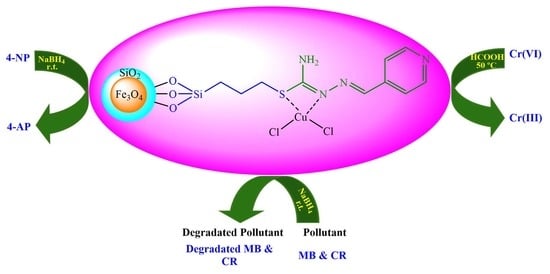
Fabrication of Copper(II)-Coated Magnetic Core-Shell Nanoparticles Fe3O4@SiO2: An Effective and Recoverable Catalyst for Reduction/Degradation of Environmental Pollutants
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Preparation of Pyridine-4-Carbaldehyde Thiosemicarbazide Ligand (L)
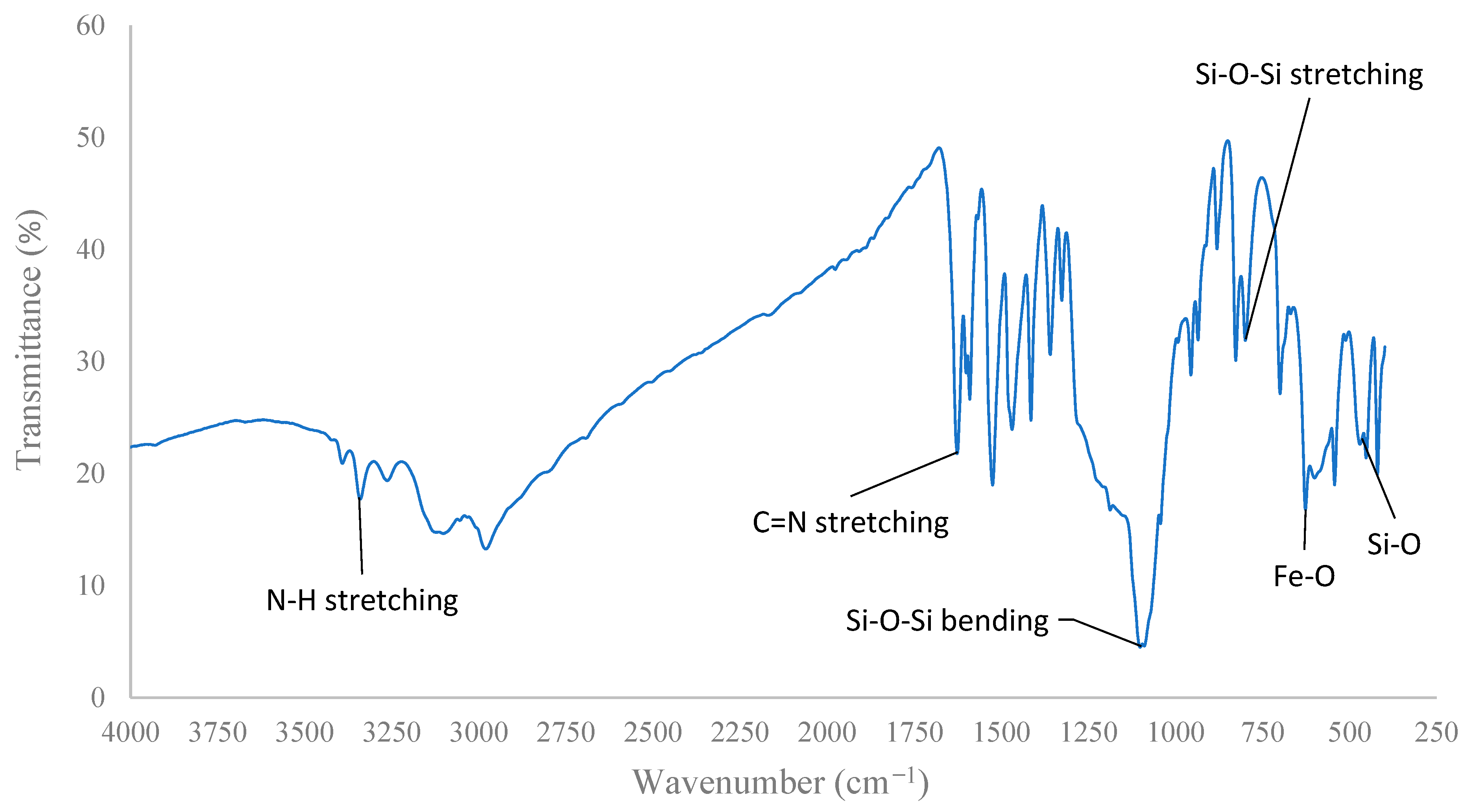
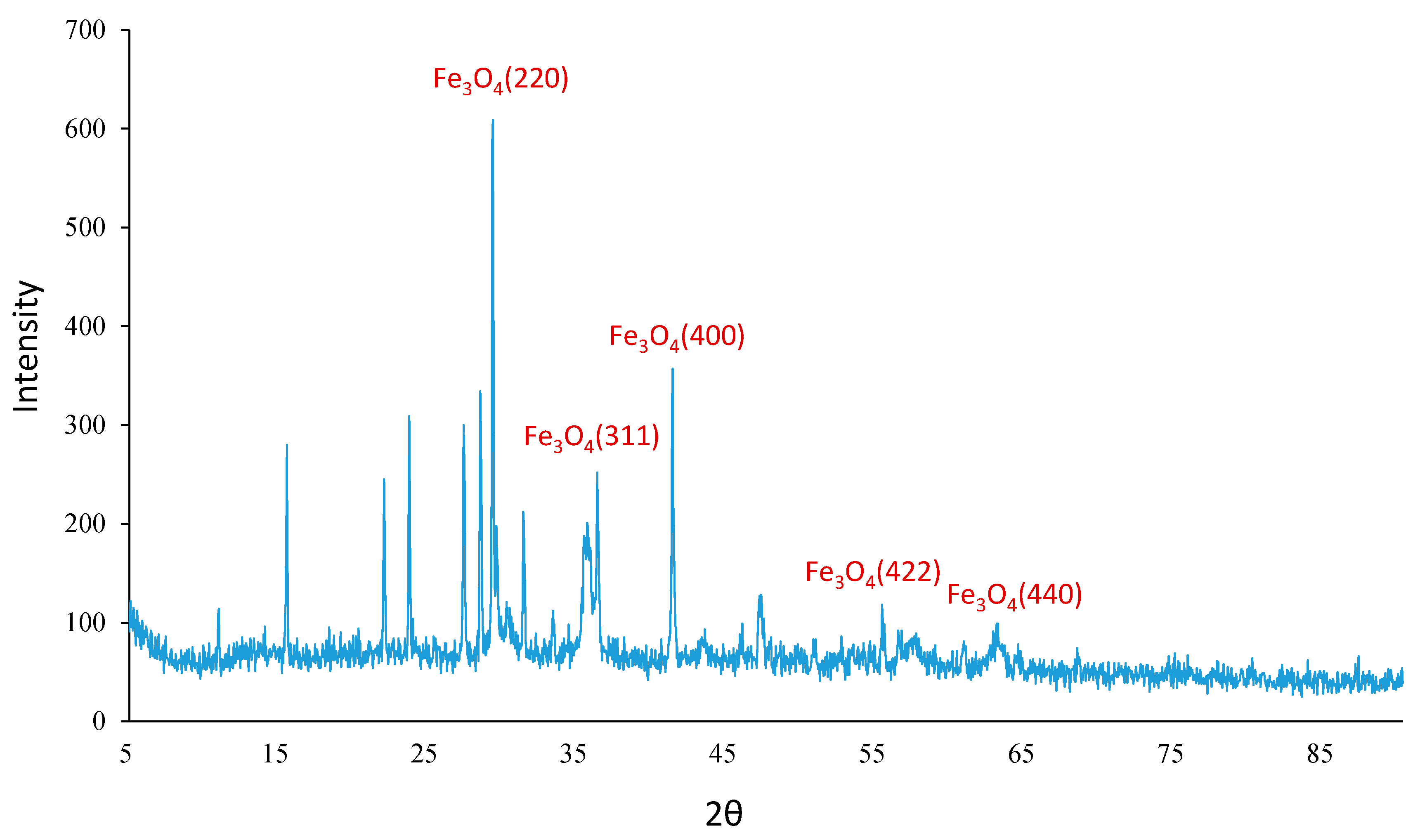
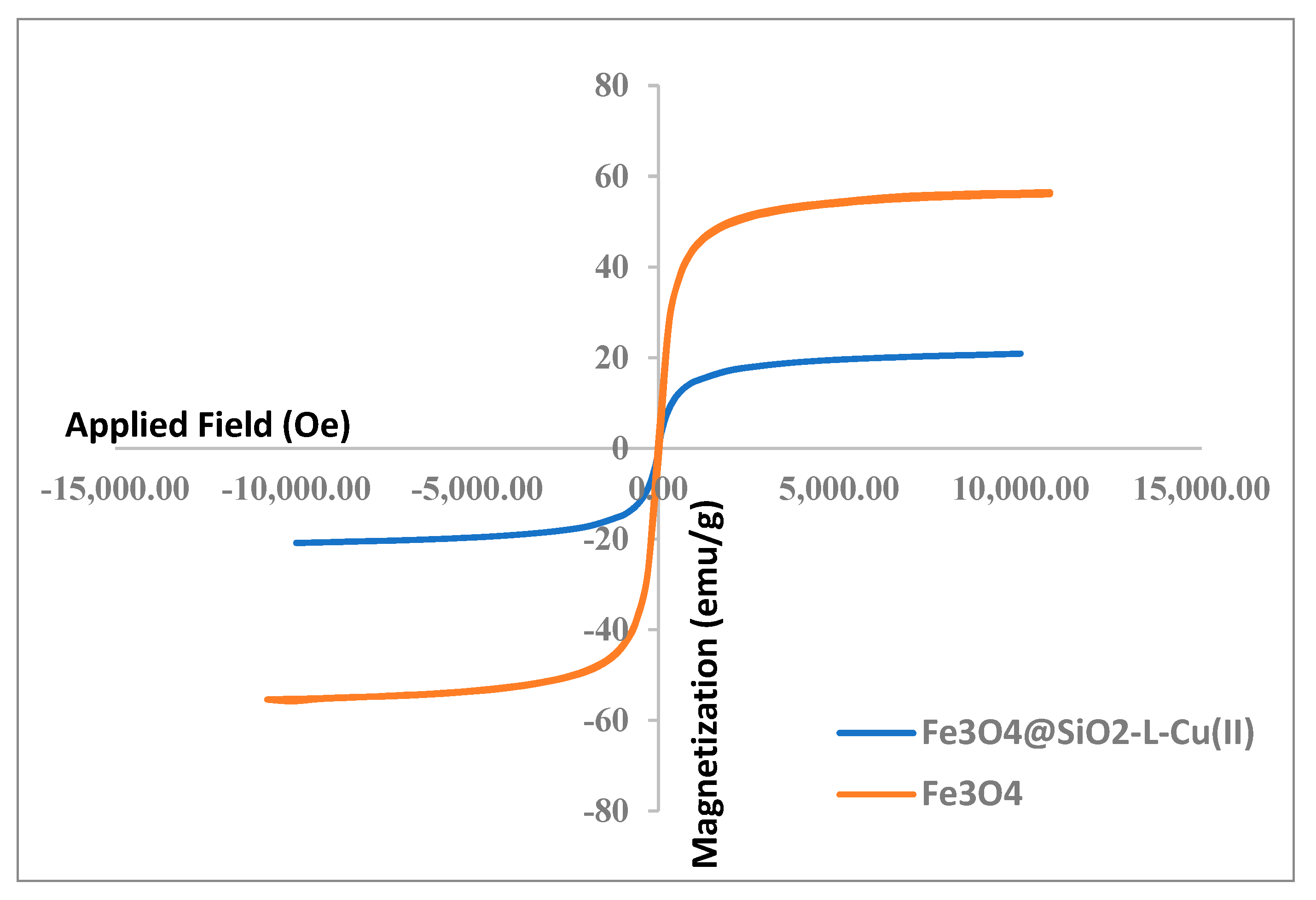
2.3. [Fe3O4@SiO2–L–Cu(II)] Catalyst Synthesis Procedure
2.4. Catalytic Activity of [Fe3O4@SiO2–L–Cu(II) for 4-NP Reduction
2.5. Catalytic Degradation of CR and MB by Employing [Fe3O4@SiO2–L–Cu(II)] Complex
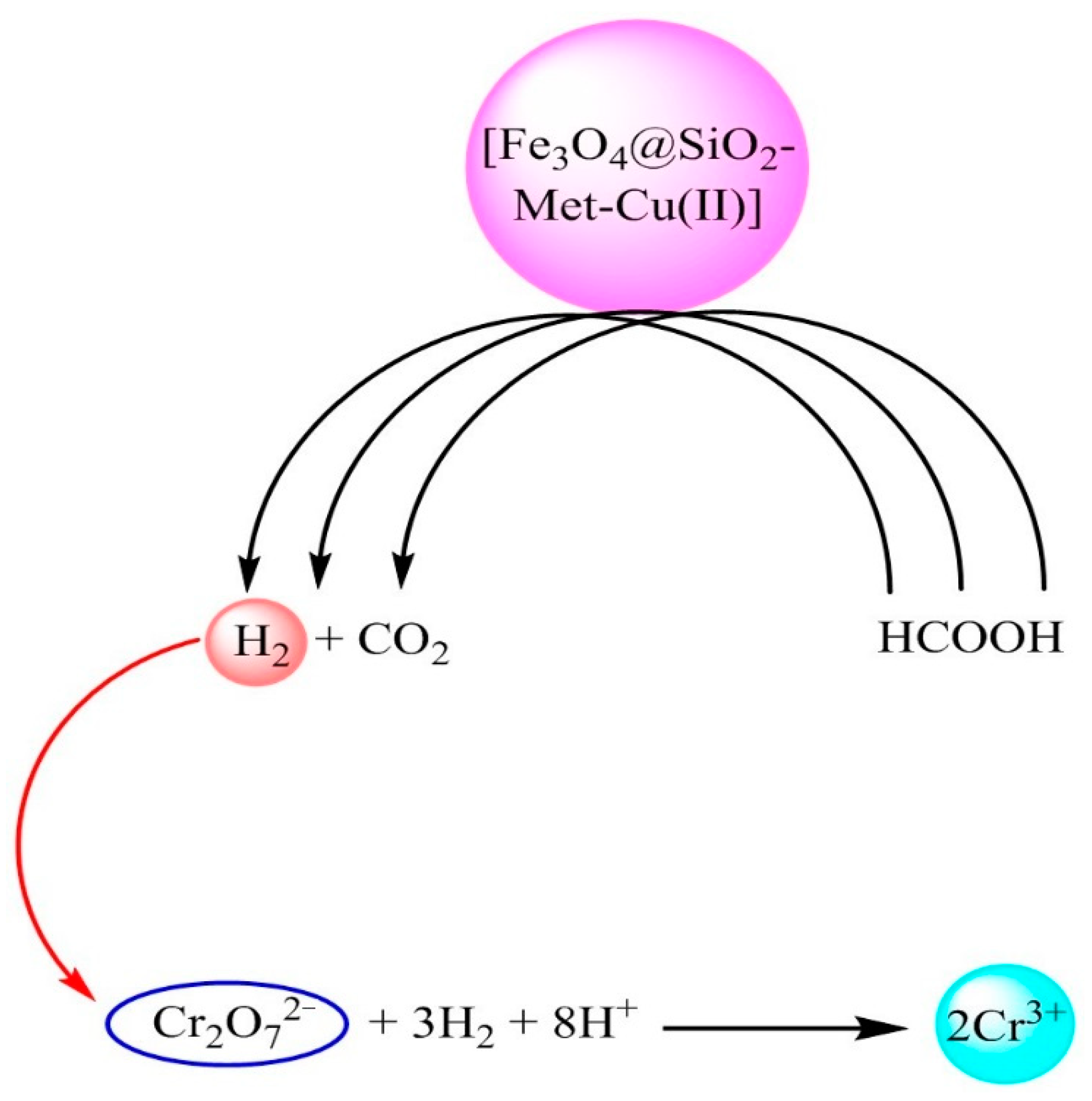
2.6. Catalytic Activity of [Fe3O4@SiO2–L–Cu(II)] for Cr(VI) Reduction
3. Results and Discussion
3.1. Catalytic Reduction of 4-NP by [Fe3O4@SiO2–L–Cu(II] Catalyst
3.2. Catalytic Reduction of Cr (VI)by [Fe3O4@SiO2–L–Cu(II)]
3.3. Catalytic Reduction of MB and CR by Using and [Fe3O4@SiO2–L–Cu(II)]
4. Catalyst Reusability
5. Comparison of Some Catalysts
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Prabakar, D.; Suvetha, S.K.; Manimudi, V.T.; Mathimani, T.; Kumar, G.; Rene, E.R.; Pugazhendhi, A. Pretreatment technologies for industrial effluents: Critical review on bioenergy production and environmental concerns. J. Environ. Manag. 2018, 218, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Bagherzade, A.; Nemati, F.; Nahzomi, H.T.; Elhampour, A. Experimental and quantum chemical study on nano-copper immobilized on magnetic graphitic carbon nitride core shell particles; a reusable heterogeneous catalyst toward reduction of nitro arenes. J. Mol. Struct. 2019, 1185, 38–49. [Google Scholar] [CrossRef]
- Farhadi, S.; Siadatnasab, F. Synthesis and structural characterization of magnetic cadmium sulfide–cobalt ferrite nanocomposite, and study of its activity for dyes degradation under ultrasound. J. Mol. Struct. 2016, 1123, 171–179. [Google Scholar] [CrossRef]
