The Crystal Structure of Sergeysmirnovite, MgZn2(PO4)2·4H2O, and Complexity of the Hopeite Group and Related Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Single-Crystal X-ray Diffraction
2.3. Complexity Calculations
3. Results
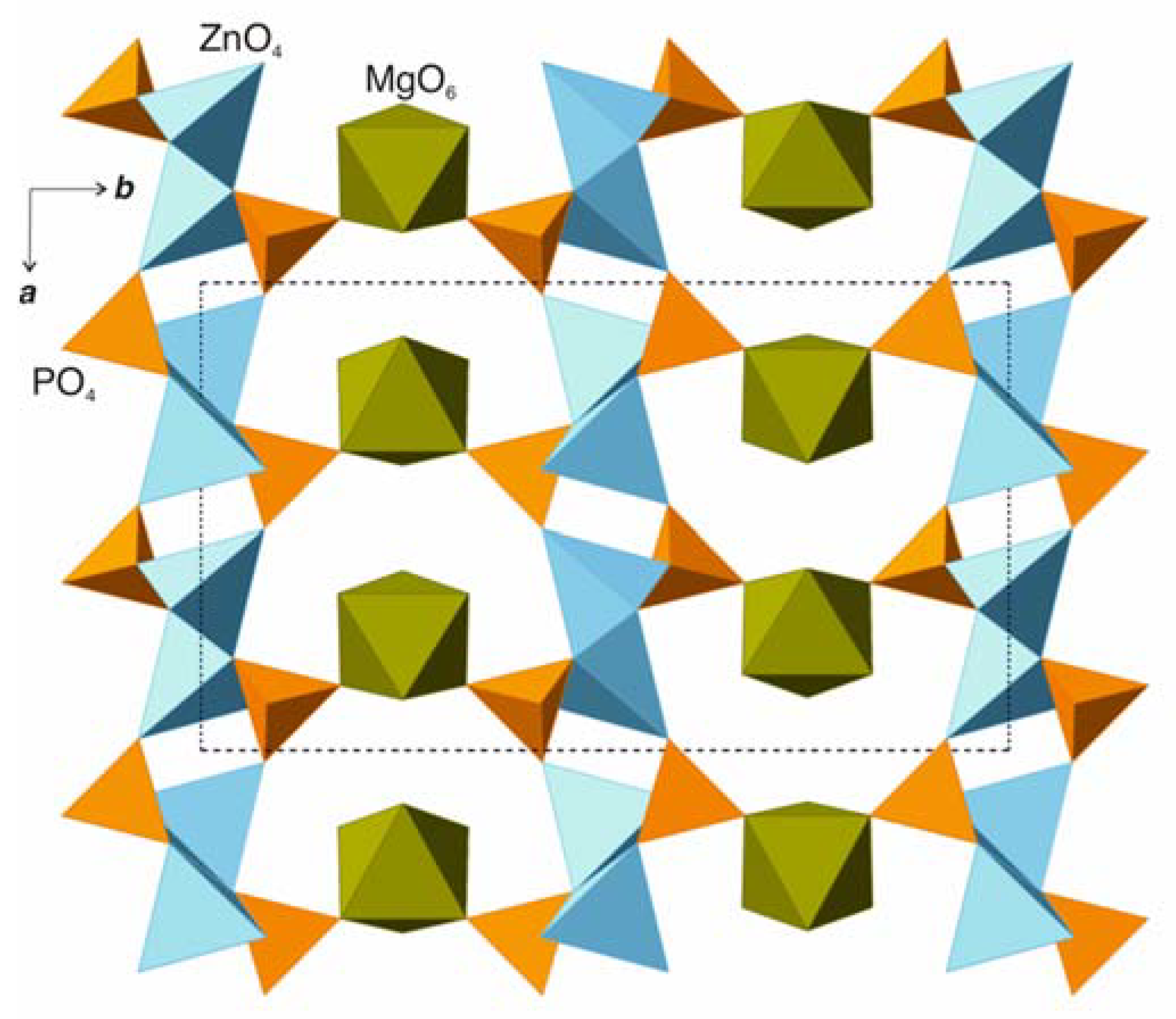
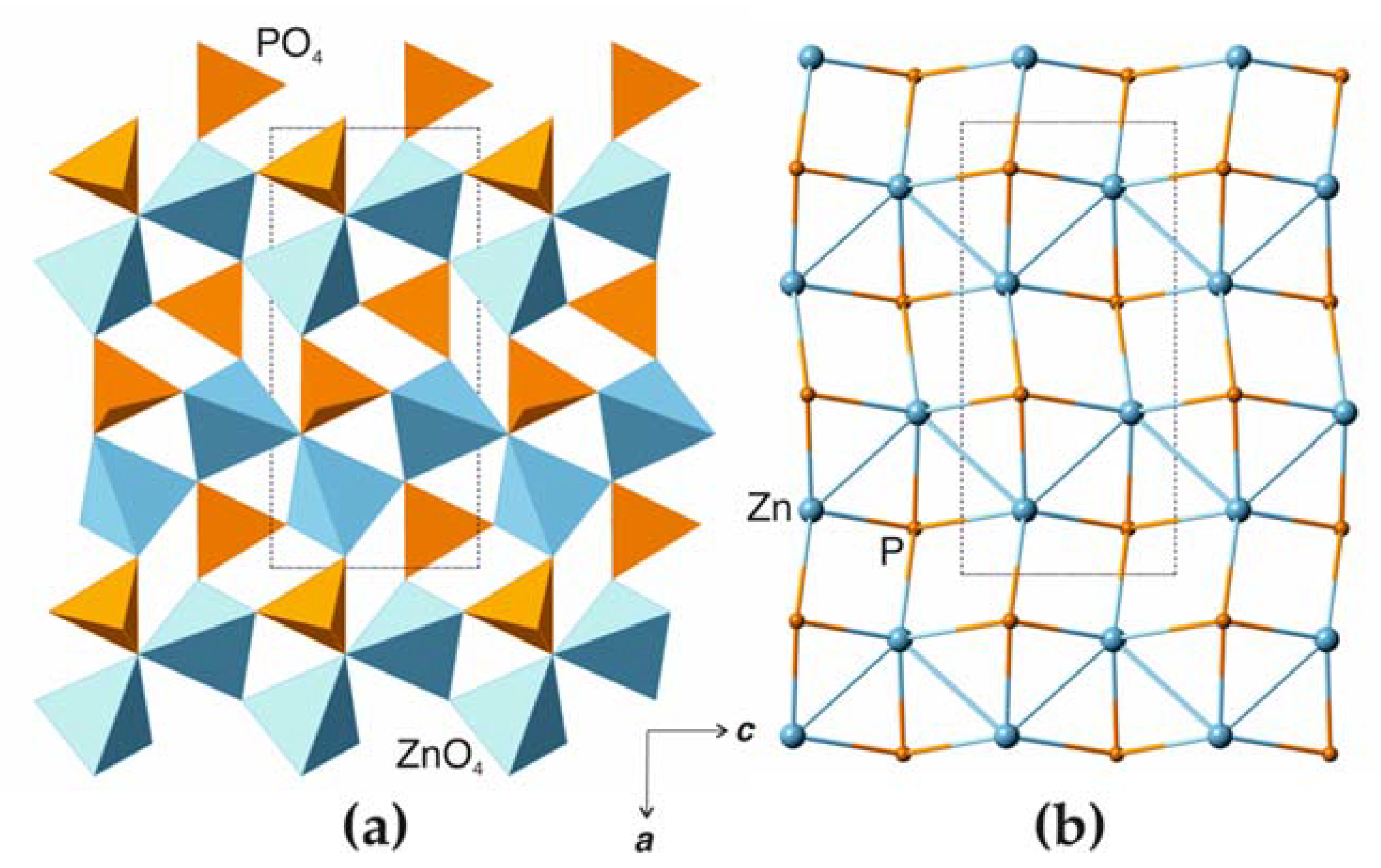
3.1. Structure Description
3.2. Hydrogen Bonding
4. Discussion
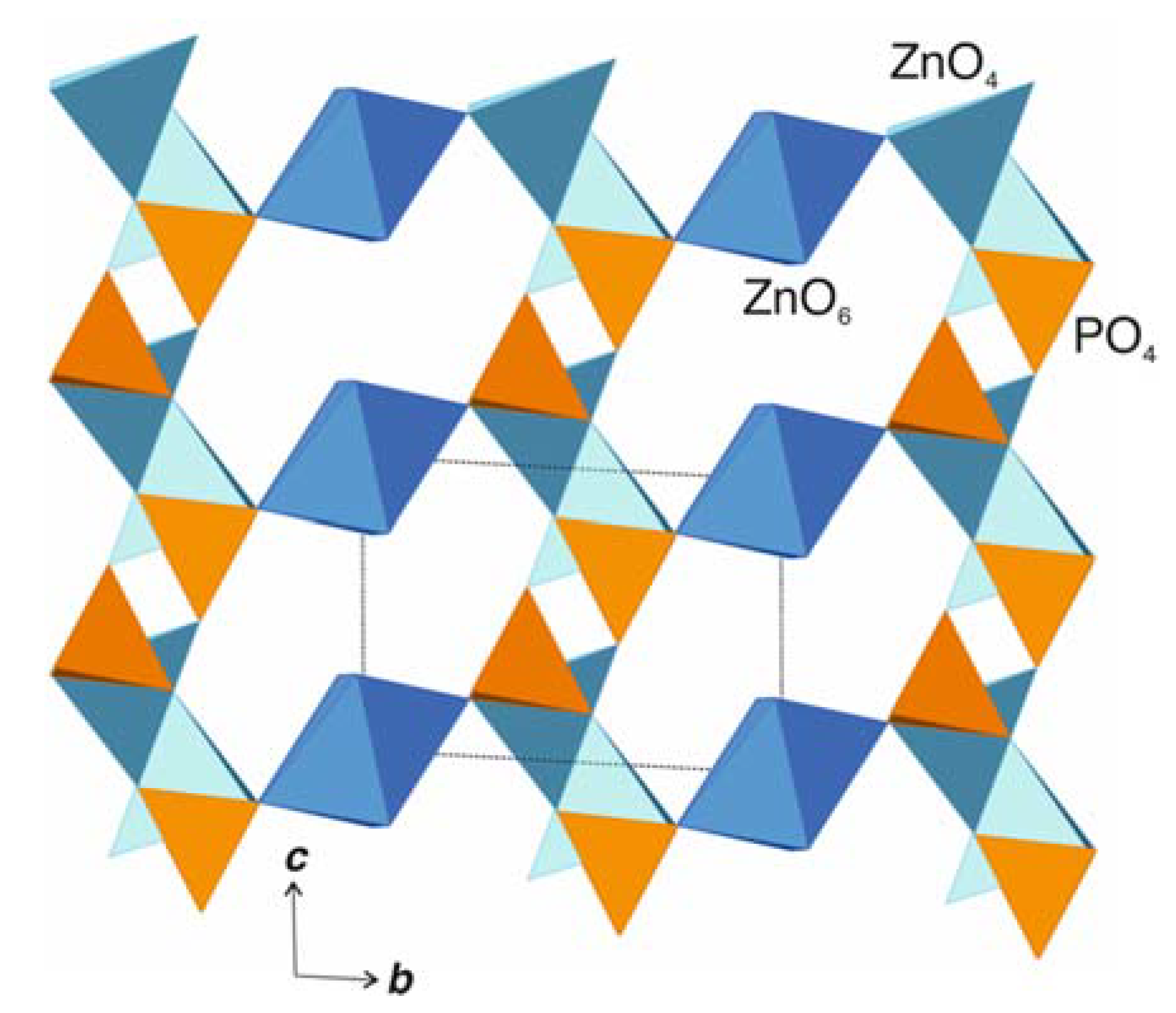
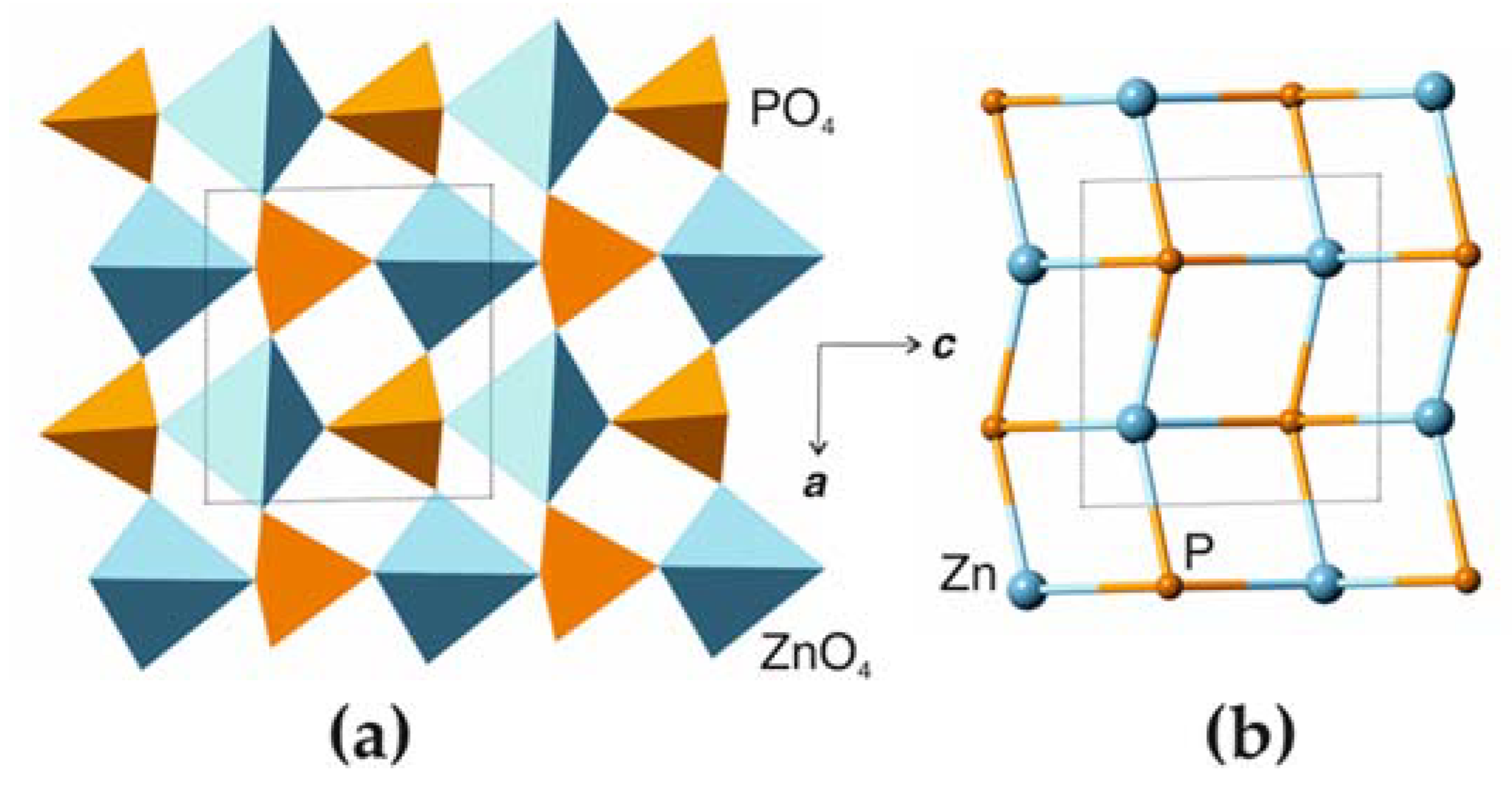
4.1. Comparison to Related Structures
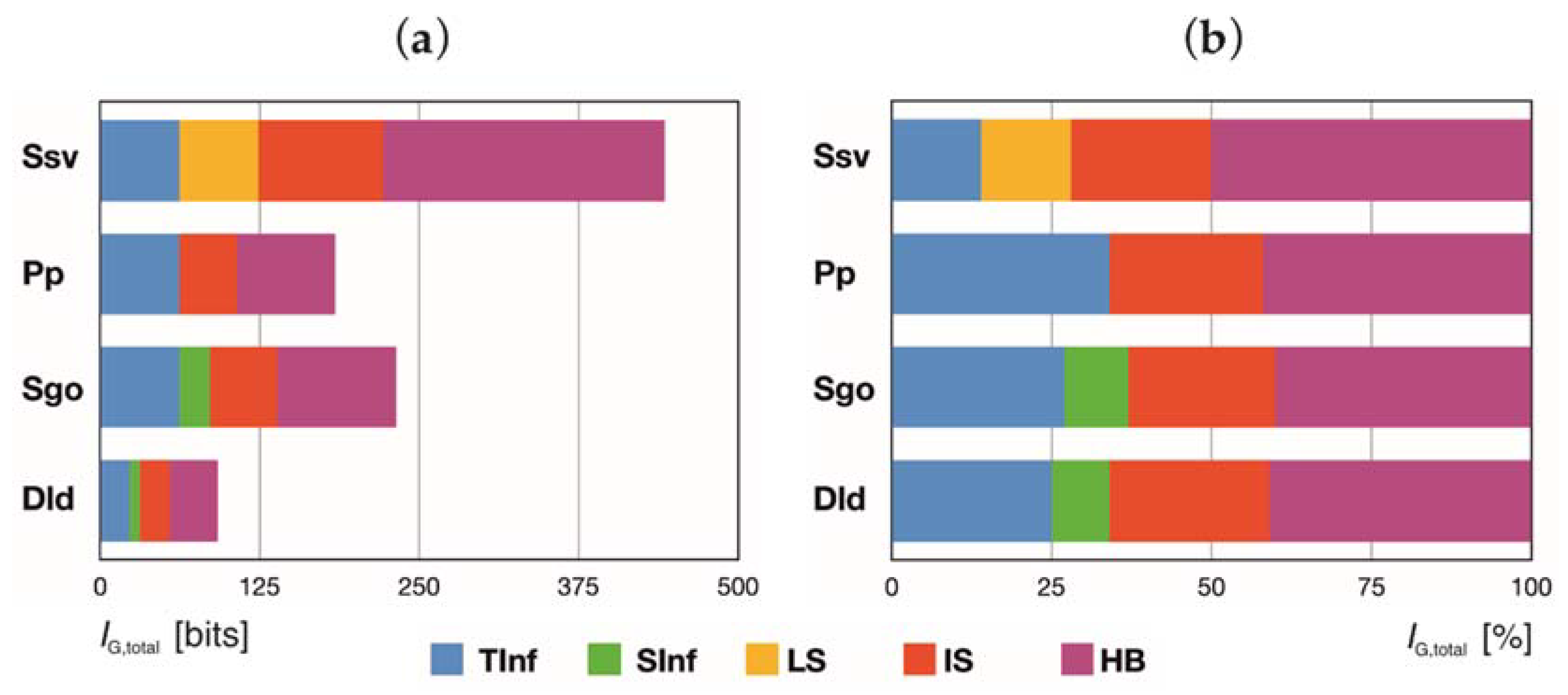
4.2. Complexity Analysis
4.3. Polymorphism in Hopeite and Related Structures
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arnaud, Y.; Sahakian, E.; Romand, M.; Charbonnier, J.C. Study of hopeite coatings. I. Pure hopeite thermal dehydration: Dihydrate, Zn3(PO4)2·2H2O, structure conformation. Appl. Surf. Sci. 1988, 32, 281–295. [Google Scholar] [CrossRef]
- Arnaud, Y.; Sahakian, E.; Lenoir, J.; Roche, A.; Charbonnier, J.C. Study of hopeite coatings. II. Study of polycationic hopeites: Structure and dehydration process. Appl. Surf. Sci. 1988, 32, 296–308. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, X. Anti-corrosion zinc phosphate coating on building steel via a facile one-step brushing method. Electrochem. Comm. 2019, 109, 106596. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.-Y.; Chen, C.-Z.; Lu, Y.-P.; Geng, X.-W. Hopeite and scholzite coatings formation on titanium via wet-chemical conversion with controlled temperature. Surf. Coat. Technol. 2020, 384, 125330. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.; Xiao, G.; Chen, C.; Lu, Y. Influence of surface post-processing on crystal refinement and characteristics of hopeite coating by phosphating. Coatings 2021, 11, 541. [Google Scholar] [CrossRef]
- Liu, J.; Jian, M.; Chen, L.; Xu, N.; Li, D.; Zhao, S. Effects of polyethylene glycol additive on zinc phosphate conversion coating of carbon steel. Chem. Papers 2022, 76, 409–416. [Google Scholar] [CrossRef]
- Riyas, A.H.; Geethanjali, C.V.; Arathy, S.; Anil, A.; Shibli, S.M.A. Exploration and tuning of Al2O3/Mo composite for enhancement of anti-corrosion and tribological characteristics in zinc phosphate conversion coatings. Appl. Surf. Sci. 2022, 593, 153370. [Google Scholar] [CrossRef]
