Abstract
The objective of this study is to use the geopolymer technique to solidify/stabilize heavy metal contaminated soil. There are over 739,700 square meters of heavy-metal-contaminated sites in Taiwan; most sites are soil farmlands. These heavy metal contaminants in soil can also infiltrate into groundwater and cause more serious pollution problems. This study explores the possibility of using the geopolymer technique to solidify heavy metal contaminated soil (CS), stabilize heavy metal, and produce good mechanical and physical properties. The ground granulated blast furnace slag (GGBFS) was activated by an alkali solution to form a geopolymer binder that can be used to solidify CS and stabilize the heavy metal. The effect of GGBFS and CS mixing ratio on the mechanical and physical properties and the TCLP test was investigated. The test results show that the compressive strength of specimens made with a 1.5 CS/GGBFS ratio can reach 46.61 MPa and 47.66 MPa after curing for 14 and 28 days, respectively. TCLP tests show only 2 ppm Cu was detected from a geopolymer-treated contaminated soil sample. The influence of alkali solution, such as the molarity of the NaOH, SiO2/Na2O, and SiO2/Al2O3 molar ratio, were also evaluated. The specimens prepared with 8 M NaOH, 0.96 SiO2/Na2O, and 1.28 SiO2/Al2O3 molar ratio alkali solution have a compressive strength of 51.74 MPa and 58.63 MPa after 14 and 28 days of curing. The TCLP tests show no heavy metal ions leached from the sample.
1. Introduction
According to the Taiwan EPA investigation, over 739,700 square meters of soil are contaminated by heavy metals, and most are soil-contaminated farmlands. These sites present a challenge to land reclaimers and the government with the presence of potentially toxic heavy metals in the environment. These contaminants, As, Cd, Cr, Hg, Pb, Cu, Ni, and Zn, in the soil can also infiltrate into groundwater and cause more serious pollution problems.
Many in situ and ex situ remediation techniques have been developed to rectify the heavy metal contaminated sites [1,2]. In situ means in the original place, while ex situ means outside the original place. While in situ methods are applicable for large populations, ex situ methods are applicable for small populations. Ex situ remediation is more expensive than in situ ways. Among these techniques, Ex situ solidification is a well-established soil remediation technology that has been implemented in 200 soil remediation projects at a treatment cost ranging from USD120 to USD220 per cubic meter of soil in the U.S. [3]. However, it is timely and efficient, but the scale-up operation has a noticeable drawback of significantly increasing waste volume.
In the past few years, some research has been conducted to encapsulate heavy metal ions using the geopolymer technique. These studies show promising results of solidifying heavy metal ions into a geopolymer matrix through physical encapsulating and chemical bonding. In order to solve the above-mentioned problem, the geopolymer technique was used in this study to solidify heavy metal contaminated soil in a more cost-effective way. The geopolymer specimens fabricated with good physical and mechanical properties can be used for further engineering applications.
Amorphous to semi-crystalline three-dimensional aluminum–silicate materials called geopolymer was first named by Joseph Davidovits in 1978. Geopolymerization involves a chemical reaction between various aluminum–silicate oxides _Al3q in IV–V fold coordination, with silicates under highly alkaline conditions, yielding polymeric Si–O–Al–O bonds [4,5,6,7,8].
Geopolymer materials have the advantages of high compressive strength, fire resistance, low CO2 emission, and chemical resistance [9,10]. In the process of preparing geopolymer, aluminum–silicate material is readily dissolved in the alkaline solution to form AlO4 and SiO4 tetrahedral units [11]. These tetrahedral frameworks are linked to yield polymeric precursors (–SiO4–AlO4–, or –SiO4–AlO4–SiO4–, or –SiO4–AlO4–SiO4–SiO4–) by sharing all oxygen atoms between two tetrahedral units, while water molecules are released [12,13,14].
The excellent performances of geopolymers could be achieved in the immobilization of certain chemical species [15]. In order to use geopolymer binders for heavy metal stabilization and solidification, geopolymers might capture hazardous elements within the three-dimensional framework of the geopolymeric matrix and convert semi-solid waste into an adhesive solid (Sefiu et al. 2019) [16]. Zheng et al. 2010 also suggested that heavy metals might be immobilized through three general steps (Zheng et al. 2010) [17]:
- Metal ions are taken into the geopolymer network;
- Metal ions are bound into the structure for charge balancing roles, and
- A precipitate containing heavy metals is physically encapsulated.
Bassam et al. (2012) indicated that the adsorption of Cu(II) and Pb(II) onto geopolymers did not decrease with the competition with other metal ions [18]. However, these matrices exhibited different compressive strengths and leaching behavior. These results implied that these metals might be taken into the geopolymer network as roles for charge balancing and possibly bound into the structure of amorphous regions of geopolymer matrices [19]. Palacios and Palomo (2004) also found that the Pb compound formed in the fly ash matrix is a highly insoluble silicate Pb3SiO5, identified by XRD, implying that the immobilization of Pb in fly-ash-based geopolymers is mainly by encapsulation of Pb3SiO5 [20].