- Singh, K.; Arora, S. Removal of Synthetic Textile Dyes from Wastewaters: A Critical Review on Present Treatment Technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Serrà, A.; Artal, R.; Pozo, M.; Garcia-Amorós, J.; Gómez, E. Simple Environmentally-Friendly Reduction of 4-Nitrophenol. Catalysts 2020, 10, 458. [Google Scholar] [CrossRef] [Green Version]
- Subhan, A.; Jhuma, S.S.; Saha, P.C.; Alam, M.M.; Asiri, A.M.; Al-Mamun, M.; Attia, S.A.; Emon, T.H.; Azad, A.K.; Rahman, M.M. Efficient selective 4-aminophenol sensing and antibacterial activity of ternary Ag2O3·SnO2·Cr2O3 nanoparticles. New J. Chem. 2019, 43, 10352–10365. [Google Scholar] [CrossRef]
- Nameni, M.; Moghadam, M.R.A.; Arami, M. Adsorption of hexavalent chromium from aqueous solutions by wheat bran. Int. J. Environ. Sci. Technol. 2008, 5, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Sarkar, A.; Sen, S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; Gu, Y.; Song, X.; Zhao, J. Carcinogenicity of chromium and chemoprevention: A brief update. OncoTargets Ther. 2017, 10, 4065–4079. [Google Scholar] [CrossRef] [Green Version]
- Barceloux, D.G. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.R. Review article. Adverse hematological effects of hexavalent chromium: An overview. Interdiscip. Toxicol. 2016, 9, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, L.; Mourad, W.; Rophael, M. Photocatalytic reduction of environmental pollutant Cr(VI) over some semiconductors under UV/visible light illumination. Appl. Catal. B Environ. 1998, 17, 267–273. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chaudhuri, B.; Bhattacharjee, S.; Ray, A.K.; Dutta, B.K. Photo-reduction of hexavalent chromium in aqueous solution in the presence of zinc oxide as semiconductor catalyst. Chem. Eng. J. 2009, 153, 86–93. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Guan, J. Applications of Magnetic Nanomaterials in Heterogeneous Catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Albonetti, S.; Mazzoni, R.; Cavani, F. CHAPTER 1. Homogeneous, Heterogeneous and Nanocatalysis. Transit. Met. Catal. Aerob. Alcohol Oxid. 2014, 1–39. [Google Scholar]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Garcia, H. Crossing the Borders Between Homogeneous and Heterogeneous Catalysis: Developing Recoverable and Reusable Catalytic Systems. Top. Catal. 