| α/° | 75.280(3) | 90 | 90 | 90 |
| β/° | 87.202(4) | 90 | 103.780(4) | 108.81(15) |
| γ/° | 83.880(3) | 90 | 90 | 90 |
| Volume/Å3 | 1640.3(4) | 2103.9 | 2321.29 | 2092.42 |
| Z | 2 | 16 | 16 | 8 |
| ρcalcg/cm3 | 1.220 | 1.302 | 1.283 | 1.191 |
| μ/mm−1 | 0.239 | 0.104 | 0.10 | 2.847 |
| F(000) | 656.0 | 896 | 976 | 816 |
| Crystal size/mm3 | 0.15 × 0.15 × 0.12 | 0.4 × 0.35 × 0.2 | 0.4 × 0.25 × 0.2 | 0.025 × 0.18 × 0.19 |
| Radiation | MoKα, λ = 0.71073 | MoKα, λ = 0.71073 | MoKα | CuKα, λ = 1.54187 |
| 2Θ range for data collection/° | 4.464 to 52.748 | 8.32 to 57.4 | 6.06 to 56.56 | to 140.0 |
| Index ranges | −8 ≤ h ≤ 8, −14 ≤ k ≤ 14, −28 ≤ l ≤ 28 | −15 ≤ h ≤ 15, −13 ≤ k ≤ 13, −25 ≤ l ≤ 25 | −26 ≤ h ≤ 26, −14 ≤ k ≤ 14, −14 ≤ l ≤ 14 | 0 ≤ h ≤ 24, 0 ≤ k ≤ 13, −11 ≤ l ≤ 11 |
| Reflections collected | 73,131 | 2712 | 23,107 | 1892 |
| Independent reflections | 6672 [Rint = 0.0338, Rsigma = 0.0175] | 2475 [Rint = 0.0314] | 2311 [Rint = 0.041] | 1394 |
| Data/restraints/parameters | 6672/0/393 | 2475/0/181 | 2891/0/196 | |
| Goodness-of-fit on F2 | 1.032 | 1.06 | 1.033 | 2.06 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0472, wR2 = 0.1218 | 0.041/0.1117 | 0.0422/0.1095 | 0.065 |
| Final R indexes [all data] | R1 = 0.0522, wR2 = 0.1262 | 0.0445/0.11448 | 0.0548/0.1190 | 0.065/0.071 |
| Largest diff. peak/hole/e Å−3 | 0.60/−0.46 | |||
| CCDC number/Code | 2,179,317 | MEDLUE–0 to 19 | MEDMAL | FINVAZ |
| D | H | A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/ |
|---|---|---|---|---|---|---|
| O33 | H33A | Cl2 | 0.84 | 2.34 | 3.165(2) | 169.3 |
| O33 | H33B | Cl2 1 | 0.87 | 2.28 | 3.141(2) | 170.1 |
| C152 | H152 | O61 2 | 0.98 | 3.10 | 3.767(2) | 126.9 |
| C42 | H42A | O33 3 | 0.97 | 2.56 | 3.503(3) | 163.8 |
| C42 | H42B | Cl1 3 | 0.97 | 2.65 | 3.562(2) | 156.1 |
| C41 | H41A | Cl2 | 0.97 | 3.13 | 3.763(2) | 124.2 |
| C41 | H41B | Cl1 4 | 0.97 | 2.98 | 3.787(2) | 141.1 |
| C41 | H41B | O23 4 | 0.97 | 3.02 | 3.881(4) | 148.8 |
| C31 | H31A | Cl1 4 | 0.96 | 3.17 | 3.895(2) | 133.8 |
| C31 | H31A | O43 2 | 0.96 | 2.89 | 3.81(2) | 160.7 |
| C31 | H31B | O61 2 | 0.96 | 3.12 | 3.723(3) | 122.7 |
| C11 | H11E | Cl2 | 0.96 | 2.96 | 3.668(3) | 131.4 |
| C11 | H11E | O33 | 0.96 | 2.98 | 3.849(3) | 150.8 |
| C11 | H11F | Cl2 2 | 0.96 | 2.98 | 3.899(2) | 159.7 |
| C11 | H11G | O23 4 | 0.96 | 3.01 | 3.892(4) | 153.7 |
| O13 | H13E | O33 | 0.85 | 1.95 | 2.789(4) | 169.0 |
| C12 | H12A | O33 3 | 0.96 | 2.85 | 3.709(4) | 150.1 |
| C12 | H12A | O13 3 | 0.96 | 3.04 | 3.645(5) | 122.3 |
| C12 | H12C | O13 5 | 0.96 | 2.87 | 3.657(4) | 140.2 |
| C12 | H12C | O43 5 | 0.96 | 3.00 | 3.68(2) | 129.6 |
| C32 | H32A | O43 5 | 0.96 | 2.33 | 3.13(2) | 140.3 |
| O23 | H23A | O13 | 0.85 | 2.24 | 2.851(6) | 128.4 |
| O23 | H23B | Cl1 | 0.85 | 2.28 | 3.121(3) | 168.4 |
| N22 | H22 | Cl2 | 0.85(2) | 2.27(2) | 3.0715(19) | 158(2) |
| N21 | H21 | O62 | 0.84(2) | 2.01(2) | 2.750(2) | 147(2) |
| N71 | H71 | Cl1 4 | 0.80(2) | 2.62(2) | 3.3321(16) | 149.3(19) |
| N72 | H72 | Cl1 3 | 0.83(2) | 2.43(2) | 3.2507(15) | 172.9(19) |
| O43 | H43A | O13 | 0.85 | 1.86 | 2.572(16) | 140.7 |
| O43 | H43A | O23 6 | 0.85 | 2.42 | 2.949(17) | 121.2 |
| O43 | H43B | Cl1 6 | 0.85 | 2.35 | 3.202(16) | 175.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chayrov, R.; Kalfin, R.; Lazarova, M.; Tancheva, L.; Sbirkova-Dimitrova, H.; Shivachev, B.; Stankova, I. Development of N,N-Dimethylglycine-Amantadine for Adjunctive Dopaminergic Application: Synthesis, Structure and Biological Activity. Crystals 2022, 12, 1227. https://doi.org/10.3390/cryst12091227
Chayrov R, Kalfin R, Lazarova M, Tancheva L, Sbirkova-Dimitrova H, Shivachev B, Stankova I. Development of N,N-Dimethylglycine-Amantadine for Adjunctive Dopaminergic Application: Synthesis, Structure and Biological Activity. Crystals. 2022; 12(9):1227. https://doi.org/10.3390/cryst12091227
Chicago/Turabian StyleChayrov, Radoslav, Reni Kalfin, Maria Lazarova, Lyubka Tancheva, Hrisitna Sbirkova-Dimitrova, Boris Shivachev, and Ivanka Stankova. 2022. "Development of N,N-Dimethylglycine-Amantadine for Adjunctive Dopaminergic Application: Synthesis, Structure and Biological Activity" Crystals 12, no. 9: 1227. https://doi.org/10.3390/cryst12091227
APA StyleChayrov, R., Kalfin, R., Lazarova, M., Tancheva, L., Sbirkova-Dimitrova, H., Shivachev, B., & Stankova, I. (2022). Development of N,N-Dimethylglycine-Amantadine for Adjunctive Dopaminergic Application: Synthesis, Structure and Biological Activity. Crystals, 12(9), 1227. https://doi.org/10.3390/cryst12091227