For ground granulated blast furnace slag (GGBFS)-based geopolymer, the composition of GGBFS is essentially an over-charge-balanced calcium aluminosilicate framework. The GGBFS-based geopolymer contains at least two-thirds by mass of glassy slag and possesses hydraulic properties when suitably activated [21]. Its chemical composition is mainly a CaO-SiO2-MgO-Al2O3 system. The key glass network forming cations are Si4+ and Al3+, and the divalent Ca2+ and Mg2+ act as network modifiers along with any alkalis present [22,23].
This study explores the possibility of using the geopolymer technique to solidify heavy metal contaminated soil (CS). The GGBFS was activated by an alkali solution to form a geopolymer binder that can be used to solidify CS and stabilize the heavy metals. Two CS were prepared in this study; one is acquired from the contaminated site that contains Zn 3855 ppm, Cu 2062 ppm, and Pb 1545 ppm. Because of its low heavy metal concentrations, the second sample was prepared by adding heavy metal ions Zn, Cu, and Ni into the soil to increase its concentration to 10,000 ppm. Several operating parameters, such as GGBFS/CS mixing ratio, NaOH molarity, solid/liquid ratio, SiO2/Na2O, and SiO2/Al2O3 ratio of alkali solutions were investigated to optimize the process for better performance of the specimens prepared.
2. Materials and Methods
2.1. Materials
The ground granulated blast furnace slag (GGBFS) used in this study was collected from the CHC Resources Corporation located in central Taiwan. The GGBFS obtained has been ground to fine powder form, and no further treatment was needed. The contaminated soil (CS) was obtained from a pollution control site announced by the government in eastern Taiwan. The chemical compositions of CS and GGBFS were analyzed and listed in Table 1. The CS mainly contains SiO2, Al2O3, and CaO. The GGBFS contains 57.62% CaO. The concentration of heavy metals in soil is listed in Table 2. It includes EPA announced value and soil samples excavated on site. The difference is due to the sampling by this study being about two years after EPA announcement. The particle size analysis shows the d50s (mean particle size) of CS and GGBFS are 224.0 μm and 13.17 μm, as shown in Figure 1. The phase analyses of spectrum of CS and GGBFS are shown in Figure 2. The amorphous phase and particle size of GGBFS can be clearly identified in Figure 2 and Figure 3.

Table 1.
The XRF chemical composition analysis of CS and GGBFS.

Table 2.
The heavy metal analysis of CS.
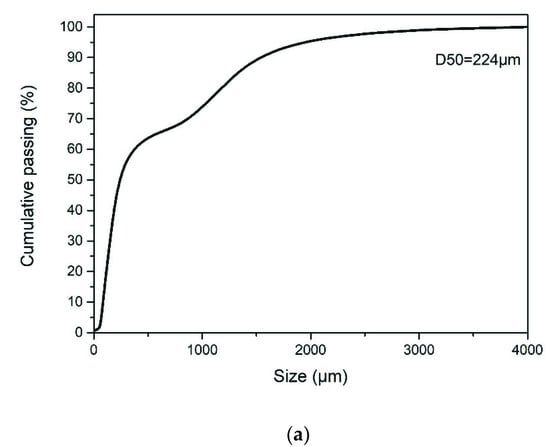
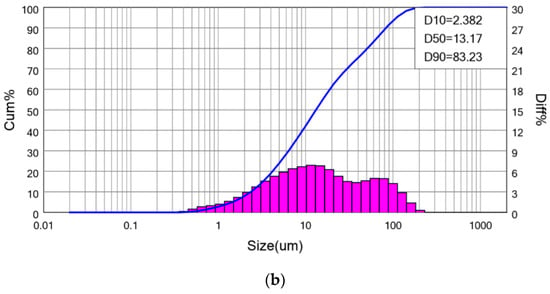
Figure 1.
The particle size distribution curve of (a) Contaminated Soil, and (b) ground granulated blast furnace slag.
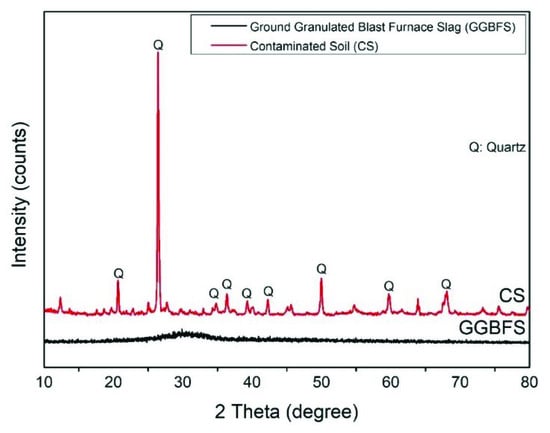
Figure 2.
The XRD (X-ray diffractometer) spectrum of CS and GGBFS.
