Adsorption of Pesticides, Antibiotics and Microcystin-LR by Graphene and Hexagonal Boron Nitride Nano-Systems: A Semiempirical PM7 and Theoretical HSAB Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Model
2.2. Computational Method
2.3. HSAB Global Descriptor
3. Results and Discussion
3.1. Non-Covalent Interaction (NCI)
3.2. The Reaction Enthalpy, Equilibrium Structure and Atomic Charge
3.3. The Co-Adsorption of Microcystin-LR
3.4. The Hard and Soft Acid and Base (HSAB) Global Descriptor
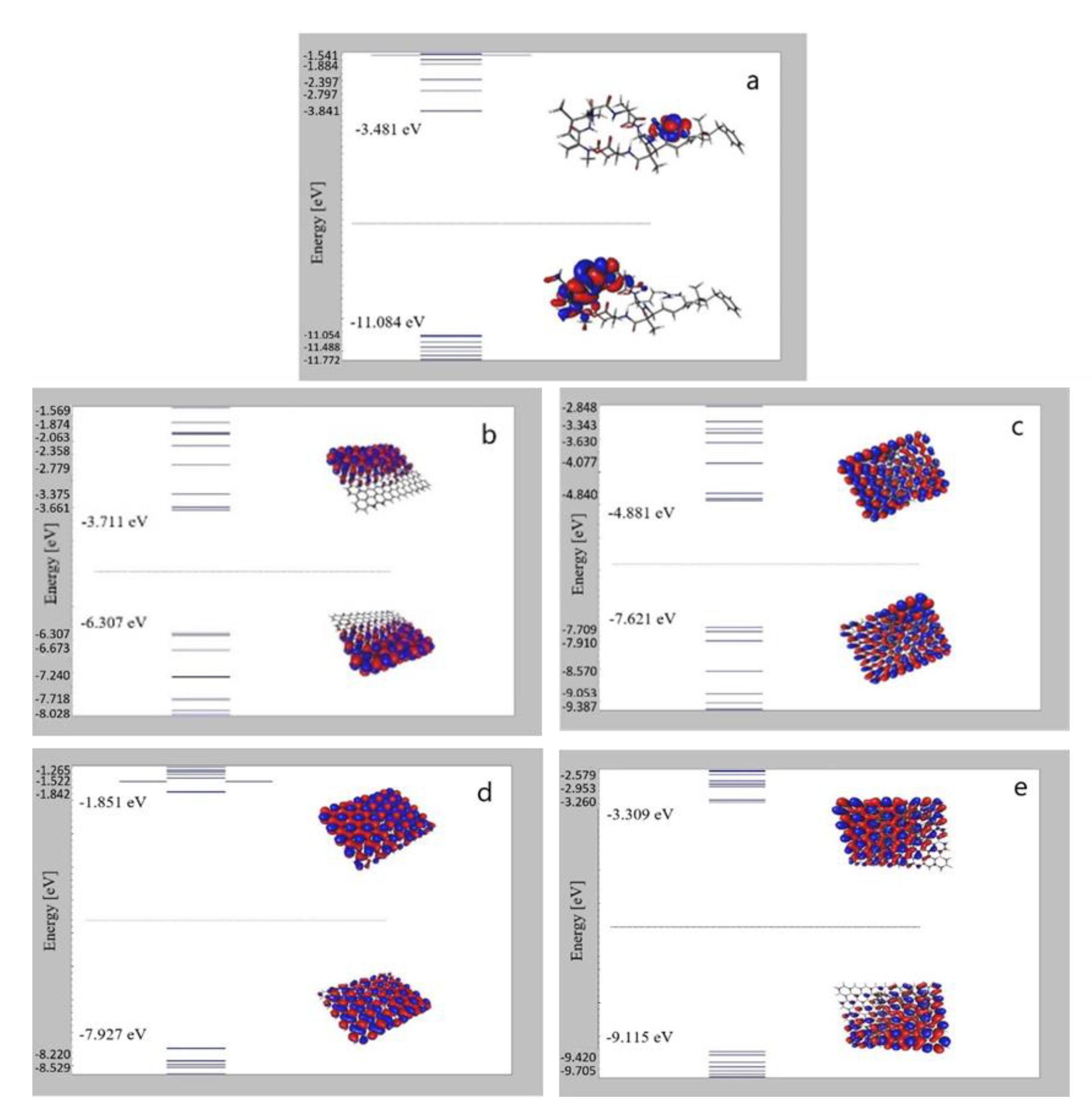
3.5. The Frontier Molecular Orbital (FMO)
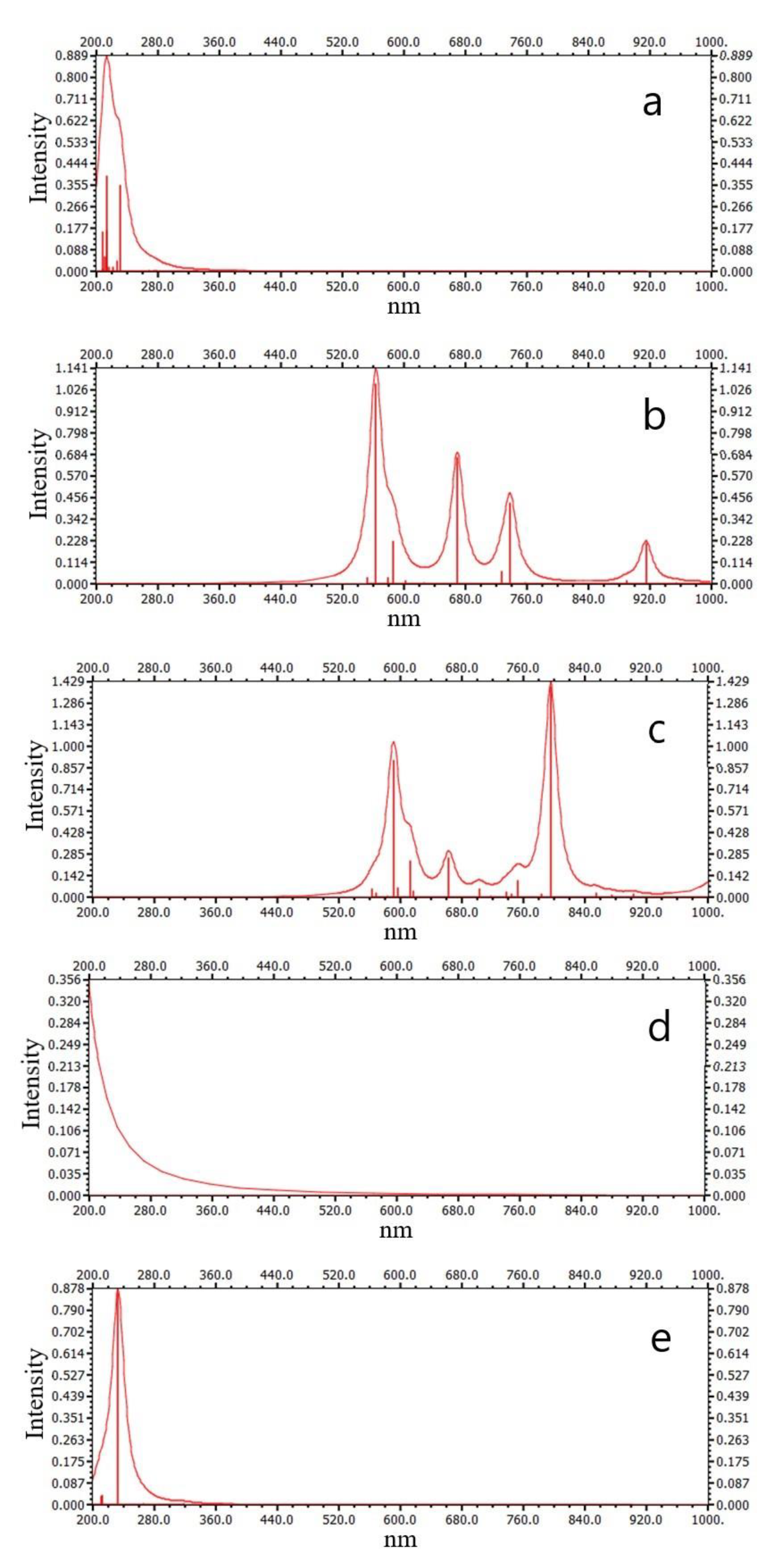
3.6. The Electronic Excitation Spectrum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Sadeghi, N.; Tyagi, I.; Gupta, V.K.; Fakhri, A. Adsorption of toxic carbamate pesticide oxamyl from liquid phase by newly synthesized and characterized graphene quantum dots nanomaterials. J. Colloid Interface Sci. 2016, 478, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Mehmeti, V.; Halili, J.; Berisha, A. Which is better for Lindane pesticide adsorption, graphene or graphene oxide? An experimental and DFT study. J. Mol. Liq. 2022, 347, 118345. [Google Scholar] [CrossRef]
- Paramasivan, T.; Sivarajasekar, N.; Muthusaravanan, S.; Subashini, R.; Prakashmaran, J.; Sivamani, S.; Ajmal Koya, P. Graphene Family Materials for the Removal of Pesticides from Water. In A New Generation Material Graphene: Applications in Water Technology; Naushad, M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 309–327. [Google Scholar]
- Pellenz, L.; da Silva, L.J.S.; Mazur, L.P.; Figueiredo, G.M.d.; Borba, F.H.; Ulson de Souza, A.A.; Guelli Ulson de Souza, S.M.A.; da Silva, A. Functionalization of graphene with nitrogen-based groups for water purification via adsorption: A review. J. Water Process Eng. 2022, 48, 102873. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, L.; Xu, Q.; Tang, S.; Yu, Y. Using Post-graphene 2D Materials to Detect and Remove Pesticides: Recent Advances and Future Recommendations. Bull. Environ. Contam. Toxicol. 2020, 107, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef]