- Rumyantsev, E.; Rumyantseva, V.; Konovalova, V. White Phosphate Coatings Obtained on Steel from Modified Cold Phosphating Solutions. Coatings 2022, 12, 70. [Google Scholar] [CrossRef]
- Margerit, J.; Cluzel, B.; Leloup, J.M.; Nurit, J.; Pauvert, B.; Terol, A. Chemical characterization of in vivo aged zinc phosphate dental cements. J. Mater. Sci. Mater. Med. 1996, 7, 623–628. [Google Scholar] [CrossRef]
- Osorio, R.; Cabello, I.; Toledano, M. Bioactivity of zinc-doped dental adhesives. J. Dent. 2014, 42, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Rolland, C.; Guidoin, R.; Ledoux, R.; Zerguini, A.; Roy, P.-E. Carbonate-hydroxylapatite, hopeite, and parascholzite in fibrous capsules surrounding silicone breast implants. Can. Mineral. 1991, 29, 337–345. [Google Scholar]
- Herschke, L.; Rottstegge, J.; Lieberwirth, I.; Wegner, G. Zinc phosphate as versatile material for potential biomedical applications Part 1. J. Mater. Sci. Mater. Med. 2006, 17, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Herschke, L.; Lieberwirth, I.; Wegner, G. Zinc phosphate as versatile material for potential biomedical applications Part II. J. Mater. Sci. Mater. Med. 2006, 17, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Brewster, D. VI. Description of Hopeite, a New Mineral, from Altenberg near Aix-la-Chapelle. Trans. Roy. Soc. Edinb. 1826, 10, 107–111. [Google Scholar] [CrossRef]
- Damour, M. Nouveaux essais sur la hopéite. Bull. Soc. Franc. Minér. 1879, 2, 131. [Google Scholar] [CrossRef]
- Friedel, L.; Sarasin, F. Sur la composition de la hopéite. Bull. Soc. Franc. Minér. 1879, 2, 153. [Google Scholar] [CrossRef]
- Mamedov, K.S.; Gamidov, R.; Belov, N.V. Crystal structure of hopeite, Zn3(PO4)2·4H2O. Sov. Phys. Crystallogr. 1961, 6, 91–94. [Google Scholar]
- Gamidov, R.; Golovachev, V.P.; Mamedov, K.S.; Belov, N.V. Crystal structure of hopeite, Zn3[PO4]2·2H2O. Dokl. Acad. Sci. USSR 1965, 150, 106–109. [Google Scholar]
- Liebau, F. Zur Kristallstruktur des Hopeits, Zn3[PO4]2·4H2O. Acta Crystallogr. 1965, 18, 352–354. [Google Scholar] [CrossRef]
- Kawahara, A.; Takano, Y.; Takahashi, M. The structure of hopeite. Mineral. J. 1973, 7, 289–297. [Google Scholar] [CrossRef]