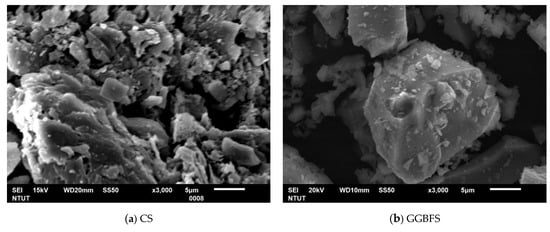
Figure 3.
The SEM (Scanning Electron Microscope) morphologies of (a) Contaminated Soil, (b) ground granulated blast furnace slag.
2.2. Preparation of Alkali Solution
In order to investigate the influence of alkali solution on the stabilization of heavy metal ions, NaOH solutions with 4, 6, and 8 molarity were prepared based on previous research works. The alkali solution was made by mixing Sodium Hydroxide, Sodium Silicate, and Sodium Aluminate at various. The molar ratio of SiO2/Na2O varied from 0.96 to 1.28, and SiO2/Al2O3 varied from 0 to 70, as shown in Table 3.

Table 3.
The experimental design for geopolymer specimen preparation.
2.3. Preparation of Geopolymer Materials
From previous studies, the optimal mixing ratio of CS and GGBFS is 60:40 [24]. The Geopolymer Materials were prepared in this study by mixing contaminated Soil (CS) and ground granulated blast furnace slag (GGBFS) at 1.5 ratio. These mixtures were then activated by alkali solutions prepared with different NaOH molarity, as given in Table 3. The liquid–solid ratio (L/S) was fixed at 0.35. As shown in Table 3, the first group is prepared by changing the molarity of NaOH. The second group changes SiO2/Na2O molar ratio, and the third group vary the SiO2/Al2O3 molar ratio.
The Cs, GGBFS, and alkali solutions were poured into mortar mixer and thoroughly mixed for 5 min; the geopolymer paste was then cast into a Ф50 × 100 mm acrylic mold. The specimens can be stored at ambient temperature.
The cast geopolymer specimens were demolded after 3 days of curing. These specimens were then kept in ambient environment for the following tests. The physical and mechanical properties of the geopolymer specimens were tested on different curing days (3, 7, 14, and 28 days). The test results are the average value of three specimens tested.
2.4. Apparatus and Testing
2.4.1. Apparatus
* XRD (X-ray diffractometer)
D2 PHASER X-ray diffractometer was used in this study for phase analysis of Ground granulate blast furnace slag (GGBFS) and heavy metal contaminated soil (CS).
* XRF (X-ray Fluorescence)
The chemical composition of the GGBFS and soil samples used in this study was analyzed using X-ray Fluorescence Spectrometer (X-MET 5100, OXFORD INSTRUMENTS Company).
* Compression Tester
Semi-Automatic compression tester (WIZARD2: 50-A22A03) with maximum load of 200 metric tons was used to measure the compressive strength of geopolymer specimens prepared.
* Mortar mixer
Mortar mixer with 25 L capacity, model KOM-01, from Ke-Zao Company in Taiwan was used for the mixing of GGBFS, CS and alkali solution.
* SEM (Scanning Electron Microscope)
JEOL Scanning Electron Microscope, model JSM-6510LV, is used to analyze the morphology of the geopolymer specimen.
* Particle size analysis
Bettersizer S2 is used to analyze the particle size distribution of GGBFS and heavy metal contaminated soil.
2.4.2. Testing
* Mechanical property testing
According to CNS 1232 “Method of test for compressive strength of cylindrical concrete specimens” [25,26], compressive strength test was conducted on the geopolymer after 3, 7, 14, and 28 days of curing at ambient temperature. The size of specimen is Ф50 × 100 mm. The pressurization rate of compression tester was set to 1 mm/min. The load is applied until the specimen fails.
* Physical properties testing
The physical properties of geopolymer, such as bulk density, apparent specific gravity, porosity, and water adsorption, were measured according to the CNS619/R3013 ‘‘Method of Test for Apparent Porosity, Water Absorption and Specific Gravity of Refractory Bricks’’ [27]. The equations are listed as follows:
Bulk Density, B (g/cm3) = D/V
Apparent Specific Gravity, T = D/(D − S)
Porosity, P (%) = (W − D)/V
Water Absorption, A (%) = (W − D)/D
Where D is dry weight, S is Suspended Weight in water, W is Saturated Weight.
* SEM (Scanning Electron Microscope) analysis
SEM analysis is to examine and compare the degree of geopolymerization of the specimens prepared with different alkali solutions by identifying the fissures, geopolymer matrix and dense/loose structure on the specimen surface. The solidifying of CS in the geopolymer gel can also be visualized through SEM morphologies. The fragment obtained from the compressive strength tests were used for this analysis because it can reveal the inner structure of geopolymer but the surface of cylindrical specimen [28,29]. The sample size is about 5 mm. It was dried in the oven and then Au sputter coating for conductivity. The magnitude is at 1000 time. The accelerating voltage is set at 15 KV.