- Lazarević-Pašti, T.; Anićijević, V.; Baljozović, M.; Anićijević, D.V.; Gutić, S.; Vasić, V.; Skorodumova, N.V.; Pašti, I.A. The impact of the structure of graphene-based materials on the removal of organophosphorus pesticides from water. Environ. Sci. Nano 2018, 5, 1482–1494. [Google Scholar] [CrossRef]
- Ihsanullah, I. Boron nitride-based materials for water purification: Progress and outlook. Chemosphere 2021, 263, 127970. [Google Scholar] [CrossRef]
- Bangari, R.S.; Sinha, N. Adsorption of tetracycline, ofloxacin and cephalexin antibiotics on boron nitride nanosheets from aqueous solution. J. Mol. Liq. 2019, 293, 111376. [Google Scholar] [CrossRef]
- Singla, P.; Goel, N.; Singhal, S. Affinity of boron nitride nanomaterials towards antibiotics established by exhaustive experimental and theoretical investigations. Chem. Eng. J. 2016, 299, 403–414. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Wang, S.; Wang, Y.; Song, H.; Lv, S.; Li, C. Adsorption performance and mechanism of antibiotics from aqueous solutions on porous boron nitride–carbon nanosheets. Environ. Sci. Water Res. Technol. 2020, 6, 1568–1575. [Google Scholar] [CrossRef]
- Song, Q.; Fang, Y.; Liu, Z.; Li, L.; Wang, Y.; Liang, J.; Huang, Y.; Lin, J.; Hu, L.; Zhang, J.; et al. The performance of porous hexagonal BN in high adsorption capacity towards antibiotics pollutants from aqueous solution. Chem. Eng. J. 2017, 325, 71–79. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Pang, H.; Zhang, R.; Song, W.; Fu, D.; Hayat, T.; Wang, X. Boron nitride-based materials for the removal of pollutants from aqueous solutions: A review. Chem. Eng. J. 2018, 333, 343–360. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Chen, J.; Wu, P.; Wu, X.; Li, H.; Han, C.; Yan, S. Development of novel graphene-like layered hexagonal boron nitride for adsorptive removal of antibiotic gatifloxacin from aqueous solution. Green Chem. Lett. Rev. 2014, 7, 330–336. [Google Scholar] [CrossRef]
- Goli, A.; Alinezhad, H.; Ganji, M.D. Theoretical insights into the performance of graphene derivatives, h-BN and BNC heterostructures in the adsorption and elimination of atrazine: An all-electron DFT study. Diam. Relat. Mater. 2020, 108, 107967. [Google Scholar] [CrossRef]
