Effect of Cobalt Substitution on the Structural and Magnetic Properties of Bismuth Ferrite Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pure and Co-BFO Powders
2.3. Materials Characterization
3. Results and Discussion
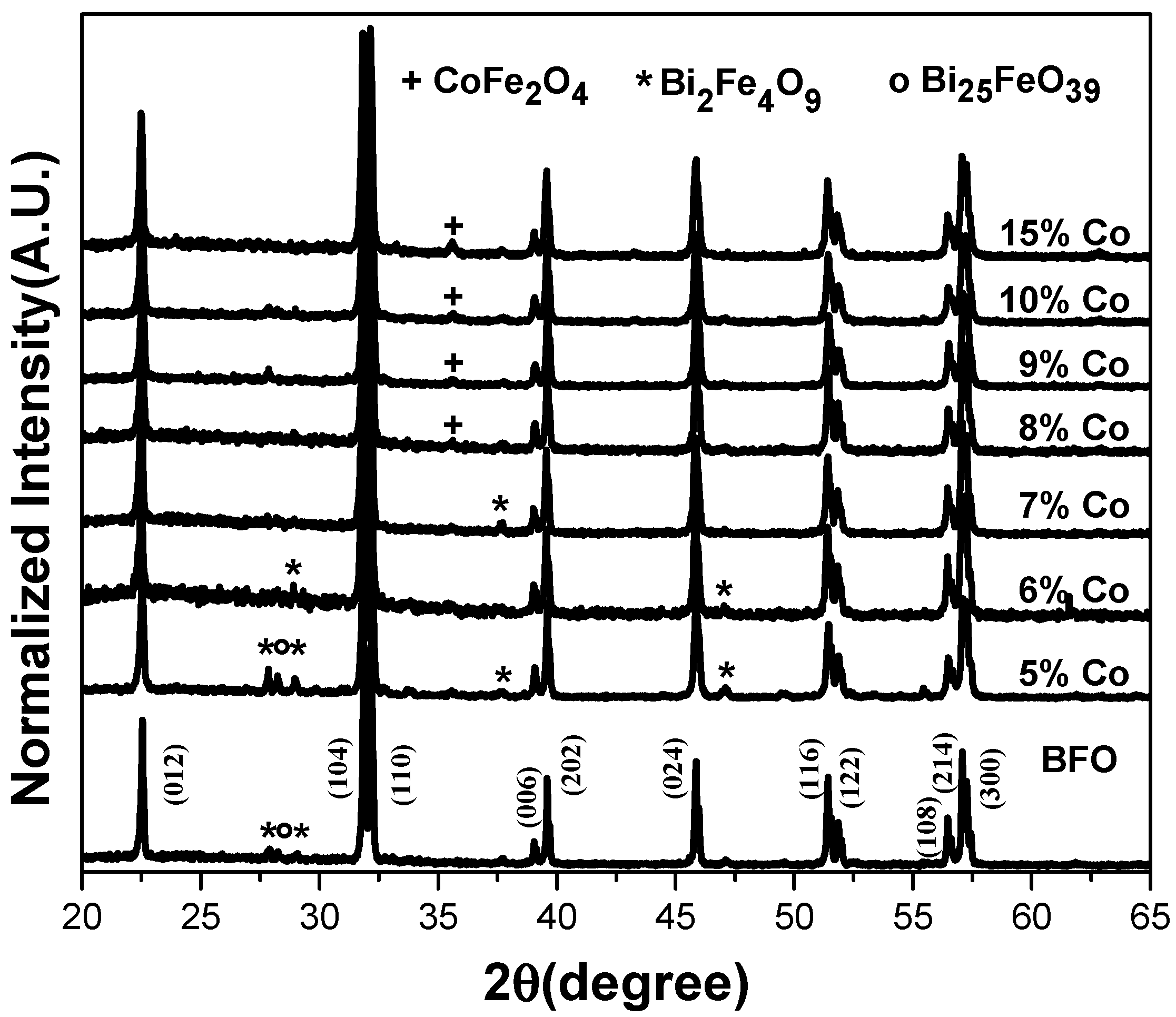
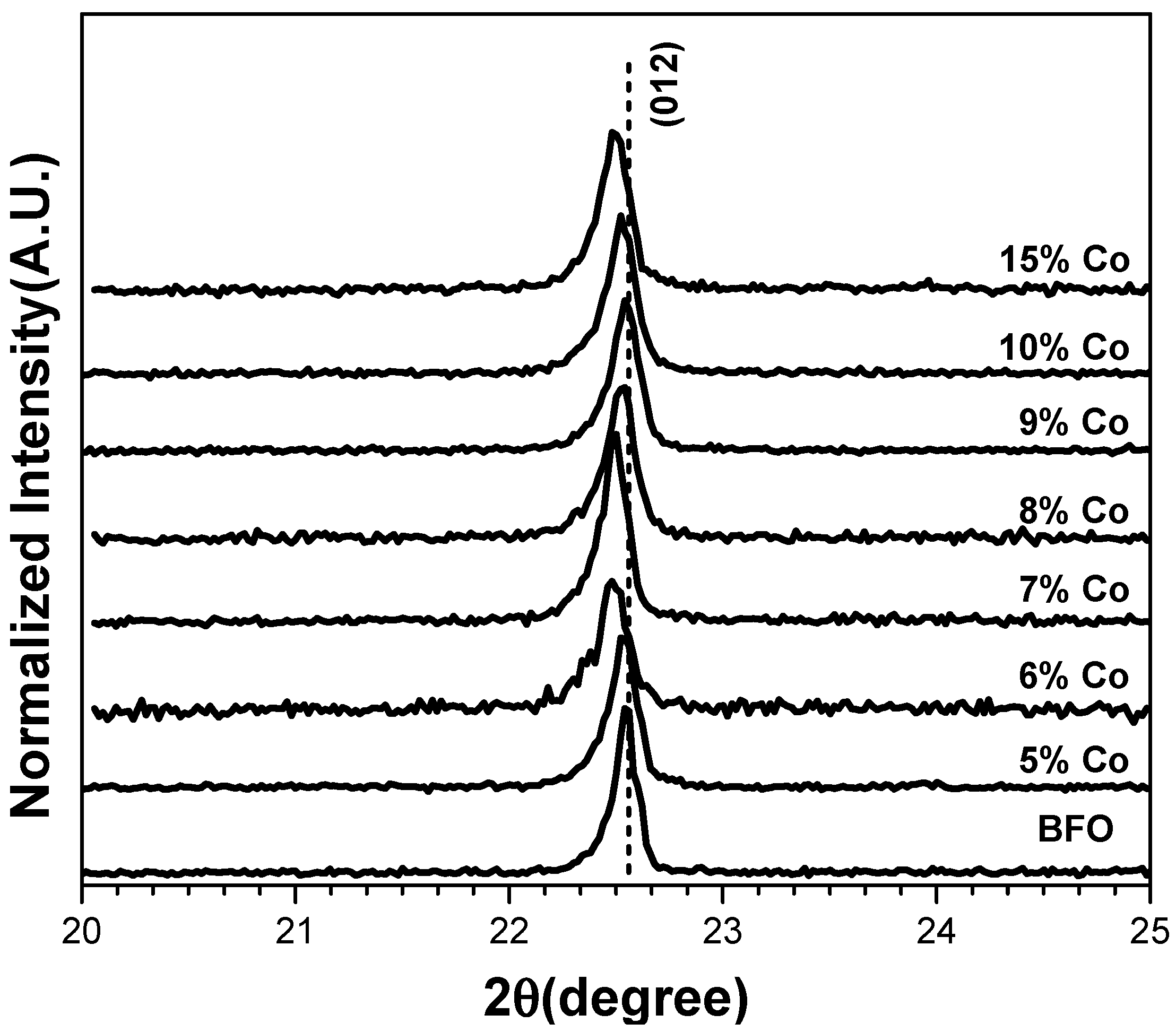
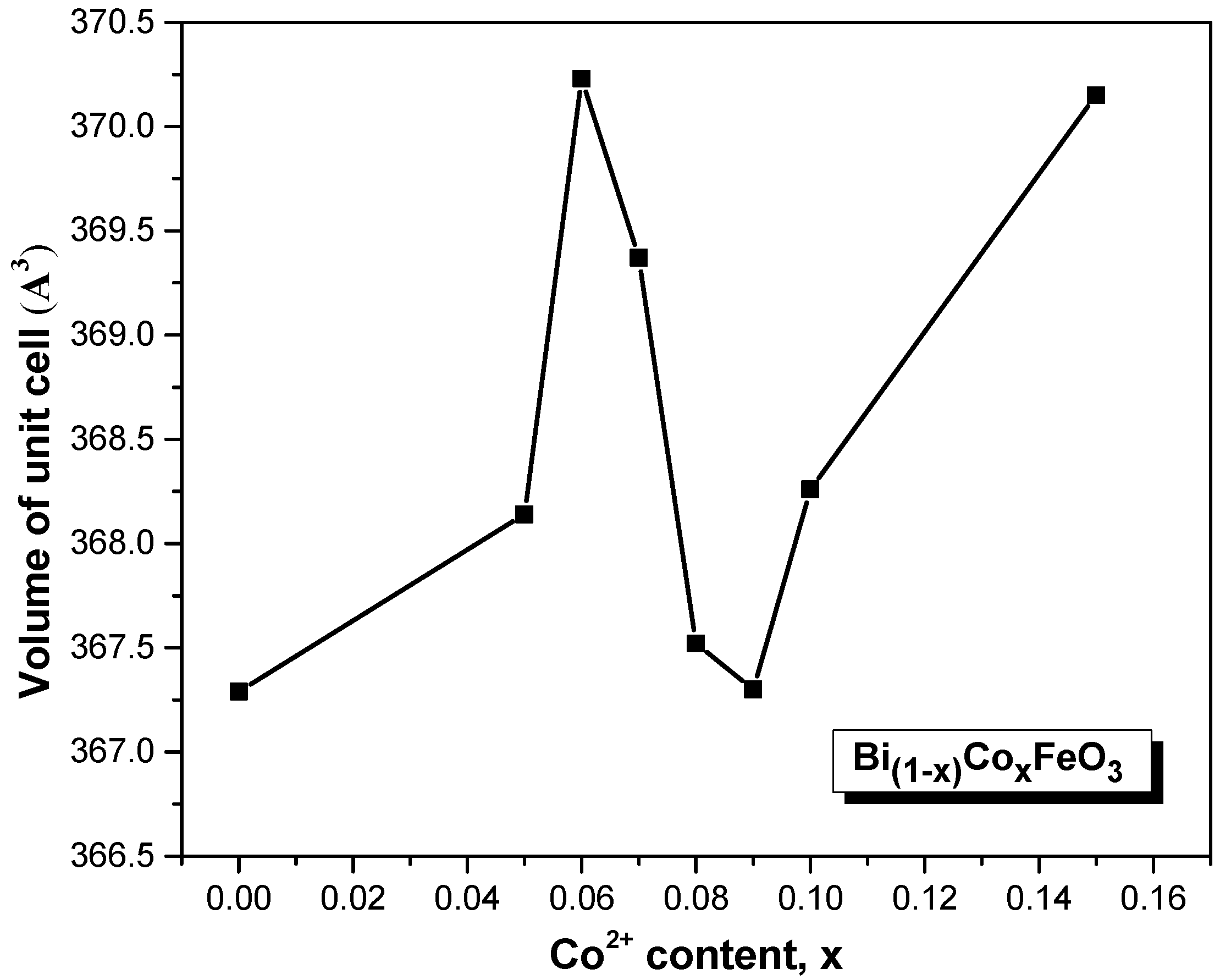
3.1. XRD Analysis
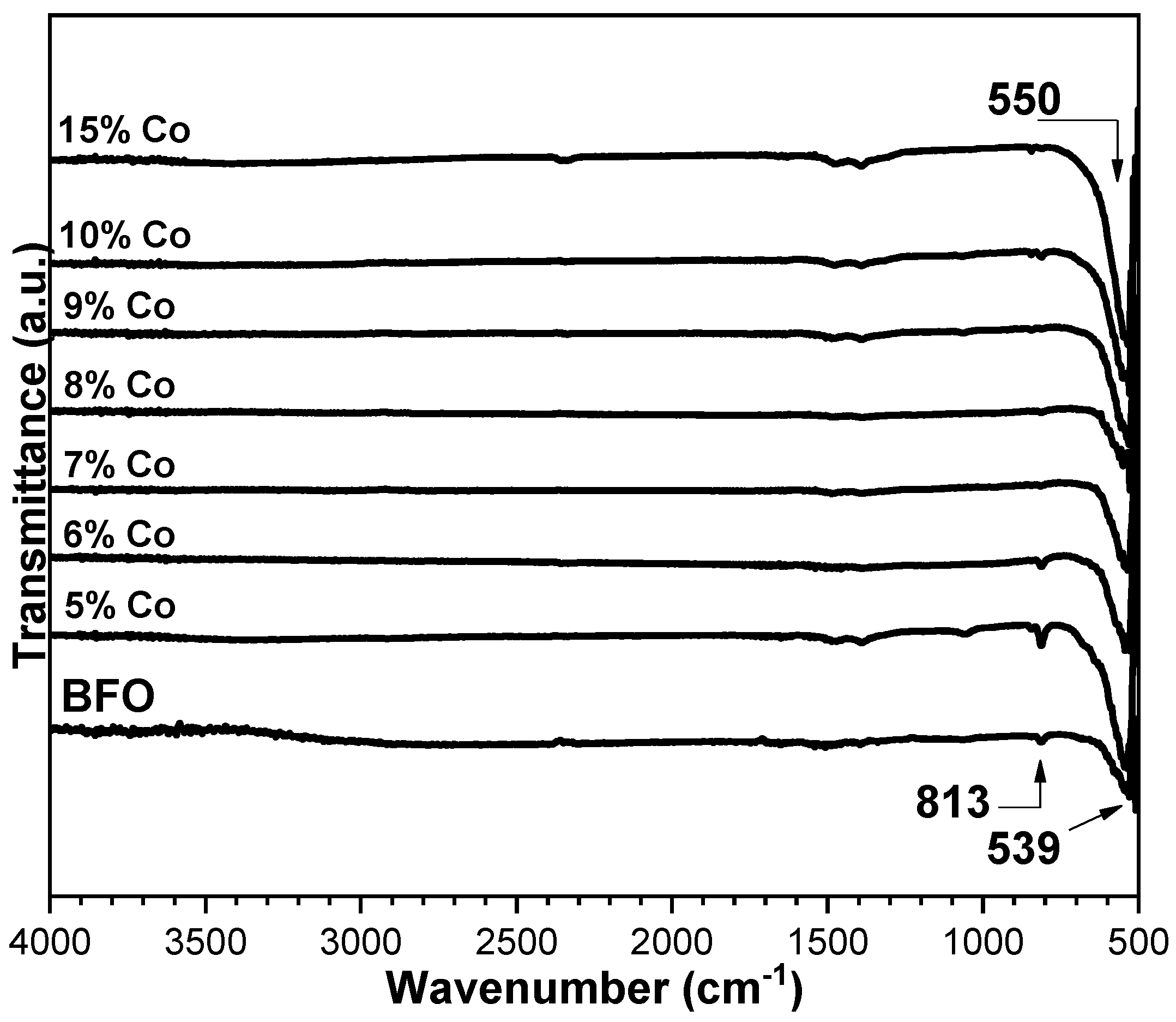
3.2. FTIR Analysis
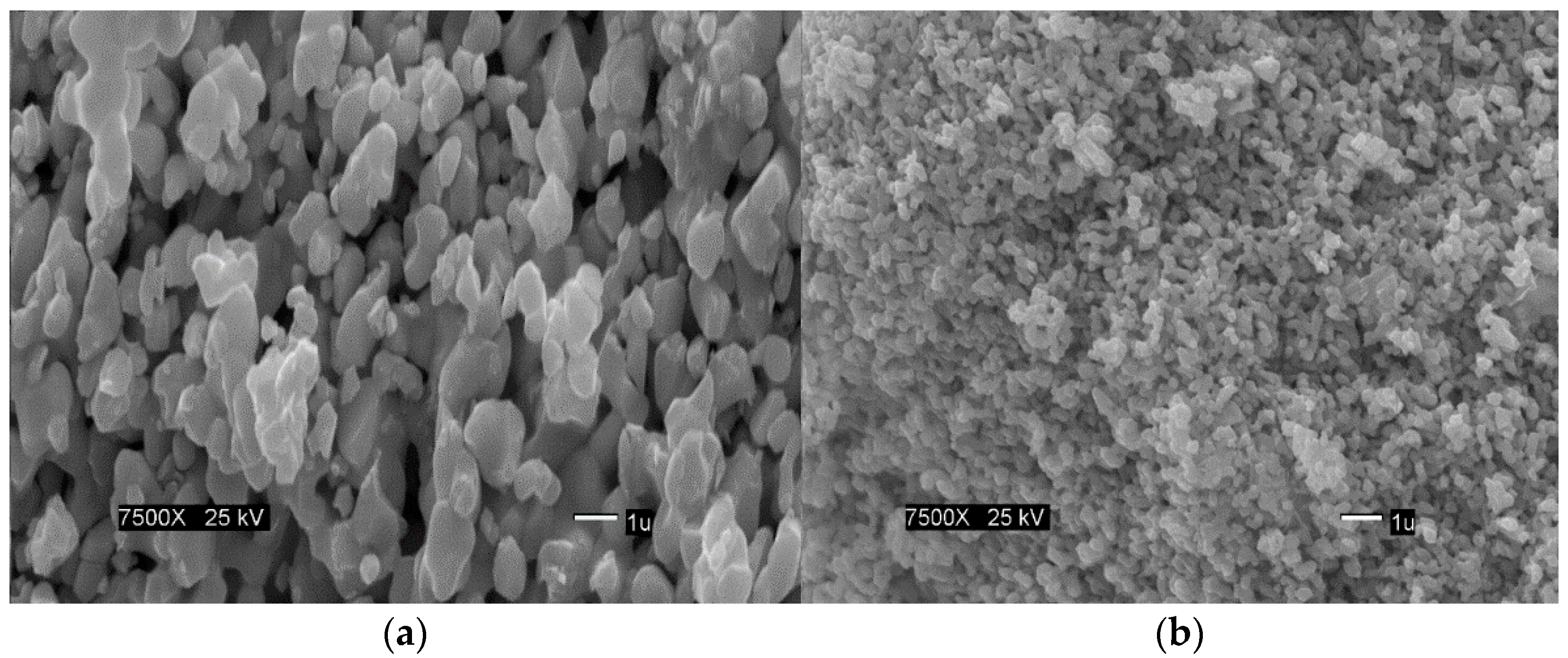
3.3. Scanning Electron Microscopy (SEM) Analysis
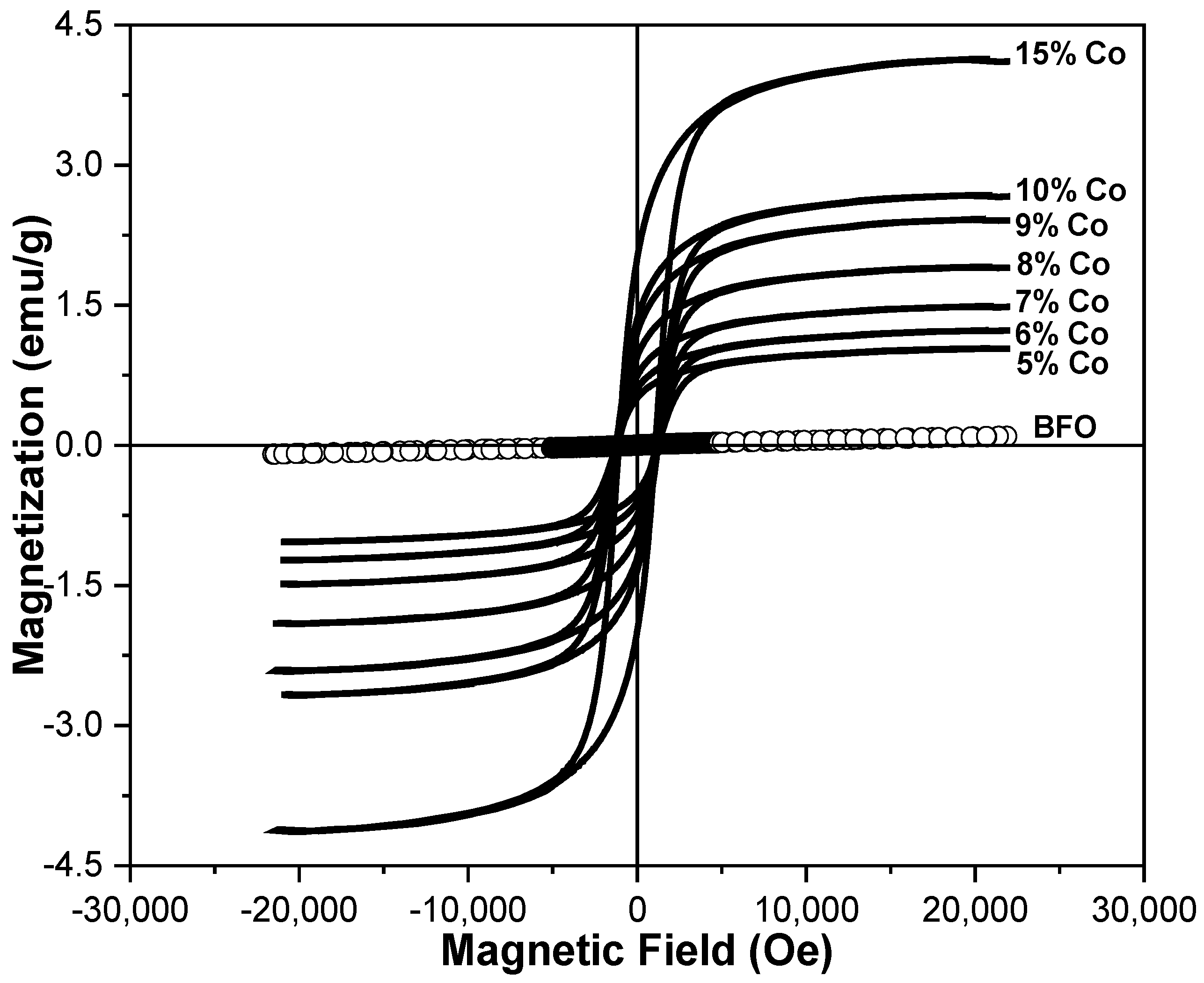
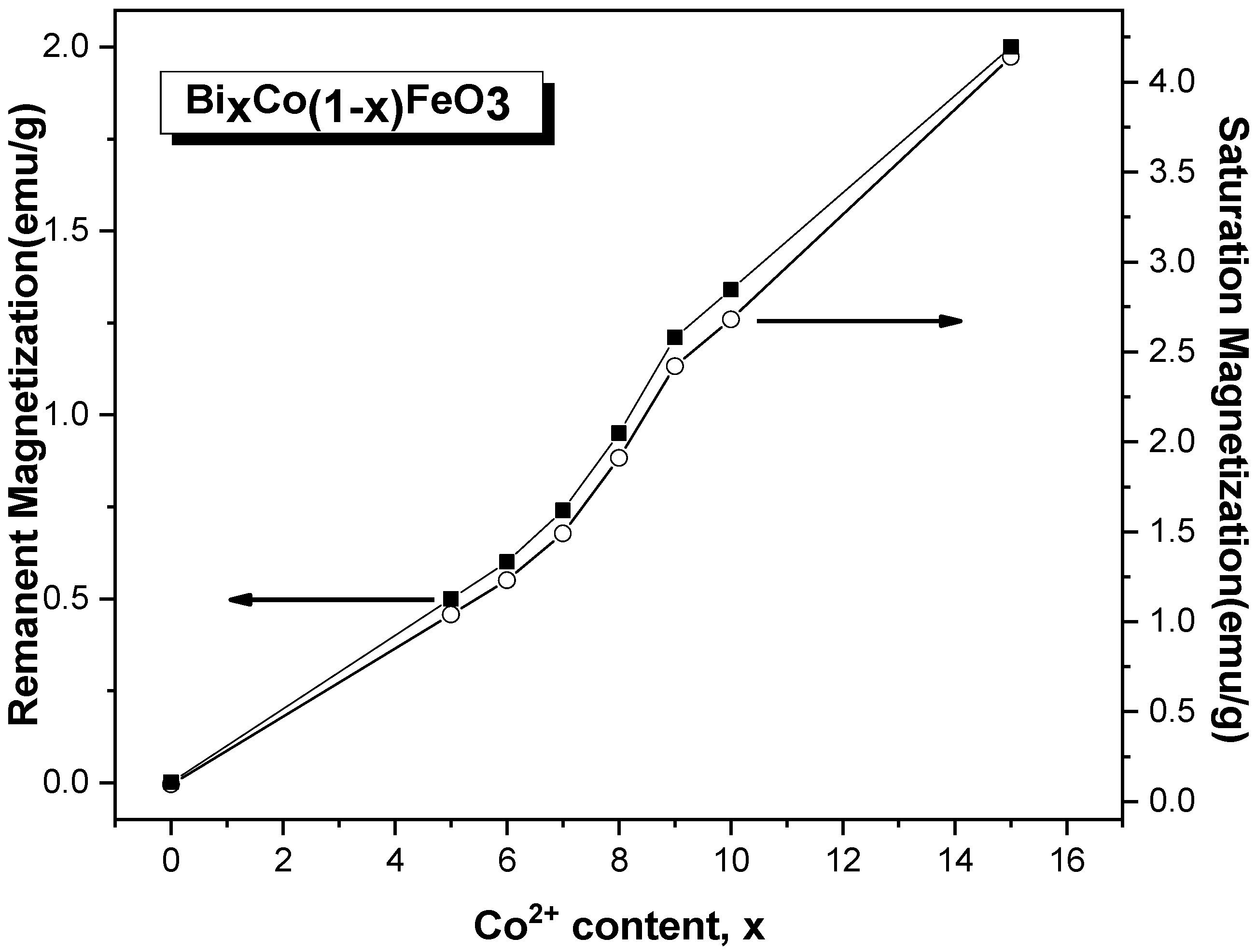
3.4. M-H Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaldin, N.; Cheong, S.; Ramesh, R. Multiferroics: Past, present, and future. Phys. Today 2010, 63, 38. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.; Kothari, D.; Gupta, A.; Gupta, S. Study of weak ferromagnetism in polycrystalline multiferroic Eu doped bismuth ferrite. Appl. Phys. Lett. 2009, 94, 10–13. [Google Scholar] [CrossRef]