* TCLP Test (Toxicity characteristic leaching procedure)
TCLP is a chemical analysis procedure to determine the leachability of hazardous elements present in waste. In this study, the encapsulation capability of geopolymer on the heavy metal contaminated soil is tested using TCLP. The TCLP procedure includes (1) Separation of liquid wastes; (2) Particle size reduction; (3) Extraction of solid material; (4) Extraction of solid material; (5) Elemental analysis in ppm.
3. Results and Discussion
3.1. Effect of the Molarity of NaOH on the Physical and Mechanical Characteristics of Geopolymer Materials
According to the previous studies, the molarity of NaOH can determine the dissolution of Si and Al ions from GGBFS and the geopolymerization process. The more the Si and Al ions dissolved from GGBFS, the higher the degree of Si–O–Al 3D structure can be formed. The alkali solutions used in this study were prepared with NaOH solution of 4 M, 6 M to 8 M, and mixed with sodium silicate and sodium aluminate. These solutions were then used to activate GGBFS and form a geopolymer precursor. The geopolymer specimens were made with a 1.5 CS/GGBFS ratio. The liquid (alkali solution)-solid ratio was fixed at 0.35. From previous experience, a 0.35 L/S ratio may result in better workability of the mixing paste. The influence of the molarity of NaOH solutions on the characteristics of geopolymer specimens is then determined based on their physical and mechanical properties test results.
3.1.1. The Physical Properties of Different Molarity of NaOH Geopolymer Materials
The physical properties of geopolymer prepared with 4 M, 6 M to 8 M NaOH were tested after 3 days of curing. The molar ratios of SiO2/Na2O and SiO2/Al2O3 of the solution were adjusted to 1.28 and 50, respectively. As shown in Table 4, the bulk density of Na04, Na06, and Na08 specimens are in the ranges of 1.64 g/cm3~1.71 g/cm3. The apparent specific gravity of the specimens increases from 2.48 to 2.73 as the molarity of NaOH increases from 4 M to 8 M. This is because more Si, Al, and Ca ions were dissolved from the GGBFS surface to facilitate the geopolymerization process. The higher degree of geopolymerization can form a more dense 3D structure that leads to higher sp.gr. [30,31,32].

Table 4.
The physical properties of different molarity of NaOH geopolymer materials.
The porosity of the specimens increases from 34% to 39% as the NaOH concentration increases from 4 M to 8 M after 3 days of curing. This is probably due to the specimens prepared with higher alkali solution also hardening faster and hindering the evaporation of water existing in the structure. The porosity of specimens prepared with 8 M NaOH decrease from 39% to 31% as the curing day increases from 3 to 28 days. This is probably due to the slower hardening process of the remaining geopolymer gel that reduces the size of the pore. The water absorption of all specimens tested is between 21% to 24 %. These results are in accordance with the porosity measurement, i.e., the higher the porosity, the higher the water absorption.
The physical properties of Na04, Na06, and Na08 specimens were also measured after 7, 14, and 28 days of curing. The test results show the physical properties of specimens slightly improve as the curing day increases from 3 to 28 days and NaOH molarity increases from 4 M to 8 M.
3.1.2. The Mechanical Strength of Different Molarity of NaOH Geopolymer Materials
The compressive strength of specimens of Na04, Na06, and Na08 specimens are shown in Figure 4. The measurements were conducted after specimens were cured after 3, 7, 14, and 28 days of curing. After 3 days of curing, the compressive strength of all specimens is between 38.90 MPa~40.19 MPa. It demonstrates the molarity of NaOH has no obvious influence on the strength of geopolymer at the early curing stage. After 7 days of curing, the specimens prepared with higher molarity of NaOH have higher strength than those prepared with lower NaOH solutions. The strength of Na04 increases from 38.90 MPa to 43.48 MPa as curing time increases from 3 to 14 days. After being cured for 3, 7, and 14 days, Na04 and Na06 have similar strengths. This is probably due to both specimens having the same speed of geopolymerization process in the early curing stage. After 28 days of curing, NA06 develops a more dense C-A-S-H structure and results in higher strength than that of Na04. For the Na04 specimen, its strength only slightly increases from 46.6 MPa to 47.66 MPa. It can be attributed to the fewer Si and Al ions being dissolved from GGBF, thus hindering its strength development. For the Na08 specimen, its strength can reach up to 58.63 MPa after 28 days of curing. This is believed to be due to the higher molarity of NaOH can lead to more Si and Al ions dissolved from GGBFS and form more precursors that is required for geopolymerization [33,34,35].