- Song, Q.; Liang, J.; Fang, Y.; Cao, C.; Liu, Z.; Li, L.; Huang, Y.; Lin, J.; Tang, C. Selective adsorption behavior/mechanism of antibiotic contaminants on novel boron nitride bundles. J. Hazard. Mater. 2019, 364, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, K.; Li, D.; Tang, Y. Boron nitride adsorbents with sea urchin-like structures for enhanced adsorption performance. J. Am. Ceram. Soc. 2021, 104, 1601–1610. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, J.; Li, H.; Wu, P.; Li, X.; Chang, H.; He, J.; Wu, H.; Li, H.; Zhu, W. Synthesis of boron nitride nanosheets with N-defects for efficient tetracycline antibiotics adsorptive removal. Chem. Eng. J. 2020, 387, 124138. [Google Scholar] [CrossRef]
- Jalali Sarvestani, M.R.; Doroudi, Z. Nalidixic Acid Adsorption on the Surface of Boron Nitride Nanocluster (B12N12): DFT Studies. Adv. J. Chem. Sect. A 2020, 3, 740–749. [Google Scholar] [CrossRef]
- Angizi, S.; Khalaj, M.; Alem, S.A.A.; Pakdel, A.; Willander, M.; Hatamie, A.; Simchi, A. Review—Towards the Two-Dimensional Hexagonal Boron Nitride (2D h-BN) Electrochemical Sensing Platforms. J. Electrochem. Soc. 2020, 167, 126513. [Google Scholar] [CrossRef]
- Yu, X.; Huang, C.; Wu, L.; Hua, S.; Ye, J.; Meng, L.; Xu, C. Effects of the herbicides quizalofop-p-ethyl and quizalofop-ethyl on the physiology, oxidative damage, synthesis, and release of microcystin-LR in Microcystis aeruginosa. Sci. Total Environ. 2021, 776, 146036. [Google Scholar] [CrossRef]
- Wan, L.; Wu, Y.; Zhang, B.; Yang, W.; Ding, H.; Zhang, W. Effects of moxifloxacin and gatifloxacin stress on growth, photosynthesis, antioxidant responses, and microcystin release in Microcystis aeruginosa. J. Hazard. Mater. 2021, 409, 124518. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Du, Y.; Wang, L.; Qian, J.; Chen, J.; Wu, Q.; Hu, X. Toxin Release of Cyanobacterium Microcystis aeruginosa after Exposure to Typical Tetracycline Antibiotic Contaminants. Toxins 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Cerbin, S.; Kraak, M.H.S.; de Voogt, P.; Visser, P.M.; Van Donk, E. Combined and single effects of pesticide carbaryl and toxic Microcystis aeruginosa on the life history of Daphnia pulicaria. Hydrobiologia 2010, 643, 129–138. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, B.; Yue, Q.; Guan, Y.; Wang, Y.; Huang, L. Influences of two antibiotic contaminants on the production, release and toxicity of microcystins. Ecotoxicol. Environ. Saf. 2012, 77, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Malécot, M.; Guével, B.; Pineau, C.; Holbech, B.F.; Bormans, M.; Wiegand, C. Specific Proteomic Response of Unio pictorum Mussel to a Mixture of Glyphosate and Microcystin-LR. J. Proteome Res. 2013, 12, 5281–5292. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Zhang, J. Antibiotic contaminants reduced the treatment efficiency of UV-C on Microcystis aeruginosa through hormesis. Environ. Pollut. 2020, 261, 114193. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Tretyakov, E.V. Determination of graphene’s edge energy using hexagonal graphene quantum dots and PM7 method. Phys. Chem. Chem. Phys. 2018, 20, 14740–14752. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Majumder, M.; Tiwari, A.K. Oxygen-Induced Dissociation of a Single Water Molecule in Confined 2-D Layers: A Semiempirical Study. Chemphyschem 2022, e202200242. [Google Scholar] [CrossRef]
- Trogen, G.-B.; Annila, A.; Eriksson, J.; Kontteli, M.; Meriluoto, J.; Sethson, I.; Zdunek, J.; Edlund, U. Conformational Studies of Microcystin-LR Using NMR Spectroscopy and Molecular Dynamics Calculations. Biochemistry 1996, 35, 3197–3205. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Allouche, A.-R. Gabedit—A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G.; Songstad, J. Application of the Principle of Hard and Soft Acids and Bases to Organic Chemistry. J. Am. Chem. Soc. 1967, 89, 1827–1836. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Parr, R.G. Density functional theory of atoms and molecules. In Horizons of Quantum Chemistry; Springer: Berlin/Heidelberg, Germany, 1980; pp. 5–15. [Google Scholar]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Anicijevic, V.; Jelić, M.; Jovanović, A.; Potkonjak, N.; Pasti, I.; Lazarević-Pašti, T. Organophosphorous pesticide removal from water by graphene-based materials -Only adsorption or something else as well? J. Serb. Chem. Soc. 2021, 86, 699–710. [Google Scholar] [CrossRef]