- Sando, D.; Barthélémy, A.; Bibes, M. BiFeO3 epitaxial thin films and devices: Past, present and future. J. Phys. Condens. Matter. 2014, 26, 473201. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.J.R.; Silva, L.M.A.; Lopez, J.A.R.; Sizov, A.S.; Emelianov, N.A. Influence of the order of layers deposition and annealing temperature on the multiferroic properties of multilayer BiFeO3-CoFe2O4 nanocomposites. Ferroelectrics 2019, 543, 107–114. [Google Scholar] [CrossRef]
- Nazario-Naveda, R.; Rojas-Flores, S.; Juárez-Cortijo, L.; Gallozzo-Cardenas, M.; Díaz, F.N.; Angelats-Silva, L.; Benites, S.M. Effect of x on the Electrochemical Performance of Two-Layered Cathode Materials xLi2MnO3–(1− x) LiNi0.5Mn0.5O2. Batteries 2022, 8, 63. [Google Scholar] [CrossRef]
- Flores, S.J.R.; Cardenas, M.M.G.; Naveda, R.R.N.; Cortijo, L.A.J.; Yupanqui, M.R.R.; Silva, L.M.A.; Odar, F.E.U. Influence of cobalt ferrite on the magnetoelectric properties of thin films of bismuth ferrite deposited by spin coating. Matéria 2020, 25, 1. [Google Scholar] [CrossRef] [Green Version]