- Nriagu, J.O. Solubility equilibrium constant of α-hopeite. Geochim. Cosmochim. Acta 1973, 37, 2357–2361. [Google Scholar] [CrossRef]
- Whitaker, A. The crystal structure of hopeite, Zn3(PO4)2·4H2O. Acta Crystallogr. 1975, B31, 2026–2035. [Google Scholar] [CrossRef]
- Hill, R.J.; Jones, J.B. The crystal structure of hopeite. Amer. Mineral. 1976, 61, 987–995. [Google Scholar]
- Haussühl, S.; Middendorf, B.; Dörffel, M. Structure and properties of hopeites (MgxZn1−x)3(PO4)2·4H2O. J. Solid State Chem. 1991, 93, 9–16. [Google Scholar] [CrossRef]
- Haussühl, S.; Friedrich, M. Crystal Growth and Elastic Properties of Hopeite, Zn3(PO4)·4H2O. Cryst. Res. Technol. 1993, 28, 437–440. [Google Scholar] [CrossRef]
- Pawlig, O.; Trettin, R. Synthesis and characterization of α-hopeite, Zn3(PO4)·4H2O. Mater. Res. Bull. 1999, 34, 1959–1966. [Google Scholar] [CrossRef]
- Schofield, P.F.; Knight, K.S.; Hodson, M.E.; Lanfranco, A.M. Thermal expansion of deuterated hopeite, Zn3(PO4)·4D2O. Amer. Mineral. 2007, 92, 1038–1047. [Google Scholar] [CrossRef]
- Parhi, P.; Manivannan, V.; Kohli, S.; McCurdy, P. Room temperature metathetic synthesis and characterization of α-hopeite, Zn3(PO4)·4H2O. Mater. Res. Bull. 2008, 43, 1836–1841. [Google Scholar] [CrossRef]
- Spencer, L.J. On hopeite and other zinc phosphates and associated minerals from the Broken Hill mines, North-Western Rhodesia. Mineral. Mag. 1908, 15, 1–38. [Google Scholar] [CrossRef]
- Herschke, L.; Enkelmann, V.; Lieberwirth, I.; Wegner, G. The role of hydrogen bonding in the crystal structures of zinc phosphate hydrates. Chem. Eur. J. 2004, 10, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Kumbasar, I.; Finney, J.J. The crystal structure of parahopeite. Mineral. Mag. 1968, 36, 621–624. [Google Scholar] [CrossRef][Green Version]
- Chao, G.Y. Refinement of the crystal structure of parahopeite. Z. Kristallogr. 1969, 130, 261–266. [Google Scholar] [CrossRef]
- Kampf, A.R.; Falster, A.U.; Simmons, W.B.; Whitmore, R.W. Nizamoffte, Mn2+Zn2(PO4)2(H2O)4, the Mn analogue of hopeite from the Palermo No. 1 pegmatite, North Groton, New Hampshire. Am. Mineral. 2013, 98, 1893–1898. [Google Scholar] [CrossRef]