- Yadav, S.; Goel, N.; Kumar, V.; Singhal, S. Graphene Oxide as Proficient Adsorbent for the Removal of Harmful Pesticides: Comprehensive Experimental Cum DFT Investigations. Anal. Chem. Lett. 2019, 9, 291–310. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Gao, Z.; Zhang, F.; Wang, J. Emerging role of graphene oxide as sorbent for pesticides adsorption: Experimental observations analyzed by molecular modeling. J. Mater. Sci. Technol. 2021, 63, 192–202. [Google Scholar] [CrossRef]
- Duverger, E.; Picaud, F. Theoretical study of ciprofloxacin antibiotic trapping on graphene or boron nitride oxide nanoflakes. J. Mol. Modeling 2020, 26, 135. [Google Scholar] [CrossRef]
- Beheshtian, J.; Sadeghi, A.; Neek-Amal, M.; Michel, K.H.; Peeters, F.M. Induced polarization and electronic properties of carbon-doped boron nitride nanoribbons. Phys. Rev. B 2012, 86, 195433. [Google Scholar] [CrossRef]
- Seyed-Talebi, S.M.; Neek-Amal, M. The different adsorption mechanism of methane molecule onto a boron nitride and a graphene flakes. J. Appl. Phys. 2014, 116, 153507. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Sillanpää, M.; Makgwane, P.R.; Kumar, S.; Kumari, K. Carbon nano-structures and functionalized associates: Adsorptive detoxification of organic and inorganic water pollutants. Inorg. Chem. Commun. 2022, 141, 109579. [Google Scholar] [CrossRef]
- Farmanzadeh, D.; Rezaeinejad, H. Adsorption of parathion and chlorpyrifos organophosphoros pesticides with the iron doped boron nitride nanotubes; A theoretical study. J. Appl. Chem. 2017, 12, 215–232. [Google Scholar] [CrossRef]
- Farmanzadeh, D.; Rezainejad, H. DFT Study of Adsorption of Diazinon, Hinosan, Chlorpyrifos and Parathion Pesticides on the Surface of B36N36 Nanocage and Its Fe Doped Derivatives as New Adsorbents. Acta Phys. Chim. Sin. 2016, 32, 1191–1198. [Google Scholar] [CrossRef]
- Doroudi, Z.; Jalali Sarvestani, M.R. Boron nitride nanocone as an adsorbent and senor for Ampicillin: A Computational Study. J. Chem. Rev. Lett. 2020, 3, 110–116. [Google Scholar] [CrossRef]
- Chao, Y.; Tang, B.; Luo, J.; Wu, P.; Tao, D.; Chang, H.; Chu, X.; Huang, Y.; Li, H.; Zhu, W. Hierarchical porous boron nitride with boron vacancies for improved adsorption performance to antibiotics. J. Colloid Interface Sci. 2021, 584, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Asencio, L.; García-Hernández, E.; Salazar-Villanueva, M.; Chigo-Anota, E. B12N12 nanocages with homonuclear bonds as a promising material in the removal/degradation of the insecticide imidacloprid. Phys. E: Low-Dimens. Syst. Nanostructures 2021, 126, 114456. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Zhang, J.; Chen, H.; Shao, B. Determination of pesticide residues and microcystin in drinking water using on-line SPE liquid chromatography-mass spectrometry. J. Hyg. Res. 2012, 41, 235–239. [Google Scholar]
- Cao, Q.; Steinman, A.D.; Yao, L.; Xie, L. Effects of light, microorganisms, farming chemicals and water content on the degradation of microcystin-LR in agricultural soils. Ecotoxicol. Environ. Saf. 2018, 156, 141–147. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhang, J.; Gao, B. Growth, microcystin-production and proteomic responses of Microcystis aeruginosa under long-term exposure to amoxicillin. Water Res. 2016, 93, 141–152. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Ashok Kumar, S.S.; Bashir, S.; Ramesh, K.; Ramesh, S. A review on graphene and its derivatives as the forerunner of the two-dimensional material family for the future. J. Mater. Sci. 2022, 57, 12236–12278. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Zheng, X.; Liu, C.; Cui, X.; Zheng, W. Size distribution-controlled preparation of graphene oxide nanosheets with different C/O ratios. Mater. Chem. Phys. 2013, 139, 8–11. [Google Scholar] [CrossRef]
- Yu, F.; Sun, Y.; Yang, M.; Ma, J. Adsorption mechanism and effect of moisture contents on ciprofloxacin removal by three-dimensional porous graphene hydrogel. J. Hazard Mater 2019, 374, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Sinha, T.K.; Mukherjee, S.; Adhikari, B.; Ray, S.K. Enhanced and Selective Photodetection Using Graphene-Stabilized Hybrid Plasmonic Silver Nanoparticles. Plasmonics 2016, 11, 1297–1304. [Google Scholar] [CrossRef]
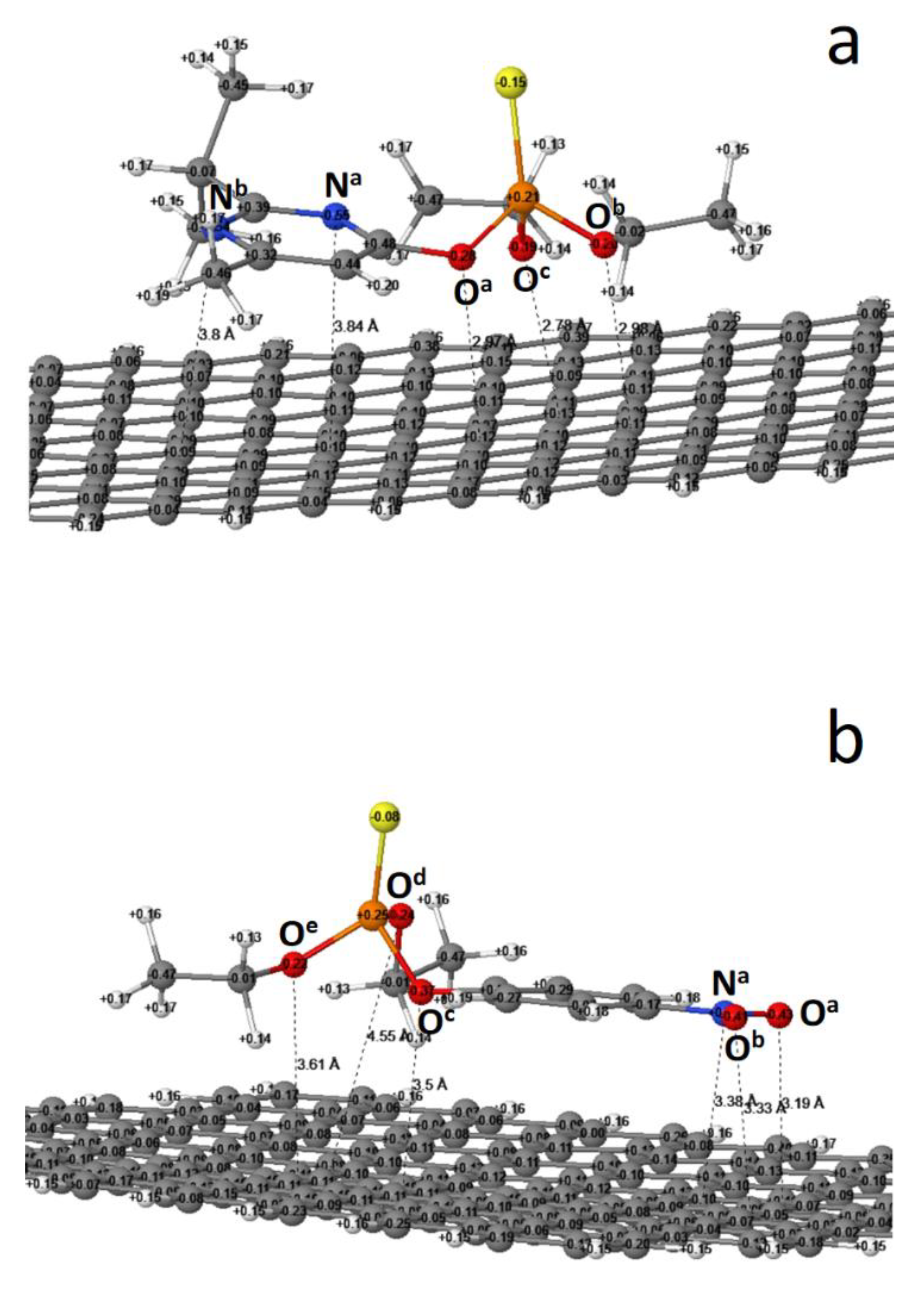
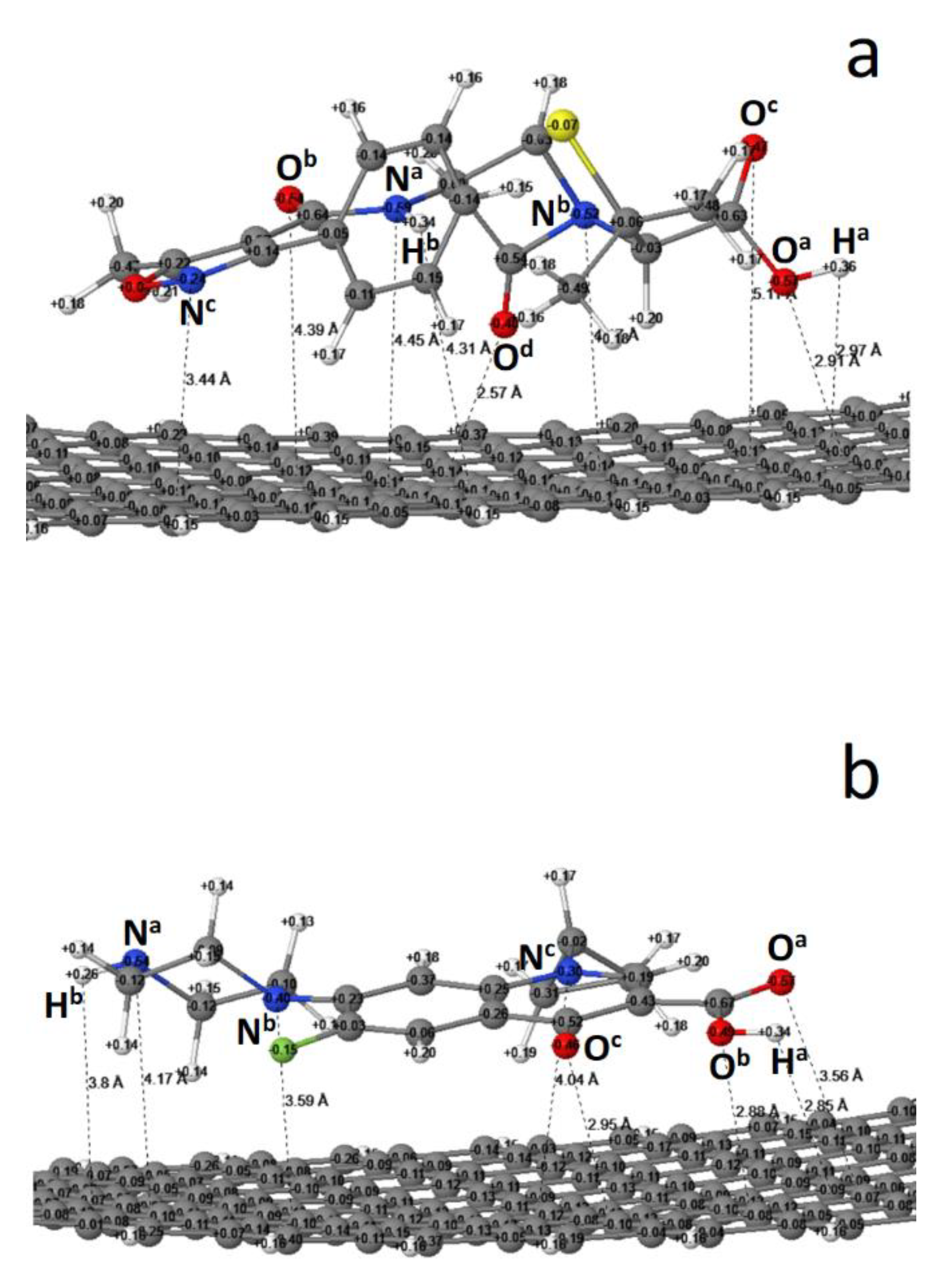


| Reaction | ∆Hf (kcal/mol) |
|---|---|
| C210 H40 [CC] + C12 H21 N2 O3 S P [dia] → C222 H61 N2 O3 S P [CC_dia] | −39.7466 |
| C210 H40 [CC] + C10 H14 N O5 S P [par] → C220 H54 N O5 S P [CC_par] | −41.6135 |
| C210 H40 [CC] + C19 H19 N3 O5 S [oxa] → C229 H59 N3 O5 S [CC_oxa] | −48.1507 |
| C210 H40 [CC] + C17 H18 N3 O3 F [cip] → C227 H58 N3 O3 F [CC_cip] | −54.6826 |