2008, 48, 8–31. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.; Lim, S.; Lee, H. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, L.; Fu, K.; Zhu, F.; Yang, T.; Yang, T.; Luo, J.; Wu, M.; Yu, D. Ultrastable MOF-Based Foams for Versatile Applications. Nano Res. 2021, 15, 2961–2970. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, M.; Xu, L.; Wang, S.; Yang, T.; Wu, M.; Lu, W.; Li, Y.; Yu, D. Unraveling Timescale-Dependent Fe-MOFs Crystal Evolution for Catalytic Ozonation Reactivity Modulation. J. Hazard. Mater. 2022, 431, 128575. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.-H.; Hahm, E.; Kim, H.-M.; Son, B.S.; Jo, A.; An, J.; Thi, T.A.T.; Nguyen, D.Q.; Jun, B.-H. Silica-Coated Magnetic Iron Oxide Nanoparticles Grafted onto Graphene Oxide for Protein Isolation. Nanomaterials 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Das, T.K.; Ganguly, S.; Ghosh, S.; Remanan, S.; Ghosh, S.K.; Das, N.C. In-situ synthesis of magnetic nanoparticle immobilized heterogeneous catalyst through mussel mimetic approach for the efficient removal of water pollutants. Colloids Interface Sci. Commun. 2019, 33, 100218. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Kalantari, E.; Khalilzadeh, M.A.; Zareyee, D.; Shokouhimehr, M. Catalytic degradation of organic dyes using green synthesized Fe3O4-cellulose-copper nanocomposites. J. Mol. Struct. 2020, 1218, 128488. [Google Scholar] [CrossRef]
- Kianfar, A.H.; Arayesh, M.A. Synthesis, characterization and investigation of photocatalytic and catalytic applications of Fe3O4/TiO2/CuO nanoparticles for degradation of MB and reduction of nitrophenols. J. Environ. Chem. Eng. 2019, 8, 103640. [Google Scholar] [CrossRef]
- Shammi, Z.M.; Kianfar, A.H.; Momeni, M.M. Hydrothermal synthesis and characterization of CuO–CoO/TiO2 for photocatalytic degradation of methylene blue under visible light and catalytic reduction of P-nitrophenol. J. Mater. Sci. Mater. Electron. 2020, 31, 14810–14822. [Google Scholar] [CrossRef]
- Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Gurau, D.; Ficai, A.; Ficai, D.; Andronescu, E. SYNTHESIS AND APPLICATIONS OF Fe3O4/SiO2 CORE-SHELL MATERIALS. Curr. Pharm. Des. 2015, 21, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Sun, S. Chemical Synthesis of Magnetic Nanoparticles for Permanent Magnet Applications. Chem. A Eur. J. 2019, 26, 6757–6766. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.; Miaskowski, A.; Jenkins, S.I.; Lim, J.; Dobson, J. Remote manipulation of magnetic nanoparticles using magnetic field gradient to promote cancer cell death. Appl. Phys. A Mater. Sci. Process. 2019, 125, 226. [Google Scholar] [CrossRef] [Green Version]
- Knežević, N.; Gadjanski, I.; Durand, J.-O. Magnetic nanoarchitectures for cancer sensing, imaging and therapy. J. Mater. Chem. B 2018, 7, 9–23. [Google Scholar] [CrossRef]
- Mirza, S.; Ahmad, M.S.; Shah, M.I.A.; Ateeq, M. Magnetic Nanoparticles: Drug Delivery and Bioimaging Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Sanaei-Rad, S.; Ghasemzadeh, M.A.; Aghaei, S.S. Synthesis and structure elucidation of ZnFe2O4/IRMOF-3/GO for the drug delivery of tetracycline and evaluation of their antibacterial activities. J. Organomet. Chem. 2021, 960, 122221. [Google Scholar] [CrossRef]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Bruls, D.M.; Evers, T.H.; Kahlman, J.A.H.; van Lankvelt, P.J.W.; Ovsyanko, M.; Pelssers, E.G.M.; Schleipen, J.J.H.B.; de Theije, F.K.; Verschuren, C.A.; van der Wijk, T.; et al. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip 2009, 9, 3504–3510. [Google Scholar] [CrossRef]
- Star, B. Supplementary Information SUPPLEMENTARY INFORMATION Albertsen. Suppl. Infomation. 2013, 22, 1–12. [Google Scholar]
- Fatima, H.; Kim, K.-S. Magnetic nanoparticles for bioseparation. Korean J. Chem. Eng. 2017, 34, 589–599. [Google Scholar] [CrossRef]
- Lee, D.-W.; Fatima, H.; Kim, K.-S. Preparation of Silica Coated Magnetic Nanoparticles for Bioseparation. J. Nanosci. Nanotechnol. 2018, 18, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Pourian, E.; Javanshir, S.; Dolatkhah, Z.; Molaei, S.; Maleki, A. Ultrasonic-Assisted Preparation, Characterization, and Use of Novel Biocompatible Core/Shell Fe3O4@GA@Isinglass in the Synthesis of 1,4-Dihydropyridine and 4H-Pyran Derivatives. ACS Omega 2018, 3, 5012–5020. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.A.; Dehkordi, R.B. Novel and green preparation of Fe3O4@TiO2-Immobilized-ILs Based on DABCO for highly synthesis of Primido [4,5-d] prymidines. Chemistryselect 2020, 5, 9097–9104. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Mirhosseini-Eshkevari, B.; Dadashi, J. IRMOF-3 Functionalized GO/CuFe2O4 A new and recyclable catalyst for the synthesis of Dihydropyrano [2,3-c] Pyrazoles under ultrasound irradiations. J. Mol Struct. 2022, 1261, 132843. [Google Scholar] [CrossRef]
- Cui, B.; Peng, H.; Xia, H.; Guo, X.; Guo, H. Magnetically recoverable core–shell nanocomposites γ-Fe2O3@SiO2@TiO2–Ag with enhanced photocatalytic activity and antibacterial activity. Sep. Purif. Technol. 2013, 103, 251–257. [Google Scholar] [CrossRef]
- Shasha, C.; Krishnan, K.M. Nonequilibrium Dynamics of Magnetic Nanoparticles with Applications in Biomedicine. Adv. Mater. 2020, 33, e1904131. [Google Scholar] [CrossRef]
- Mehta, R. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology. Mater. Sci. Eng. C 2017, 79, 901–916. [Google Scholar] [CrossRef]
- Abu-Reziq, R.; Alper, H.; Wang, D.; Post, M.L. Metal Supported on Dendronized Magnetic Nanoparticles: Highly Selective Hydroformylation Catalysts. J. Am. Chem. Soc. 2006, 128, 5279–5282. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Magnetic and Dendritic Catalysts. Accounts Chem. Res. 2015, 48, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Abolfathi, P. Nickel embedded on triazole-modified magnetic nanoparticles: A novel and sustainable heterogeneous catalyst for Hiyama reaction in fluoride-free condition. Catal. Commun. 2018, 103, 92–95. [Google Scholar] [CrossRef]
- Asl, E.A.; Haghighi, M.; Talati, A. Enhanced simulated sunlight-driven magnetic MgAl2O4-AC nanophotocatalyst for efficient degradation of organic dyes. Sep. Purif. Technol. 2020, 251, 117003. [Google Scholar] [CrossRef]