- Segundo, R.F.; La Cruz-Noriega, D.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Díaz, F. Golden Berry Waste for Electricity Generation. Fermentation 2022, 8, 256. [Google Scholar] [CrossRef]
- Flores, S.R.; Pérez-Delgado, O.; Naveda-Renny, N.; Benites, S.M.; de la Cruz–Noriega, M.; Narciso, D.A.D. Generation of Bioelectricity Using Molasses as Fuel in Microbial Fuel Cells. Environmental Research. Eng. Manag. 2022, 78, 19–27. [Google Scholar]
- Coey, J. Magnetism and Magnetic Materials; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.; Rocha, L.; Cortes, J.; Ramirez, M.; Longo, E.; Simoes, A. Enhancement of ferromagnetic and ferroelectric properties in calcium doped BiFeO3 by chemical synthesis. Ceram. Int. 2015, 41, 9265–9275. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, K.; Xu, J.; Wang, L.; Bian, L.; Xu, F. Structure-Dependent Electrical, Optical and Magnetic Properties of Mn-Doped BiFeO3 Thin Films Prepared by the Sol-Gel Process. J. Mater. Sci. Res. 2013, 2, 75–81. [Google Scholar] [CrossRef]
- Montes, G.M.; Perales-Perez, O.; Rentería, B.; Gálvez, M. Tuning of Magnetic Properties in Cobalt-Doped Nanocrystalline Bismuth Ferrite Gina. MRS Proc. 2011, 1368, 1135. [Google Scholar] [CrossRef]
- Chaudhari, Y.A.; Mahajan, C.M.; Jagtap, P.P.; Bendre, S.T. Structural, magnetic and dielectric properties of nano-crystalline Ni-doped BiFeO3 ceramics formulated by self-propagating high-temperature synthesis. J. Adv. Ceram. 2013, 2, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Tang, P.; Kuang, D.; Yang, S.; Zhang, Y. Preparation and enhanced ferromagnetic properties in Co doped BiFeO 3 nanoparticles prepared by sol–gel method. Ferroelectrics 2016, 505, 123–129. [Google Scholar] [CrossRef]
- Han, J.-T.; Huang, Y.-H.; Wu, X.-J.; Wu, C.-L.; Wei, W.; Peng, B.; Huang, W.; Goodenough, J.B. Tunable synthesis of bismuth ferrites with various morphologies. Adv. Mater. 2006, 18, 2145–2148. [Google Scholar] [CrossRef]