| C210 H40 [CC] + C49 H75 N10 O12 [mc] → C259 H115 N10 O12 [CC_mc] | −131.988 |
| B105 N105 H40 [BN] + C12 H21 N2 O3 S P [dia] → C12 H61 N107 O3 B105 S P [BN_dia] | −44.5491 |
| B105 N105 H40 [BN] + C10 H14 N O5 S P [par] → C10 H54 N106 O5 B105 S P [BN_par] | −44.2583 |
| B105 N105 H40 [BN] + C19 H19 N3 O5 S [oxa] → C19 H59 N108 O5 B105 S [BN_oxa] | −59.1402 |
| B105 N105 H40 [BN] + C17 H18 N3 O3 F [cip] → C17 H58 N108 O3 B105 F [BN_cip] | −59.1552 |
| B105 N105 H40 [BN] + C49 H75 N10 O12 [mc] → C49 H115 N115 O12 B105 [BN_mc] | −142.168 |
| C259 H115 N10 O12 [CC_mc] + C12 H21 N2 O3 S P [dia] → C271 H136 N12 O15 S P [CC_mc_dia] | −48.6947 |
| C259 H115 N10 O12 [CC_mc] + C10 H14 N O5 S P [par] → C269 H129 N11 O17 S P [CC_mc_par] | −48.7991 |
| C259 H115 N10 O12 [CC_mc] + C19 H19 N3 O5 S [oxa] → C278 H134 N13 O17 S [CC_mc_oxa] | −61.9871 |
| C259 H115 N10 O12 [CC_mc] + C17 H18 N3 O3 F [cip] → C276 H133 N13 O15 F [CC_mc_cip] | −60.1555 |
| C49 H115 N115 O12 B105 [BN_mc] + C12 H21 N2 O3 S P [dia] → C61 H136 N117 O15 B105 S P [BN_mc_dia] | −52.1374 |
| C49 H115 N115 O12 B105 [BN_mc] + C10 H14 N O5 S P [par] → C59 H129 N116 O17 B105 S P [BN_mc_par] | −53.7237 |
| C49 H115 N115 O12 B105 [BN_mc] + C19 H19 N3 O5 S [oxa] → C68 H134 N118 O17 B105 S [BN_mc_oxa] | −69.7886 |
| C49 H115 N115 O12 B105 [BN_mc] + C17 H18 N3 O3 F [cip] → C66 H133 N118 O15 B105 F [BN_mc_cip] | −65.1636 |
| GAP (eV) | I (eV) | A (eV) | χ (eV) | η (eV) | μ (eV) | S (eV−1) | ω (eV) | |
|---|---|---|---|---|---|---|---|---|
| dia | 8.571 | 8.933 | 0.362 | 4.648 | 4.286 | −4.648 | 0.117 | 2.520 |
| par | 8.217 | 9.298 | 1.081 | 5.190 | 4.109 | −5.190 | 0.122 | 3.277 |
| oxa | 8.497 | 9.191 | 0.694 | 4.943 | 4.249 | −4.943 | 0.118 | 2.875 |
| cip | 7.983 | 8.858 | 0.875 | 4.867 | 3.992 | −4.867 | 0.125 | 2.967 |
| mc | 7.603 | 11.084 | 3.481 | 7.283 | 3.802 | −7.283 | 0.132 | 6.976 |
| CC | 2.596 | 6.307 | 3.711 | 5.009 | 1.298 | −5.009 | 0.385 | 9.665 |
| h-BN | 6.076 | 7.927 | 1.851 | 4.889 | 3.038 | −4.889 | 0.165 | 3.934 |
| CC_mc | 2.740 | 7.621 | 4.881 | 6.251 | 1.370 | −6.251 | 0.365 | 14.261 |
| h-BN_mc | 5.806 | 9.115 | 3.309 | 6.212 | 2.903 | −6.212 | 0.172 | 6.646 |
| CC_dia | 2.594 | 6.316 | 3.722 | 5.019 | 1.297 | −5.019 | 0.386 | 9.711 |
| CC_par | 2.626 | 6.343 | 3.717 | 5.030 | 1.313 | −5.030 | 0.381 | 9.635 |