- Neuhold, F.; Kolitsch, U.; Bernhardt, H.-J.; Lengauer, C.L. Arsenohopeite, a new zinc arsenate mineral from the Tsumeb mine, Namibia. Mineral. Mag. 2012, 76, 603–612. [Google Scholar] [CrossRef]
- Elliott, P. Reaphookhillite, MgZn2(PO4)2·4H2O, the Mg analogue of parahopeite from Reaphook Hill, South Australia. Mineral. Mag. 2022, in press. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Cooper, M.A.; Abdu, Y.A.; Ball, N.A.; Back, M.E.; Tait, K.T. Davidlloydite, ideally Zn3(AsO4)2(H2O)4, a new arsenate mineral from the Tsumeb mine, Otjikoto (Oshikoto) region, Namibia: Description and crystal structure. Mineral. Mag. 2012, 76, 45–57. [Google Scholar] [CrossRef]
- Ivanov, V.V.; Pyatenko, Y.A. On the so-called kësterite. Zap. Vses. Mineral. Obshch. 1958, 88, 165–168. (In Russian) [Google Scholar]
- Liu, X.; Feng, Y.; Cui, H.; Liu, F.; Hao, X.; Conibeer, G.; Mitzi, D.B.; Green, M. The current status and future prospects of kesterite solar cells: A brief review. Progr. Photovolt. Res. Appl. 2016, 24, 879–898. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Krivovichev, S.V.; Yakovenchuk, V.N.; Ivanyuk, G.Yu. The crystal structure of epifanovite. Zap. Ross. Mineral. Obshch. 2017, 146, 39–50. (In Russian) [Google Scholar]
- Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Konoplyova, N.G.; Panikorovskii, T.L.; Mikhailova, Y.A.; Bocharov, V.N.; Krivovichev, S.V.; Ivanyuk, G.Y. Epifanovite, NaCaCu5(PO4)4[AsO2(OH)2]·7H2O: A New Mineral from the Kester Deposit, Sakha (Yakutia) Republic, Russia. Geol. Ore Dep. 2018, 60, 587–593. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Konopleva, N.G.; Panikorovskii, T.L.; Bazai, A.; Mikhailova, J.A.; Bocharov, V.N.; Ivanyuk, G.Y.; Krivovichev, S.V. Batagayite, CaZn2(Zn,Cu)6(PO4)4(PO3OH)3·12H2O, a new phosphate mineral from Këster tin deposit (Yakutia, Russia): Occurrence and crystal structure. Mineral. Petrol. 2018, 112, 591–601. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Konopleva, N.G.; Panikorovskii, T.L.; Bazai, A.V.; Mikhailova, J.A.; Bocharov, V.N.; Krivovichev, S.V. Sergeysmirnovite, MgZn2(PO4)2·4H2O, a new mineral from the Kester deposit (Sakha-Yakutia, Russia). Dokl. Earth Sci. 2022, 505, 549–552. [Google Scholar]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and Chemical Complexity of Minerals: An Update. Mineral. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Hill, R.J. The crystal structure of phosphophyllite. Am. Mineral. 1977, 62, 812–817. [Google Scholar]