- Chinchay-Espino, H.; Montes-Albino, G.; Villa-Santos, C.; Perales-Pérez, O. Structural and Magnetic Properties of Pure and Mn-Doped Bismuth Ferrite Powders. MRS Adv. 2017, 2, 253–258. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Gao, B.; Chang, A.; Chen, J.; Bian, L.; Song, C. Synthesis of BiFeO3 nanoparticles by a low-heating temperature solid-state precursor method. Mater. Res. Bull. 2013, 48, 383–388. [Google Scholar] [CrossRef]
- Taylor, P.; Xie, H.; Wang, K.; Jiang, Y.; Zhao, Y.; Wang, X.; Xie, H.; Wang, K.; Jiang, Y.; Zhao, Y.; et al. An Improved Co-precipitation Method to Synthesize Three Bismuth Ferrites. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 1363–1367. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, Q.; Wen, Z.; Lang, X.; Wu, D.; Qiu, T.; Xu, M. The magnetic properties of La doped and codoped BiFeO3. J. Alloy. Compd. 2010, 499, 108–112. [Google Scholar] [CrossRef]
- Hasan, M.; Islam, M.; Mahbub, R.; Hossain, M.; Hakim, M. A soft chemical route to the synthesis of BiFeO3 nanoparticles with enhanced magnetization. Mater. Res. Bull. 2016, 73, 179–186. [Google Scholar] [CrossRef]
- Kumar, N.S.; Kumar, K.V. Synthesis and Structural Properties of Bismuth Doped Cobalt Nanoferrites Prepared by Sol-Gel Combustion Method. World J. Nano Sci. Eng. 2015, 5, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Sakar, M.; Balakumar, S.; Saravanan, P.; Jaisankar, S. Annealing temperature mediated physical properties of bismuth ferrite (BiFeO3) nanostructures synthesized by a novel wet chemical method. Mater. Res. Bull. 2013, 48, 2878–2885. [Google Scholar] [CrossRef]
- Rout, J.; Choudhary, R.; Sharma, H.; Shannigrahi, S. Effect of co-substitutions (Ca-Mn) on structural, electrical and magnetic characteristics of bismuth ferrite. Ceram. Int. 2015, 41, 9078–9087. [Google Scholar] [CrossRef]
- Vashisth, B.K.; Bangruwa, J.S.; Beniwal, A.; Gairola, S.P.; Kumar, A.; Singh, N.; Verma, V. Modified ferroelectric/magnetic and leakage current density properties of Co and Sm co-doped bismuth ferrites. J. Alloy. Compd. 