| CC_oxa | 2.654 | 6.331 | 3.677 | 5.004 | 1.327 | −5.004 | 0.377 | 9.435 |
| CC_cip | 2.691 | 6.334 | 3.643 | 4.989 | 1.346 | −4.989 | 0.372 | 9.248 |
| h-BN_dia | 6.073 | 7.950 | 1.877 | 4.914 | 3.037 | −4.914 | 0.165 | 3.975 |
| h-BN_par | 6.009 | 7.933 | 1.924 | 4.929 | 3.005 | −4.929 | 0.166 | 4.042 |
| h-BN_oxa | 6.037 | 7.945 | 1.908 | 4.927 | 3.019 | −4.927 | 0.166 | 4.020 |
| h-BN_cip | 5.945 | 7.871 | 1.926 | 4.899 | 2.973 | −4.899 | 0.168 | 4.036 |
| CC_mc_dia | 2.724 | 7.602 | 4.878 | 6.240 | 1.362 | −6.240 | 0.367 | 14.294 |
| CC_mc_par | 2.722 | 7.623 | 4.901 | 6.262 | 1.361 | −6.262 | 0.367 | 14.406 |
| CC_mc_oxa | 2.740 | 7.583 | 4.843 | 6.213 | 1.370 | −6.213 | 0.365 | 14.088 |
| CC_mc_cip | 2.704 | 7.564 | 4.860 | 6.212 | 1.352 | −6.212 | 0.370 | 14.271 |
| h-BN_mc_dia | 5.843 | 9.096 | 3.253 | 6.175 | 2.922 | −6.175 | 0.171 | 6.525 |
| h-BN_mc_par | 5.808 | 9.118 | 3.310 | 6.214 | 2.904 | −6.214 | 0.172 | 6.648 |
| h-BN_mc_oxa | 5.863 | 9.153 | 3.290 | 6.222 | 2.932 | −6.222 | 0.171 | 6.602 |
| h-BN_mc_cip | 5.877 | 9.100 | 3.223 | 6.162 | 2.939 | −6.162 | 0.170 | 6.460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, S.-C.; Lee, C.-L.; Chang, C.M. Adsorption of Pesticides, Antibiotics and Microcystin-LR by Graphene and Hexagonal Boron Nitride Nano-Systems: A Semiempirical PM7 and Theoretical HSAB Study. Crystals 2022, 12, 1068. https://doi.org/10.3390/cryst12081068
Chi S-C, Lee C-L, Chang CM. Adsorption of Pesticides, Antibiotics and Microcystin-LR by Graphene and Hexagonal Boron Nitride Nano-Systems: A Semiempirical PM7 and Theoretical HSAB Study. Crystals. 2022; 12(8):1068. https://doi.org/10.3390/cryst12081068
Chicago/Turabian StyleChi, Shu-Chun, Chien-Lin Lee, and Chia Ming Chang. 2022. "Adsorption of Pesticides, Antibiotics and Microcystin-LR by Graphene and Hexagonal Boron Nitride Nano-Systems: A Semiempirical PM7 and Theoretical HSAB Study" Crystals 12, no. 8: 1068. https://doi.org/10.3390/cryst12081068
APA StyleChi, S.-C., Lee, C.-L., & Chang, C. M. (2022). Adsorption of Pesticides, Antibiotics and Microcystin-LR by Graphene and Hexagonal Boron Nitride Nano-Systems: A Semiempirical PM7 and Theoretical HSAB Study. Crystals, 12(8), 1068. https://doi.org/10.3390/cryst12081068