- Dadashi, J.; Hanifehpour, Y.; Mirtamizdoust, B.; Abdolmaleki, M.; Jegarkandi, E.; Rezaei, M.; Joo, S. Ultrasound-Assisted Synthesis and DFT Calculations of the Novel 1D Pb (II) Coordination Polymer with Thiosemicarbazone Derivative Ligand and Its Use for Preparation of PbO Clusters. Crystals 2021, 11, 682. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Mirtamizdoust, B.; Dadashi, J.; Wang, R.; Rezaei, M.; Abdolmaleki, M.; Joo, S.W. The Synthesis and Characterization of a Novel One-Dimensional Bismuth (III) Coordination Polymer as a Precursor for the Production of Bismuth (III) Oxide Nanorods. Crystals 2022, 12, 113. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Dadashi, J.; Khaleghian, M.; Rezaei, M.; Mirtamizdoust, B.; Joo, S.W. Sonochemical synthesis of the novel 1D zig-zag Hg(II)-Iodo bridged metal-organic coordination compounds with thiosemicarbazide derivative ligand. J. Mol. Struct. 2021, 1250, 131902. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Ge, B.; Zhang, L.; Zhou, J.; Liu, S.; Jiao, T. Facile preparation of black phosphorus-based rGO-BP-Pd composite hydrogels with enhanced catalytic reduction of 4-nitrophenol performances for wastewater treatment. J. Mol. Liq. 2020, 310, 113083. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Qin, Z.; Zhang, L.; Ye, Y.; Cao, M.; Gao, L.; Jiao, T. Preparation of PdNPs doped chitosan-based composite hydrogels as highly efficient catalysts for reduction of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125889. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Z.; Qin, C.; Lv, H.; Qin, L.; Niu, W.; Zhai, S.; An, Q. Sandwich-like N-C/Cu/N-C porous beads derived from alginate with enhanced catalytic activity and excellent recyclability for 4-nitrophenol reduction. Ind. Crops Prod. 2021, 164, 113413. [Google Scholar] [CrossRef]
- Sip, Y.Y.L.; Fox, D.W.; Shultz, L.R.; Davy, M.; Chung, H.-S.; Antony, D.-X.; Jung, Y.; Jurca, T.; Zhai, L. Cu–Ag Alloy Nanoparticles in Hydrogel Nanofibers for the Catalytic Reduction of Organic Compounds. ACS Appl. Nano Mater. 2021, 4, 6045–6056. [Google Scholar] [CrossRef]
- Naseer, F.; Ajmal, M.; Bibi, F.; Farooqi, Z.; Siddiq, M. Copper and cobalt nanoparticles containing poly(acrylic acid-co-acrylamide) hydrogel composites for rapid reduction of 4-nitrophenol and fast removal of malachite green from aqueous medium. Polym. Compos. 2017, 39, 3187–3198. [Google Scholar] [CrossRef]