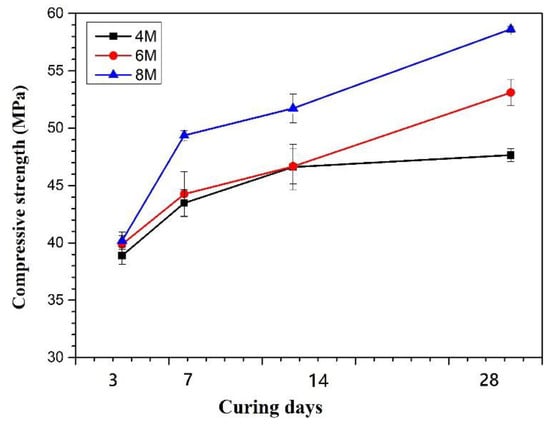
Figure 4.
Effect of the molarity of NaOH on the compressive strength (The molar ratios of SiO2/Na2O and SiO2/Al2O3 are 1.28 and 50).
The SEM morphologies of Na04, Na06, and Na08 are shown in Figure 5. For Na04, its microstructure shows more fissures than Na06 and Na08. For the sample prepared with higher molarity of NaOH solutions (Figure 5b,c), a more dense structure can be clearly identified. The higher the molarity of NaOH, the more geopolymeric gel was formed [36]. This can be attributed to the more C-A-S-H structure presented in geopolymer gel, which results in its higher strength, as shown in Figure 4.
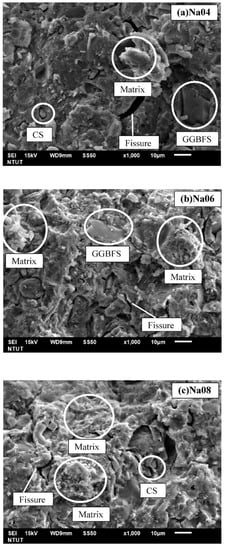
Figure 5.
SEM Images of specimens with (a) 4 M, (b) 6 M, and (c) 8 M of the molarity of NaOH.
3.1.3. TCLP Test of Different Molarity of NaOH Geopolymer Materials
Table 5 shows the TCLP test results of Na04, Na06, and Na08 specimens after 7 days of curing. The original CS contains 1987 ppm of Zn, 1267 ppm of Cu, and 629 ppm of Pb. After the geopolymer solidification process, no heavy metal can be detected in all specimens. For specimens prepared with low molarity of NaOH (Na04), it still demonstrates very good heavy metal encapsulation capability. For Na06 and Na08, they not only can encapsulate heavy metal but also present good physical and mechanical properties, as discussed above.

Table 5.
The TCLP analysis of different molarity of NaOH geopolymer materials curing 7 days.
3.2. Effect of SiO2/Na2O Molar Ratio on the Physical and Mechanical Characteristics of Geopolymer Materials
Table 6 shows the physical properties of geopolymer prepared with 4 M NaOH solution but varying the SiO2/Na2O molar ratio to 0.96, 1.28, and 1.91, as listed in Table 3. The bulk density of all specimens measured is in the range of 1.64 g/cm3~1.65 g/cm3. There is also only a slight difference in apparent specific gravity was found in all three samples. However, the porosity of the specimen decreases to 35% (SiNa096) as the SiO2/Na2O molar ratio increases from 0.96 to 1.28 and 1.91. The SiNa096 also have higher water absorption characteristic as comparing with SiNa128 and SiNa191.

Table 6.
The physical properties of different SiO2/Na2O molar ratio geopolymer materials.
3.2.1. The Mechanical Strength of Different SiO2/Na2O Molar Ratio Geopolymer Materials
The effect of the SiO2/Na2O molar ratio in the alkali solution on the compressive strength of specimens is shown in Figure 6. The SiNa096 and SiNa128 have almost the same strength in the early 3 days curing stage. After 14 days of curing, it was found that SiNa096 has much higher strength than SiNa1.28 and SiNa1.91. After 28 days of curing, the strength of SiNa096 and SiNa128 can reach 49.80 MPa and 47.66 MPa. For SiNa191, its strength is generally about 10 MPa lower than SiNa096 and SiNa128 at each curing stage. This is because too many Si ions exist in the geopolymer gel at a high SiO2/Na2O molar ratio and then quickly form short Si–O bonds or ring-type Si–O–Al bonds. These bonding cannot link to geopolymer 3D structure and thus reduce its strength [37].
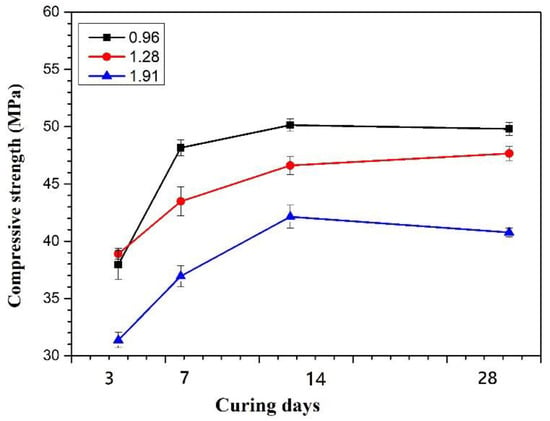
Figure 6.