- Grey, I.; Keck, E.; Kampf, A.R.; Mumme, W.G.; Macrae, C.M.; Gable, R.W.; Glenn, A.M.; Davidson, C.J. Steinmetzite, Zn2Fe3+(PO4)2(OH)·3H2O, a new mineral formed from alteration of phosphophyllite at the Hagendorf Süd pegmatite, Bavaria. Mineral. Mag. 2017, 81, 329–338. [Google Scholar] [CrossRef]
- Rieck, B.; Giester, G.; Lengauer, C.L.; Chanmuang, N.C.; Topa, D. Stergiouite, CaZn2(AsO4)2·4H2O—A new mineral from the Lavrion Mining District, Greece. Mineral. Petrol. 2020, 114, 319–327. [Google Scholar] [CrossRef]
- CrysAlisPro; Version 1.171.36.20; Agilent Technologies Ltd.: Santa Clara, CA, USA, 2012.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Gurzhiy, V.V.; Plášil, J. Structural complexity of natural uranyl sulfates. Acta Crystallogr. 2019, B75, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hornfeck, W. On an extension of Krivovichev’s complexity measures. Acta Crystallogr. 2020, A76, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Kaußler, C.; Kieslich, G. CrystIT: Complexity and configurational entropy of crystal structures via information theory. J. Appl. Crystallogr. 2021, 54, 306–316. [Google Scholar] [CrossRef]
- Hallweger, S.A.; Kaußler, C.; Kieslich, G. The structural complexity of perovskites. Phys. Chem. Chem. Phys. 2022, 24, 9196–9202. [Google Scholar] [CrossRef] [PubMed]
- Hornfeck, W. Crystallographic complexity partition analysis. Z. Kristallogr. 2022, 237, 127–134. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topology of microporous structures. Rev. Miner. Geochem. 2005, 57, 17–68. [Google Scholar] [CrossRef]
- International Tables for Crystallography. Vol. E. Subperiodic Groups; Kopsky, V., Litvin, D.B., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2002. [Google Scholar]