2017, 698, 699–705. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Sarkar, B.; Ashok, V.; de Chaudhuri, S. Enhanced magnetic and dielectric behavior in Co doped BiFeO3 nanoparticles. J. Magn. Magn. Mater. 2015, 381, 271–277. [Google Scholar] [CrossRef]
- Mukherjee, A.; Basu, S.; Green, L.; Thanh, N.; Pal, M. Enhanced multiferroic properties of Y and Mn codoped multiferroic BiFeO3 nanoparticles. J. Mater. Sci. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Lotey, G.; Verma, N. Structural, magnetic, and electrical properties of Gd-doped BiFeO3 nanoparticles with reduced particle size. J. Nanoparticle Res. 2012, 14, 742. [Google Scholar] [CrossRef]
| Samples | Coercivity (Oe) | Saturation Magnetization (emu/g) |
|---|---|---|
| pure BFO | 14.1 | 0.1 |
| 15% Co-doped BFO | 1083 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinchay-Espino, H.A.; Montes-Albino, G.M.; Morales-Cruz, C.M.; Dobbertin-Sanchez, S.E.; Rojas-Flores, S. Effect of Cobalt Substitution on the Structural and Magnetic Properties of Bismuth Ferrite Powders. Crystals 2022, 12, 1058. https://doi.org/10.3390/cryst12081058
Chinchay-Espino HA, Montes-Albino GM, Morales-Cruz CM, Dobbertin-Sanchez SE, Rojas-Flores S. Effect of Cobalt Substitution on the Structural and Magnetic Properties of Bismuth Ferrite Powders. Crystals. 2022; 12(8):1058. https://doi.org/10.3390/cryst12081058
Chicago/Turabian StyleChinchay-Espino, Hector A., Gina M. Montes-Albino, Carlex M. Morales-Cruz, Segundo E. Dobbertin-Sanchez, and Segundo Rojas-Flores. 2022. "Effect of Cobalt Substitution on the Structural and Magnetic Properties of Bismuth Ferrite Powders" Crystals 12, no. 8: 1058. https://doi.org/10.3390/cryst12081058
APA StyleChinchay-Espino, H. A., Montes-Albino, G. M., Morales-Cruz, C. M., Dobbertin-Sanchez, S. E., & Rojas-Flores, S. (2022). Effect of Cobalt Substitution on the Structural and Magnetic Properties of Bismuth Ferrite Powders. Crystals, 12(8), 1058. https://doi.org/10.3390/cryst12081058