Effect of SiO2/Na2O Molar ratio on the compressive strength (The molar ratios of SiO2/Al2O3 is 50).
Figure 7 shows the SEM morphologies of SiNa096 and SiNa191. For the SiNa096 specimen, Figure 7a, a dense geopolymer matrix can be clearly seen, and CS particles are tightly encapsulated. On the contrary, SiNa191 has a sheet- or flake-like surface and relatively loose structure. These findings can also be related to the compressive strength results shown in Figure 6.

Figure 7.
SEM Images of specimens with (a) 0.96 and (b) 1.91 of SiO2/Na2O Molar ratio.
3.2.2. The TCLP of Different SiO2/Na2O Molar Ratio Geopolymer Materials
After 7 days of curing, the TCLP test of SiNa096, SiNa128, and SiNa191 specimens show no heavy metal dissolved from the geopolymer matrix. These results indicate the heavy metal in contaminated soil can be effectively encapsulated and not affected by the SiO2/Na2O molar ratio of the alkali solution (Table 7). However, the SiNa096and SiNa128 specimens have higher strength and may also provide better engineering applications.

Table 7.
The TCLP analysis of different SiO2/Na2O molar ratio geopolymer materials curing 7 days.
3.3. Effect of SiO2/Al2O3 Molar Ratio on Physical and Mechanical Characteristics of Geopolymer Materials
Apart from the SiO2/Na2O molar ratio, the influence of the SiO2/Al2O3 molar ratio of the alkali solution on the properties of geopolymer is also evaluated. The alkali solutions were prepared with 4 M NaOH and 1.28 SiO2/Al2O3 molar ratio but varying SiO2/Al2O3 molar ratio to 0, 50, and 70, as shown in Table 4.
The physical properties of SiAl00, SiAl50, and SiAl70 specimens are listed in Table 8. SiAl50 and SiAl70 specimens have very similar bulk density, apparent specific gravity, porosity, and water absorption characteristics. SiAl00, prepared without aluminum silicate addition, has a lower density (1.56 g/cm3) and higher porosity (41%) than SiAl50 and SiAl70.

Table 8.
The physical properties of different SiO2/Al2O3 molar ratio geopolymer materials.
The compressive strength test results of SiAl00, SiAl50, and SiAl70 specimens are shown in Figure 8. After 3 days of curing, the compressive strength of SiAl00 is 28.54 MPa, but SiAl50 and SiAl70 can reach 38.9 MPa and 39.53 MPa, respectively. At every curing stage, SiAl00 also has much lower strength than SiAl50 and SiAl70. This is probably due to the low Al2O3 content (10%) of GGBFS that cannot provide sufficient Al ions to form Si–O–Al frame structure during geopolymerization reaction. Therefore, more Si–O–Si frame structure is formed but is not favorable for developing their strength [37]. For SiAl70, after 7 days of curing, its strength reduces from 44.08 MPa to 43.17 MPa at 14 days and 39.97 MPa at 28 days.
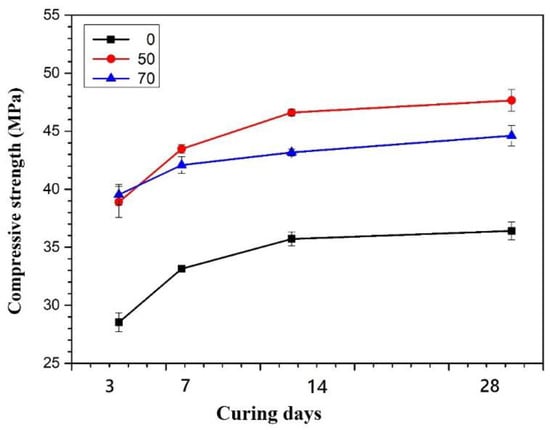
Figure 8.
Effect of SiO2/Al2O3 Molar ratio on the compressive strength (The molar ratios of SiO2/Na2O is 1.28).
The TCLP of Different SiO2/Al2O3 Molar Ratio Geopolymer Materials
The TCLP tests were also conducted on SiAl00, SiAl50, and SiAl70 samples after 7 days of curing. The same results were found as in previous tests. There is no heavy metal detected (Table 9). It is believed that the heavy metal ions can bond to the Si–O–Al and Si–O–Si frame structure and not be leached from the geopolymer matrix.

Table 9.
The TCLP analysis of different SiO2/Al2O3 molar ratio geopolymer materials curing 7 days.