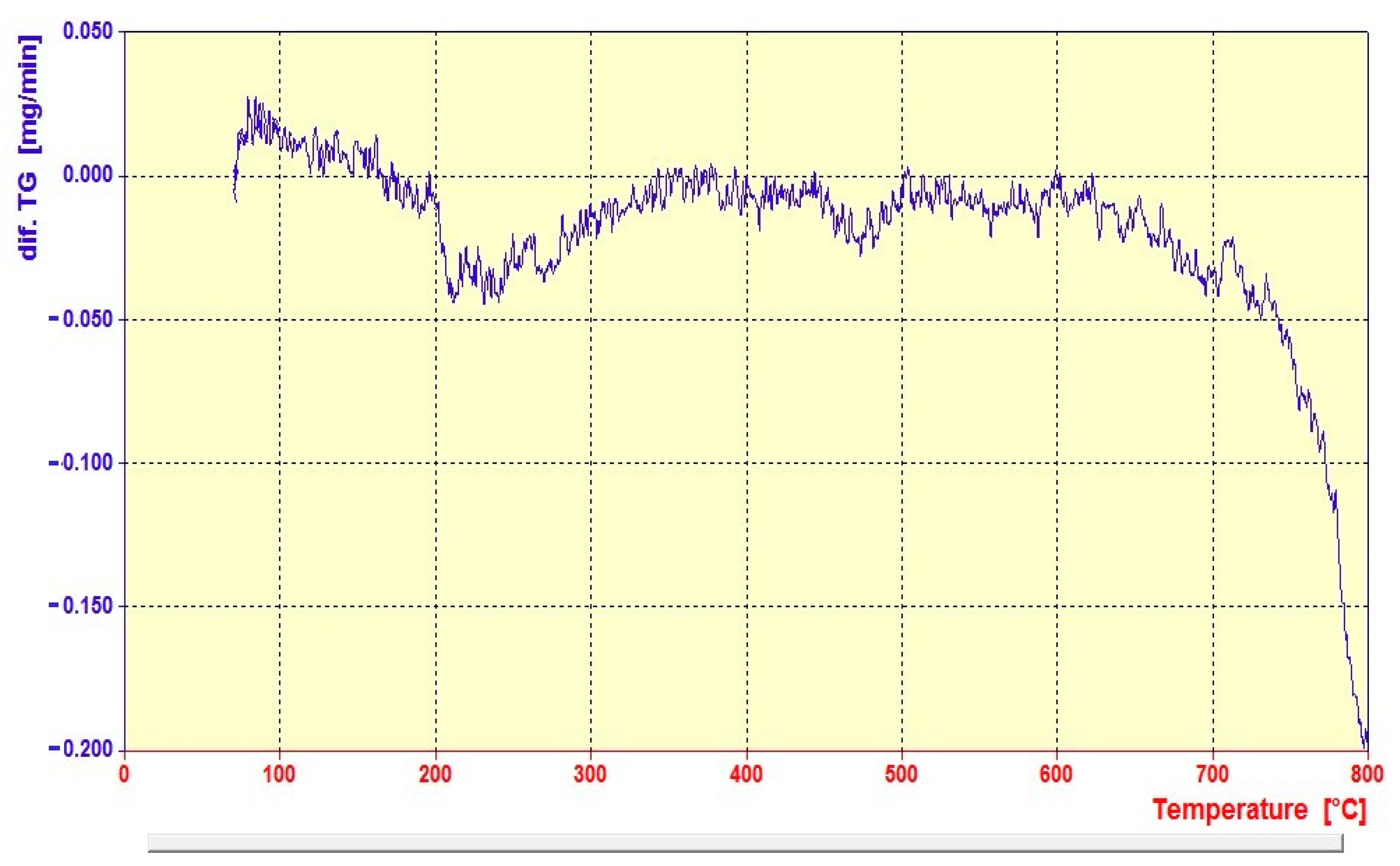
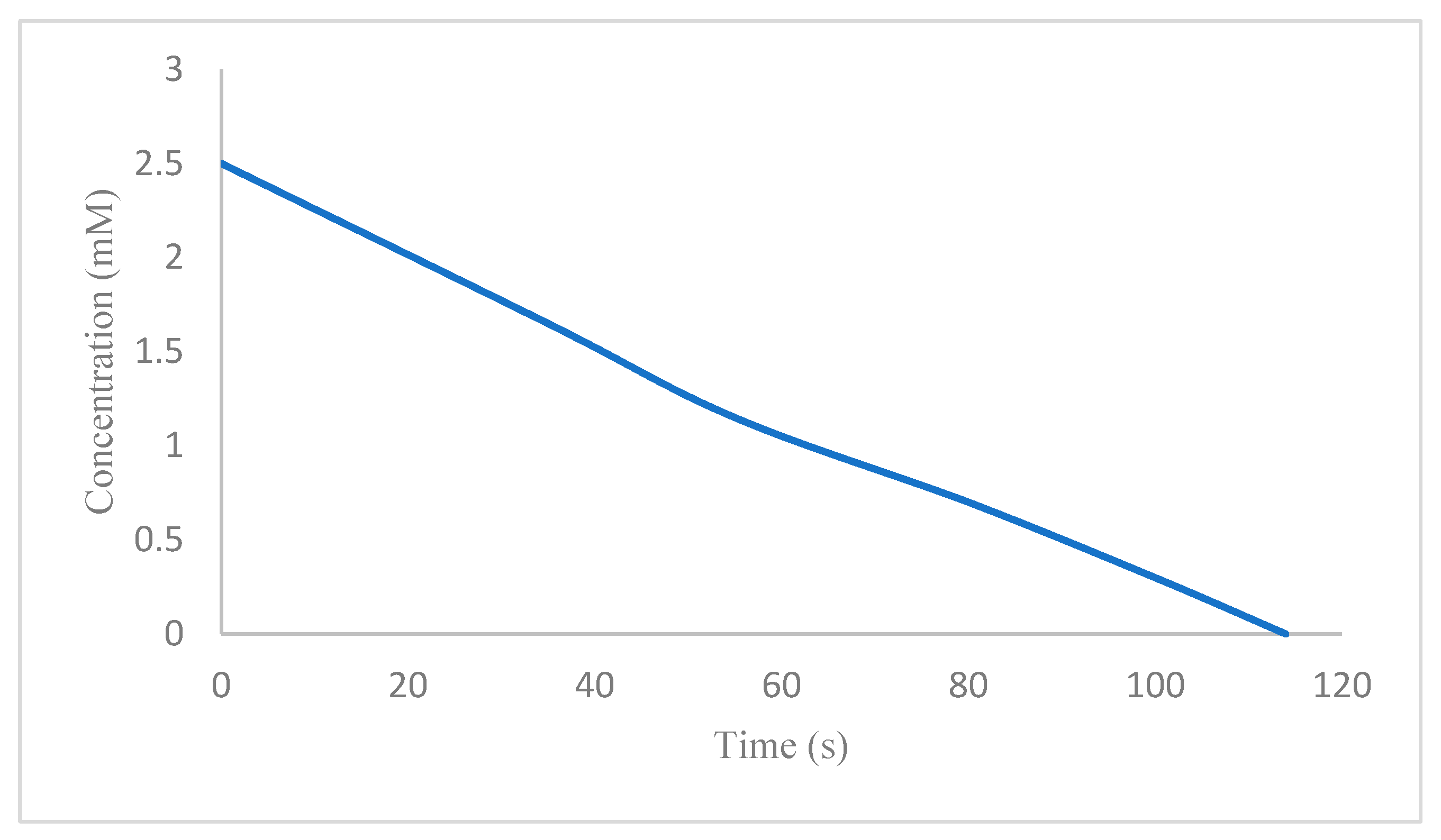

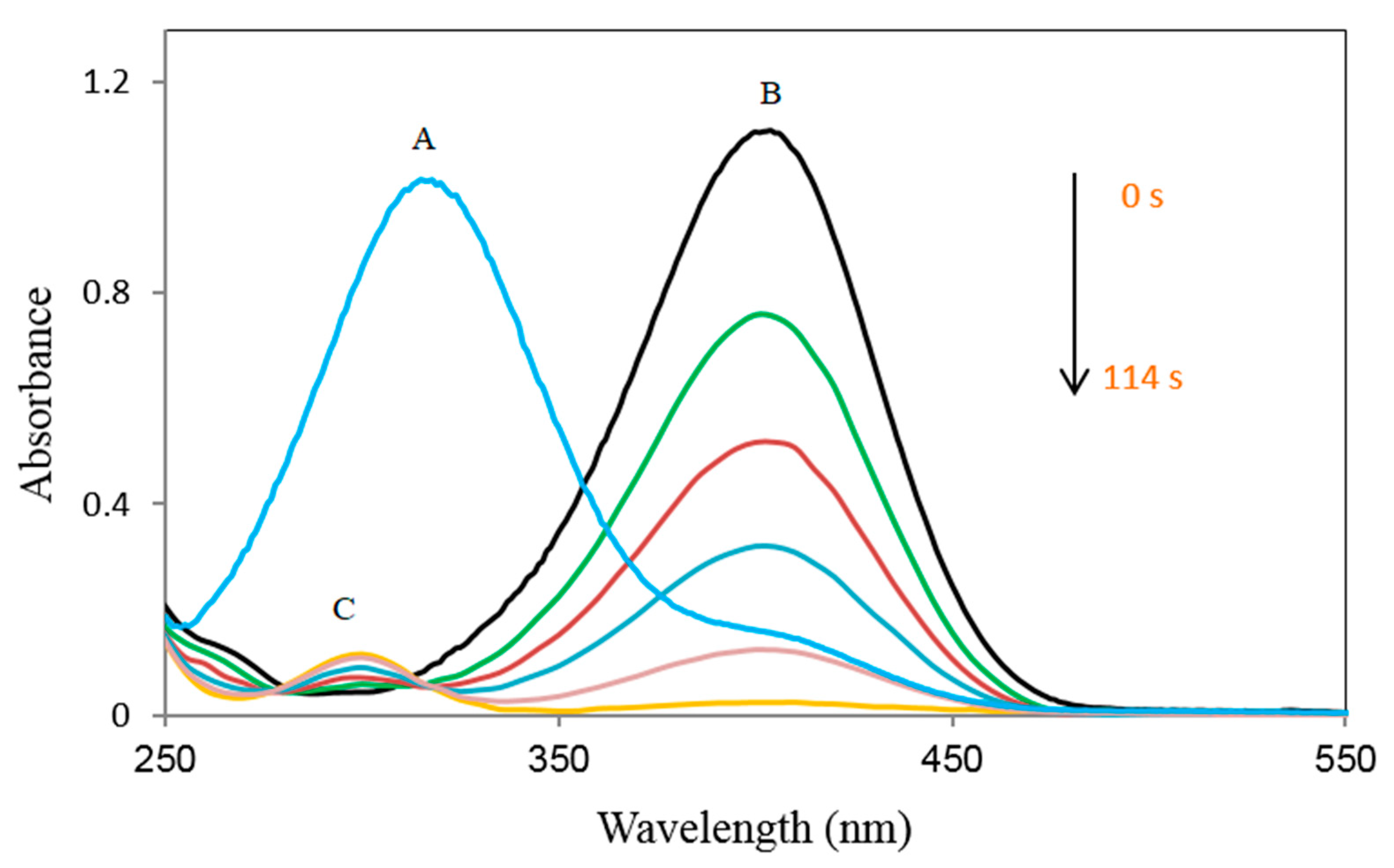
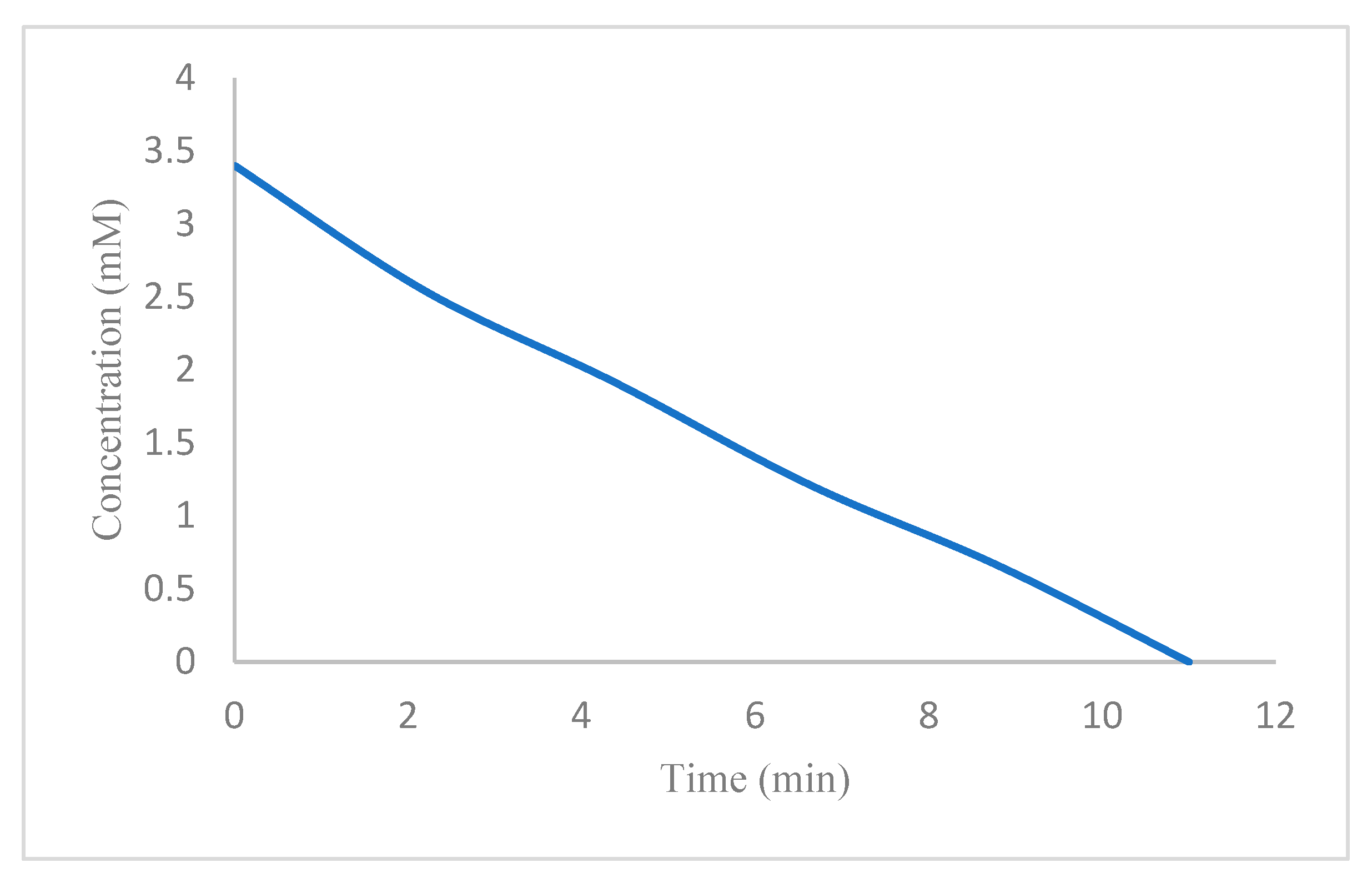

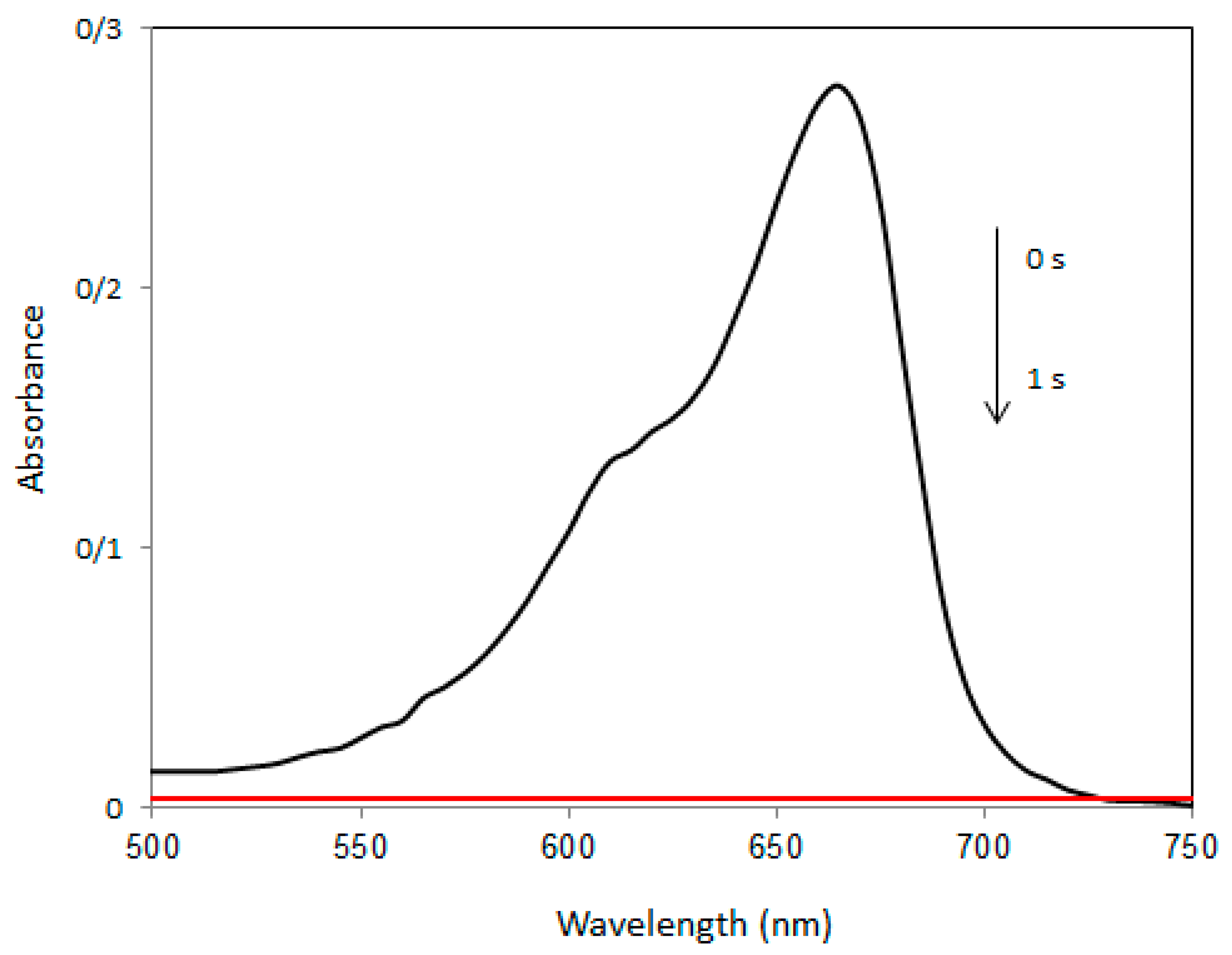

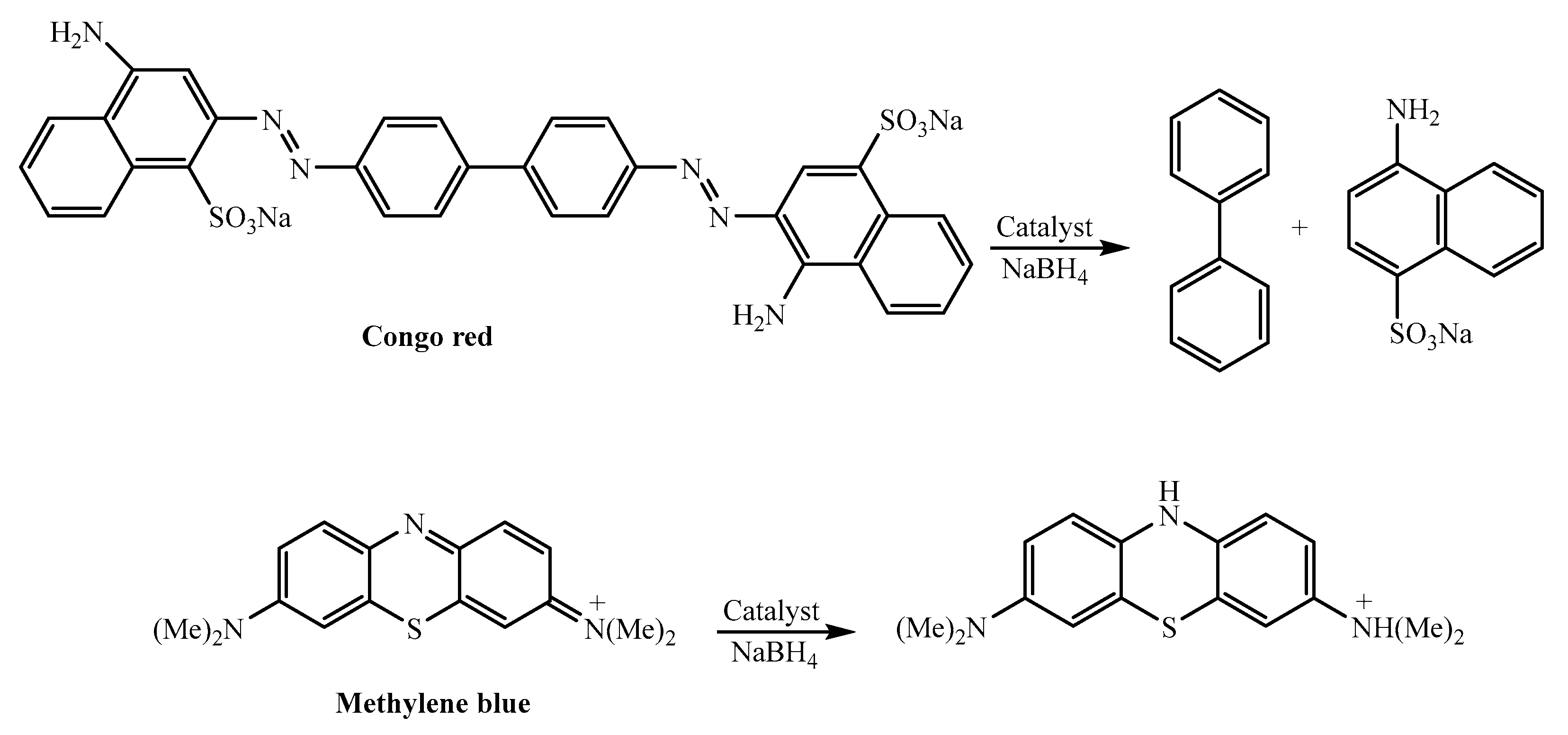

| Entry | NaBH4 (Equivalents) | Catalyst (mg) | Time |
|---|---|---|---|
| 1 | 100 | - | Not reaction |
| 2 | 100 | Fe3O4@SiO2 (7.0) | Not reaction |
| 3 | 100 | Fe3O4@SiO2-L (7.0) | Not reaction |
| 4 | - | [Fe3O4@SiO2–L–Cu(II)] (7.0) | Not reaction |
| 5 | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 114 s |
| 6 | 79 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 135 s |
| 7 | 50 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 192 s |
| 8 | 100 | [Fe3O4@SiO2–L–Cu(II)] (5.0) | 125 s |
| 9 | 79 | [Fe3O4@SiO2–L–Cu(II)] (5.0) | 159 s |
| 10 | 50 | [Fe3O4@SiO2–L–Cu(II)] (3.0) | 182 s |
| 11 | 100 | [Fe3O4@SiO2–L–Cu(II)] (3.0) | 140 s |
| Entry | Formic Acid | Catalyst (mg) | Time |
|---|---|---|---|
| 1 | 1.0 mL | - | No reaction |
| 2 | 1.0 mL | [Fe3O4@SiO2] (7.0) | No reaction |
| 3 | 1.0 mL | [Fe3O4@SiO2-L] (7.0) | No reaction |
| 4 | 1.0 mL | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 11 min |
| 5 | 1.0 mL | [Fe3O4@SiO2–L–Cu(II)] (5.0) | 19 min |
| 6 | 1.0 mL | [Fe3O4@SiO2–L–Cu(II)] (3.0) | 29 min |
| Entry | Dye | Catalyst (mg) | NaBH4 (M) | Time |
|---|---|---|---|---|
| 1 | MB | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 5.3 × 10−3 | Immediately |
| 2 | MB | [Fe3O4@SiO2–L–Cu(II)] (5.0) | 5.3 × 10−3 | 3 s |
| 3 | MB | [Fe3O4@SiO2–L–Cu(II)] (3.0) | 5.3 × 10−3 | 12 s |
| 4 | CR | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 5.3 × 10−3 | Immediately |
| 5 | CR | [Fe3O4@SiO2–L–Cu(II)] (5.0) | 5.3 × 10−3 | 6 s |
| 6 | CR | [Fe3O4@SiO2–L–Cu(II)] (3.0) | 5.3 × 10−3 | 15 s |
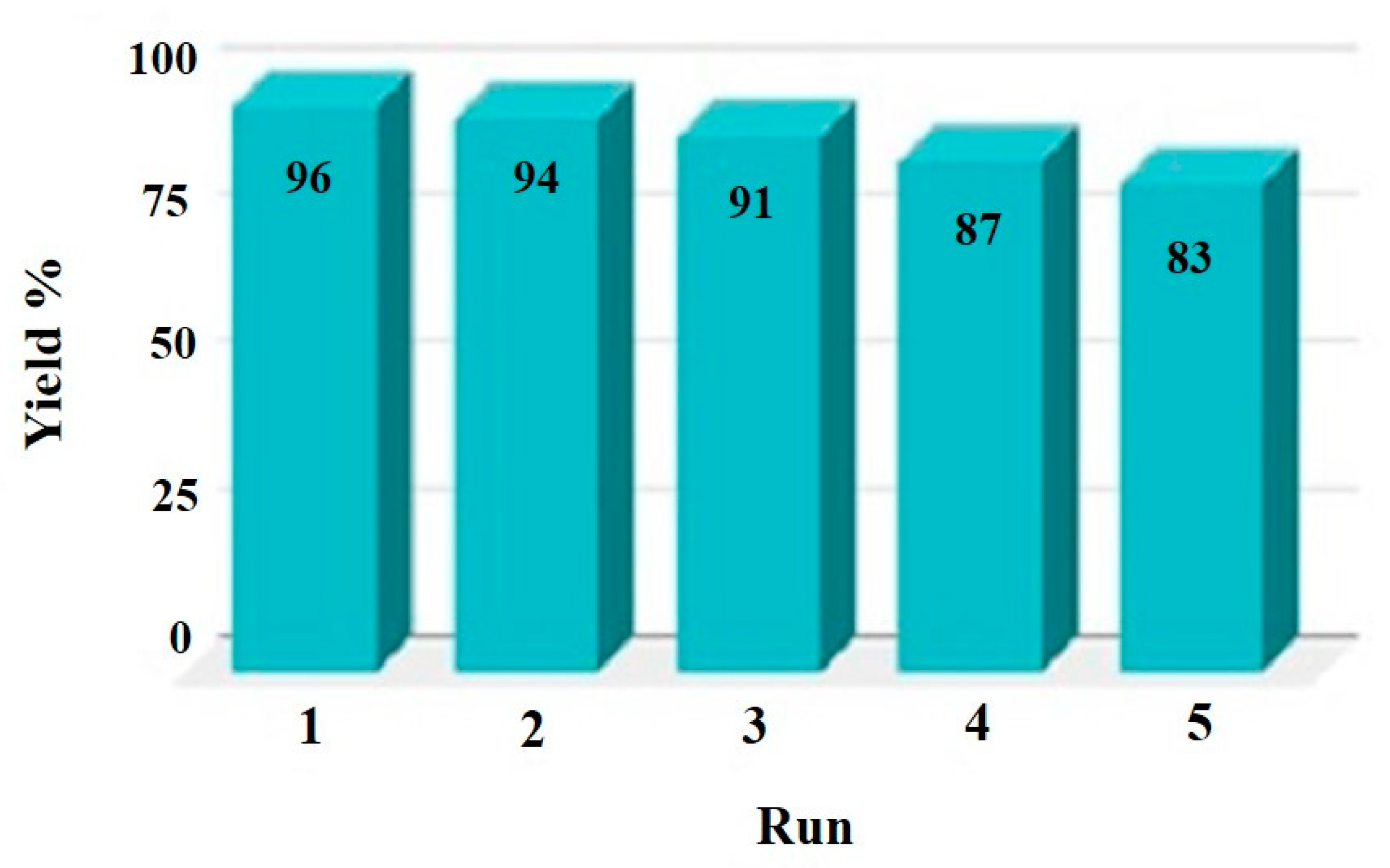
| Entry | Cycle | NaBH4 (Equivalents) | Catalyst (mg) | Time |
|---|---|---|---|---|
| 1 | Fresh | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 114 s |