- Krivovichev, S.V. Ladders of information: What contributes to the structural complexity in inorganic crystals. Z. Kristallogr. 2018, 233, 155–161. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC Approved Mineral Symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Goldsmith, J.R. A “simplexity principle” and its relation to “ease” of crystallization. J. Geol. 1953, 61, 439–451. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Hawthorne, F.C.; Williams, P.A. Structural complexity and crystallization: The Ostwald sequence of phases in the Cu2(OH)3Cl system (botallackite–atacamite–clinoatacamite). Struct. Chem. 2017, 28, 153–159. [Google Scholar] [CrossRef]
- Plášil, J. Structural complexity of uranophane and uranophane-β: Implications for their formation and occurrence. Eur. J. Mineral. 2018, 30, 253–257. [Google Scholar] [CrossRef]
- Bhakat, A.; Pal, A.; Siddaramaiah, R.; Chattopadhyay, A. Complexation-Based Super Crystalline Assembly of Zinc Oxide Quantum Dots for Sensitive Carbon Dioxide Gas Sensing. J. Phys. Chem. C 2021, 125, 12316–12323. [Google Scholar] [CrossRef]


| Mineral Name | Chemical Formula | Space Group | a [Å]/a [°] | b [Å]/b [°] | c [Å]/g [°] | V [Å3] | Ref. |
|---|---|---|---|---|---|---|---|
| Hopeite structure type | |||||||
| Hopeite | Zn3(PO4)2·4H2O | Pnma | 10.597/90 | 18.318/90 | 5.031/90 | 976.6 | [28] |
| Nizamoffite | Mn2+Zn2(PO4)2·4H2O | Pnma | 10.653/90 | 18.478/90 | 5.058/90 | 995.7 | [33] |
| Sergeysmirnovite | MgZn2(PO4)2·4H2O | Pnma | 10.629/90 | 18.370/90 | 5.021/90 | 980.3 | this work |
| Arsenohopeite | Zn3(AsO4)2·4H2O | Pnma | 10.804/90 | 19.003/90 | 5.112/90 | 1049.5 | [34] |
| Parahopeite structure type | |||||||
| Parahopeite | Zn3(PO4)2·4H2O | 5.768/93.42 | 7.550/91.18 | 5.276/91.37 | 228.3 | [32] | |
| Reaphookhillite | MgZn2(PO4)2·4H2O | 5.759/93.44 | 7.534/91.27 | 5.279/91.30 | 228.5 | [35] | |
| Davidlloydite | Zn3(AsO4)2·4H2O | 5.976/84.29 | 7.600/90.49 | 5.447/88.00 | 246.0 | [36] | |
| Related structures | |||||||
| Phosphophyllite | Fe2+Zn2(PO4)2·4H2O | P21/c | 10.378/90 | 5.084/121.14 | 10.553/90 | 476.6 | [48] |
| Steinmetzite | Fe3+Zn2(PO4)2(OH)·3H2O | 10.438/91.37 | 5.102/115.93 | 10.546/94.20 | 502.7 | [49] | |
| Stergiouite | CaZn2(AsO4)2·4H2O | Pc | 9.416/90 | 5.300/91.77 | 10.893/90 | 543.4 | [50] |
| Temperature/K | 293(2) |
| Crystal system | orthorhombic |
| Space group | Pnma |
| a/Å | 10.6286(4) |
| b/Å | 18.3700(6) |
| c/Å | 5.02060(15) |