3.4. TCLP of High Heavy Metal Content Geopolymer
The original contaminated soil samples only contented 1987 ppm Zn and 1267 ppm Cu. In order to evaluate the encapsulation capability, both Cu and Zn concentrations of the soil were adjusted to 10,000 ppm. The soil was then used to prepare geopolymer, as shown in Table 10. The TCLP test results are listed in Table 11. The Zn concentration of CS-6 (1.5 CS/GGBFS ratio) and CS-5 (50/50 CS/GGBFS ratio) were reduced to 10 ppm and6.77 ppm after 3 days of curing. After 7 days of curing, no Zn ion was detected. The Cu concentration of CS-6 and CS-5 reduced to 56.18 ppm and 32.71 ppm, respectively, after 7 days of curing. No Cu ion was detected after 14 days of curing for both CS-6 and CS-5. These test results indicate the higher GGBFS in the geopolymer mixture (50/50 CS/GGBFS ratio) can encapsulate metal ions during its early curing stage because more Si, Ca, and Al ions were dissolved from GGBFS and form more geopolymer gel [38]. However, CS-6 also has good heavy metal encapsulation characteristics at an extended long curing period. The TCLP results also proved that the geopolymer technique is capable of treating contaminated soil with high heavy metal content [15].

Table 10.
The parameters of soil-based geopolymer.

Table 11.
The TCLP analysis of geopolymer.
4. Conclusions
- It was found that geopolymer specimens prepared with 8 M NaOH have higher strength (58.63 MPa) when compared with those prepared with 4 M (43.11 MPa) and 6 M (47.66 MPa) NaOH alkali solution. This is because higher molarity of NaOH can dissolve more Si and Al ions for a geopolymerization reaction. The SEM morphologies 8 M specimens also exhibit more CASH gel matrix
- The strength of specimens prepared with an alkali solution of 0.96 and 1.29 SiO2/Na2O molar ratio have a strength of about 49 MPa after 28 days of curing. However, the specimen prepared with 1.91 SiO2/Na2O molar ratio alkali solution only has a strength of 38 MPa. This can be attributed to the higher Si ion in the solution may form a Si–O bond instead of Si–O–Si and Si–O–Al bonds.
- By varying SiO2/Al2O3 molar ratios of alkali solution, it was found the specimens prepared with 50 SiO2/Al2O3 molar ratios have better physical and mechanical properties when compared with those prepared with 0 SiO2/Al2O3 molar solution. This is because extra Al ions are needed to form a Si–O–Al structure when insufficient Al ions are dissolved from GGBFS.
- TCLP tests show 7 days curing specimens prepared with heavy metal contaminated soil have on only 2 ppm Cu ion and no Zn ion can be detected. Adding an extra 10,000 ppm of heavy metal ions in the geopolymer process also shows no heavy metal ions were found after 14 days of curing.
- According to the test results obtained from this study, the solidification/stabilization of heavy metal contaminated soil can be successfully achieved using the geopolymer technique. It is also possible to use the solidified geopolymer product for further engineering applications due to its good physical and mechanical properties.
Author Contributions
Conceptualization, Y.-C.C. and Y.-C.D.; methodology, Y.-C.D., W.-H.L. and X.L.; formal analysis, Y.-C.C., S.L. and Q.L.; writing—original draft preparation, Y.-C.C.; writing—review and editing, Y.-C.D. and W.-H.L.; data curation, Y.-C.C. and H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Helena, I.G.; Celia, D.F.; Alexandra, B.E. Overview of in situ and ex situremediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci. Total Environ. 2013, 445–446, 237–260. [Google Scholar]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Online. Available online: https://clu-in.org/techfocus/default.focus/sec/Solidification/cat/Overview/ (accessed on 6 June 2022).
- Davidovits, J. Geopolymer Chemistry and Properties. In Proceedings of the 1st International Conference on Geopolymer ‘88, Compiegne, France, 1–3 June 1988; Volume 1, pp. 25–48. [Google Scholar]
- Wang, Y.S.; Alrefaei, Y.; Dai, J.G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Struct. Mater. 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Room-Temperature Ceramic Matrix for Composites. In Ceramic Engineering and Science Proceedings, Proceedings of the 12th Annual Conference on Composites and Advanced Ceramic Materials, Cocoa Beach, FL, USA, 17–20 January 1988; The American Ceramic Society: Westerville, OH, USA; Volume 9, pp. 835–842.