| 2 | 1st recycle | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 118 s |
| 3 | 2nd recycle | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 121 s |
| 4 | 3rd recycle | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 125 s |
| 5 | 4th recycle | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 131 s |
| 6 | 5th recycle | 100 | [Fe3O4@SiO2–L–Cu(II)] (7.0) | 137 s |
| Entry | Catalyst | Application | Reaction Time | Ref |
|---|---|---|---|---|
| 1 | rGO-BP-Pd | Reduction of 4-NP | 15 min | [58] |
| 2 | Pd NPs doped chitosan | Reduction of 4-NP | 35 min | [59] |
| 3 | N-C/Cu/N-C | Reduction of 4-NP | 12 min | [60] |
| 4 | Ag−Cu bimetallic | Reduction of 4-NP | 30 min | [61] |
| 5 | p(AAc-co-AAm)-Cu | Reduction of 4-NP | 12 min | [62] |
| 6 | Fe3O4@SiO2–L–Cu(II) | Reduction of 4-NP | 114 s | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadashi, J.; Khaleghian, M.; Mirtamizdoust, B.; Hanifehpour, Y.; Joo, S.W. Fabrication of Copper(II)-Coated Magnetic Core-Shell Nanoparticles Fe3O4@SiO2: An Effective and Recoverable Catalyst for Reduction/Degradation of Environmental Pollutants. Crystals 2022, 12, 862. https://doi.org/10.3390/cryst12060862
Dadashi J, Khaleghian M, Mirtamizdoust B, Hanifehpour Y, Joo SW. Fabrication of Copper(II)-Coated Magnetic Core-Shell Nanoparticles Fe3O4@SiO2: An Effective and Recoverable Catalyst for Reduction/Degradation of Environmental Pollutants. Crystals. 2022; 12(6):862. https://doi.org/10.3390/cryst12060862
Chicago/Turabian StyleDadashi, Jaber, Mohammad Khaleghian, Babak Mirtamizdoust, Younes Hanifehpour, and Sang Woo Joo. 2022. "Fabrication of Copper(II)-Coated Magnetic Core-Shell Nanoparticles Fe3O4@SiO2: An Effective and Recoverable Catalyst for Reduction/Degradation of Environmental Pollutants" Crystals 12, no. 6: 862. https://doi.org/10.3390/cryst12060862
APA StyleDadashi, J., Khaleghian, M., Mirtamizdoust, B., Hanifehpour, Y., & Joo, S. W. (2022). Fabrication of Copper(II)-Coated Magnetic Core-Shell Nanoparticles Fe3O4@SiO2: An Effective and Recoverable Catalyst for Reduction/Degradation of Environmental Pollutants. Crystals, 12(6), 862. https://doi.org/10.3390/cryst12060862