| Volume/Å3 | 980.26(6) |
| Z | 4 |
| Dcalc, g/cm3 | 2.933 |
| μ/mm−1 | 6.130 |
| F(000) | 856.0 |
| Crystal size/mm3 | 0.23 × 0.14 × 0.12 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 7.668 to 61.952 |
| Index ranges | −8 ≤ h ≤ 15, −22 ≤ k ≤ 24, −7 ≤ l ≤ 6 |
| Reflections collected | 4164 |
| Independent reflections | 1435 [Rint = 0.0251, Rsigma = 0.0261] |
| Data/restraints/parameters | 1435/3/85 |
| Goodness-of-fit on F2 | 1.162 |
| Final R indices [I ≥ 2σ (I)] | R1 = 0.0304, wR2 = 0.0831 |
| Final R indices [all data] | R1 = 0.0359, wR2 = 0.0861 |
| Largest diff. peak/hole/e Å−3 | 0.73/−0.80 |
| Site | s.o.f. | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|---|
| Zn | Zn | 0.85696(3) | −0.00084(2) | 0.29290(7) | 0.00857(13) |
| Mg | Mg0.66Zn0.34 | 0.76076(7) | ¼ | 0.92640(16) | 0.0089(3) |
| P | P | 0.89752(7) | 0.09434(4) | 0.77589(14) | 0.00931(17) |
| O1 | O | 0.8600(2) | 0.1727(1) | 0.7173(4) | 0.0153(5) |
| O2 | O | 0.0254(2) | 0.0784(1) | 0.6446(5) | 0.0162(4) |
| O3 | O | 0.8999(3) | 0.0800(1) | 0.0749(4) | 0.0256(6) |
| O4 | O | 0.69790(19) | −0.0398(1) | 0.1428(4) | 0.0110(4) |
| Ow5 | O | 0.8906(3) | ¼ | 0.2437(6) | 0.0119(6) |
| Ow6 | O | 0.6138(3) | ¼ | 0.6449(6) | 0.0141(6) |
| Ow7 | O | 0.6635(2) | 0.3307(1) | 0.1582(5) | 0.0157(4) |
| H5A | H0.50 | 0.890421 | 0.293569 | 0.371961 | 0.021 * |
| H5B | H0.50 | 0.974113 | 0.250002 | 0.110938 | 0.021 * |
| H6A | H0.50 | 0.526323 | 0.233849 | 0.713066 | 0.021 * |
| H6B | H0.50 | 0.610751 | 0.292141 | 0.539091 | 0.021 * |
| H7A | H | 0.616338 | 0.312024 | 0.308925 | 0.024 * |
| H7B | H | 0.601176 | 0.358934 | 0.065064 | 0.024 * |
| Zn–O4 | 1.984(2) | Mg–Ow7 | 2.149(2) 2x | P–O4 | 1.575(2) |
| Zn–O4 | 1.995(2) | Mg–O1 | 2.058(2) 2x | P–O3 | 1.524(2) |
| Zn–O3 | 1.901(2) | Mg–Ow5 | 2.108(3) | P–O2 | 1.539(2) |
| Zn–O2 | 1.921(2) | Mg–Ow6 | 2.107(3) | P–O1 | 1.521(2) |
| <Zn–O> | 1.950 | <Mg–O> | 2.104 | <P–O> | 1.540 |
| O4–Zn–O4 | 102.70(6) | Ow7–Mg–Ow7 | 87.2(1) 2× | O3–P–O4 | 108.5(1) |
| O4–Zn–O3 | 105.45(10) | Ow7–Mg–O1 | 92.7(1) 2× | O3–P–O2 | 112.0(1) |
| O4–Zn–O3 | 106.59(9) | Ow7–Mg–O1 | 177.4(1) 2× | O2–P–O4 | 105.4(1) |
| O4–Zn–O2 | 121.18(11) | O1–Mg–O1 | 87.3(1) | O1–P–O4 | 110.6(1) |