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Allahverdi, A.; Kani, E.N.; Hossain, K.M.A.; Lachemi, M. Methods to control efflorescence in alkali-activated cement-based materials. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Thorston, UK, 2015; pp. 463–483. [Google Scholar]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and Ordinary Portland Cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liegeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Institut Géopolymère: Saint-Quentin, France, 2011; p. 58. [Google Scholar]
- Cai, J.; Li, X.; Tan, J.; Vandevyvere, B. Thermal and compressive behaviors of fly ash and metakaolin-based geopolymer. J. Build. Eng. 2020, 30, 101307. [Google Scholar] [CrossRef]
- Singh, A.; Bhadauria, S.S.; Mudgal, M.; Kushwah, S.S. Effect of alkali activator dosage on compressive and tensile strength of ground granulated blast furnace slag based geopolymer concrete. Can. J. Civ. Eng. 2022, 49, 73–82. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N. Mechanisms of Heavy Metal Immobilisation using Geopolymerisation Techniques—A review. J. Adv. Concr. Technol. 2018, 16, 124–136. [Google Scholar] [CrossRef]
- Sefiu, R.; Zhang, B.; Rohiverth, G.; Tiju, T.; Yang, M. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Bassam, E.E.; Mazen, A.; Rushdi, I.Y.; Imad, H.; Fawwaz, K. Adsorption of Cu(II), Ni(II), Zn(II), Cd(II) and Pb(II) onto Kaolin/Zeolite Based- Geopolymers. Adv. Mater. Phys. Chem. 2012, 2, 119–125. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. Factors affecting the immobilization of metals in geopolymerized fly ash. Metall. Mater. Trans. B 1998, 29, 283–291. [Google Scholar] [CrossRef]
- Palacios, M.; Palomo, A. Alkali-activated fly ash matrices for lead immobilisation: A comparison of different leaching tests. Adv. Cem. Res. 2004, 16, 137–144. [Google Scholar] [CrossRef]
- El Shazly, R.M.; Sadawy, M.M. Effect of Slag as a Fine Aggregate on Mechanical, Corrosion, and Nuclear Attenuation Properties of Concrete. Int. J. Sci. Eng. Res. 2017, 5, 243–250. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice Husk Ash-Based Geopolymer Binder: Compressive Strength, Optimize Composition, FTIR Spectroscopy, Microstructural, and Potential as Fire-Retardant Material. Polymers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lee, W.H.; Ding, Y.C. The Use of Recycled Aggregate Sludge for the Preparation of GGBFS and Fly Ash Based Geopolymer. Crystals 2011, 11, 1486. [Google Scholar] [CrossRef]
- Method of Test for Compressive Strength of Cylindrical Concrete Specimens. CNS 1232-2002. Available online: http://www.gbstandards.org/CNS/CNS_standard_chinese.asp?word=CNS%201232 (accessed on 2 April 2022).
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM Int West Conshohocken: Conshohocken, PA, USA, 2000.
- Method of Test for Apparent Porosity, Water Absorption and Specific Gravity of Refractory Bricks. CNS 619-1986. Available online: http://www.gbstandards.org/CNS/CNS_standard_chinese.asp?word=CNS%20619 (accessed on 2 April 2022).
- Arioz, E.; Arioz, O.; Kockar, O.M. An experimental study on the mechanical and microstructural properties of geopolymers. Procedia Eng. 2012, 42, 100–105. [Google Scholar] [CrossRef][Green Version]
- Al-Jaberi, L.; Al-Saraj, A.K.W.; Al-Saraj, A.J.S. Scanning Electron Microscopy of Metakaolin Based Geopolymer Concrete. J. Phys. Conf. Ser. 2021, 2114, 012061. [Google Scholar] [CrossRef]
- Pawan, K.K.R.; Surendra, B.V. Study on Strength of Geopolymer Concrete with Abient Temperature Curing and Low Alkali Content. Int. Res. J. Eng. Technol. (IRJET) 2016, 3, 5. [Google Scholar]
- Zhang, Y.; Sun, W.; Chen, Q.; Chen, L. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar]
- Mishra, A.; Choudhary, D.; Jain, N.; Kumar, M. Effect of concentration of alkali liquid and curing time on strength and water absorption of geopolymer concrete. J. Eng. Appl. Sci. 2008, 3, 14–18. [Google Scholar]
- Mayhoub, O.A.; Nasr, E.S.A.; Ali, Y.; Kohail, M. Properties of slag based geopolymer reactive powder concrete. Ain Shams Eng. J. 2021, 12, 99–105. [Google Scholar] [CrossRef]
- Reddy, S.; Krishna, K.V.; Rao, M.V.S.; Shrihari, S. Effect of molarity of sodium hydroxide and molar ratio of alkaline activator solution on the strength development of geopolymer concrete. Web Conf. 2021, 309, 0158. [Google Scholar]
- Verma, M.; Dev, N. Sodium hydroxide effect on the mechanical properties of flyash-slag based geopolymer concrete. Struct. Concr. 2020, 22, E368–E379. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Abdullah, M.M.A.; Binhussain, M. Kaolin-based geopolymers with various NaOH concentrations. Int. J. Miner. Metall. Mater. 2013, 20, 313–322. [Google Scholar] [CrossRef]
- Walkley, B.; Provis, J.L.; San Nicolas, R.; Sani, M.-A.; Sani, M.-A.; Van Decenter, J.S.J. Stoichiometrically controlled C-A-S-H/N-A-S-H gel blends via alkali-activation of synthetic precursors. Adv. Appl. Ceram. 2015, 114, 372–377. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Sun, S.; Yang, W.; Fang, L.; Ma, R.; Lin, C.; Li, H. Lead zinc slag-based geopolymer: Demonstration of heavy metal solidification mechanism from the new perspectives of electronegativity and ion potential. Environ. Pollut. 2022, 293, 118509. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).