| O4–Zn–O2 | 110.46(9) | O1–Mg–Ow6 | 92.2(1) 2× | O1–P–O3 | 110.9(1) |
| O3–Zn–O2 | 108.87(9) | O1–Mg–Ow5 | 92.9(1) | O1–P–O2 | 109.2(1) |
| <O–Zn–O> | 109.2 | Ow5–Mg–Ow7 | 84.5(1) 2× | <O–P–O> | 109.4 |
| Ow6–Mg–Ow7 | 90.4(1) 2× | ||||
| Ow5–Mg–Ow6 | 173.0(1) |
| D | H | A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| Ow7 | H7A | Ow6 | 0.97 | 2.04 | 2.906(3) | 148.3 |
| Ow7 | H7B | O2 | 0.96 | 1.76 | 2.695(3) | 163.8 |
| Ow6 | H6A | O1 | 1.03 | 2.12 | 3.126(4) | 162.7 |
| Ow6 | H6A | O1 | 1.03 | 2.49 | 3.126(4) | 119.1 |
| Ow6 | H6B | Ow7 | 0.94 | 2.11 | 2.906(3) | 141.1 |
| Ow5 | H5A | O1 | 1.03 | 1.87 | 2.789(3) | 147.2 |
| Mineral Name | Chemical Formula | IG [bit/atom] | IG,total [bit/cell] | r [g/cm3] | Ref. |
|---|---|---|---|---|---|
| Hopeite | Zn3(PO4)2·4H2O | 3.844 | 384.386 | 3.096 | [28] |
| Parahopeite | Zn3(PO4)2·4H2O | 3.684 * | 92.096 * | 3.304 | [42] |
| Sergeysmirnovite | MgZn2(PO4)2·4H2O | 3.950 | 442.424 | 2.933 | this work |
| Reaphookhillite | MgZn2(PO4)2·4H2O | 3.684 | 92.096 | 3.090 | [43] |
| Arsenohopeite | Zn3(AsO4)2·4H2O | 3.844 | 384.386 | 3.420 | [41] |
| Davidlloydite | Zn3(AsO4)2·4H2O | 3.684 | 92.096 | 3.661 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivovichev, S.V.; Panikorovskii, T.L.; Yakovenchuk, V.N. The Crystal Structure of Sergeysmirnovite, MgZn2(PO4)2·4H2O, and Complexity of the Hopeite Group and Related Structures. Crystals 2022, 12, 1120. https://doi.org/10.3390/cryst12081120
Krivovichev SV, Panikorovskii TL, Yakovenchuk VN. The Crystal Structure of Sergeysmirnovite, MgZn2(PO4)2·4H2O, and Complexity of the Hopeite Group and Related Structures. Crystals. 2022; 12(8):1120. https://doi.org/10.3390/cryst12081120
Chicago/Turabian StyleKrivovichev, Sergey V., Taras L. Panikorovskii, and Victor N. Yakovenchuk. 2022. "The Crystal Structure of Sergeysmirnovite, MgZn2(PO4)2·4H2O, and Complexity of the Hopeite Group and Related Structures" Crystals 12, no. 8: 1120. https://doi.org/10.3390/cryst12081120
APA StyleKrivovichev, S. V., Panikorovskii, T. L., & Yakovenchuk, V. N. (2022). The Crystal Structure of Sergeysmirnovite, MgZn2(PO4)2·4H2O, and Complexity of the Hopeite Group and Related Structures. Crystals, 12(8), 1120. https://doi.org/10.3390/cryst12081120








