Role of Water in Defining the Structure and Properties of B-Form DNA
Abstract
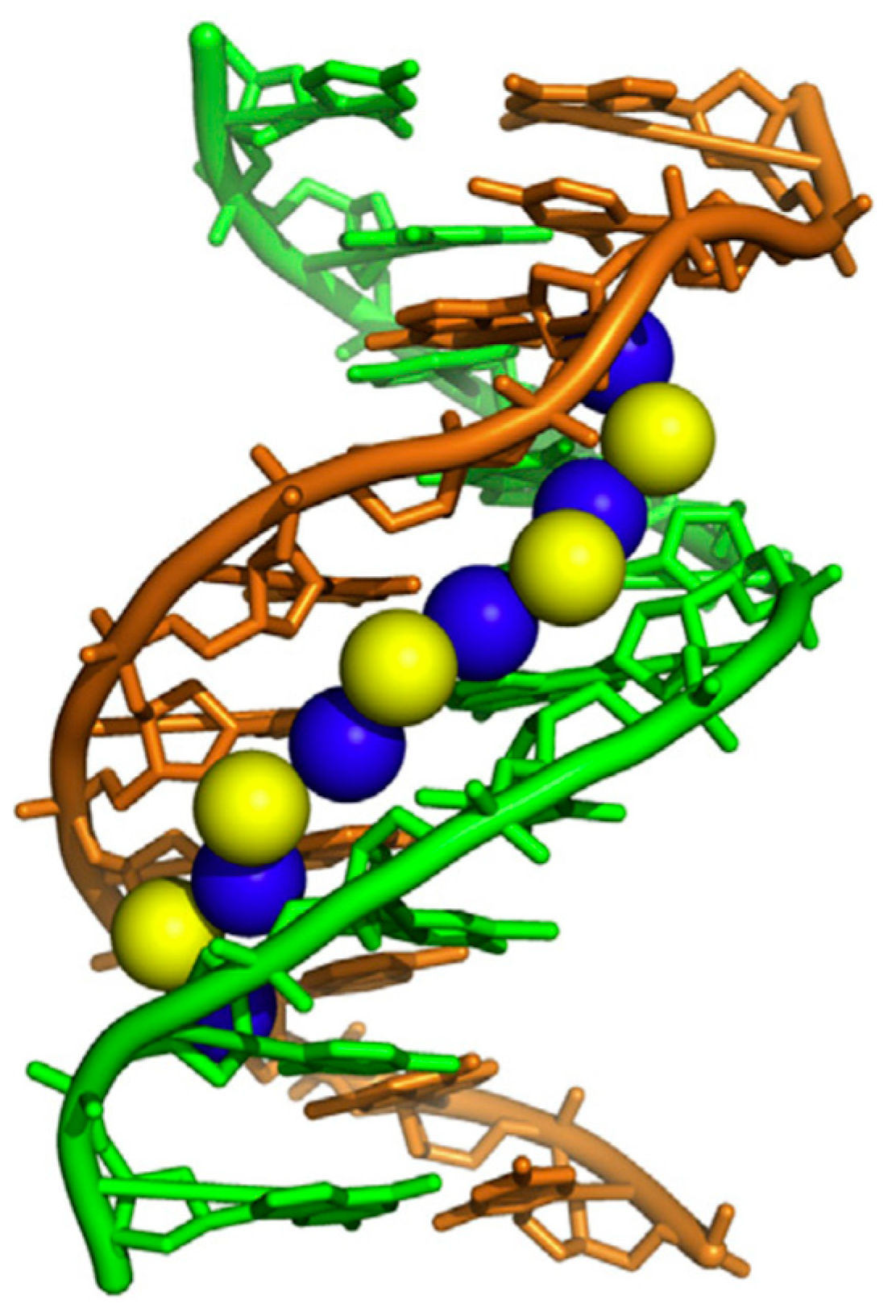
:1. Crystallographic Studies
2. Calorimetric Studies
3. Thermodynamic Characteristics of the Individual Base Pairs
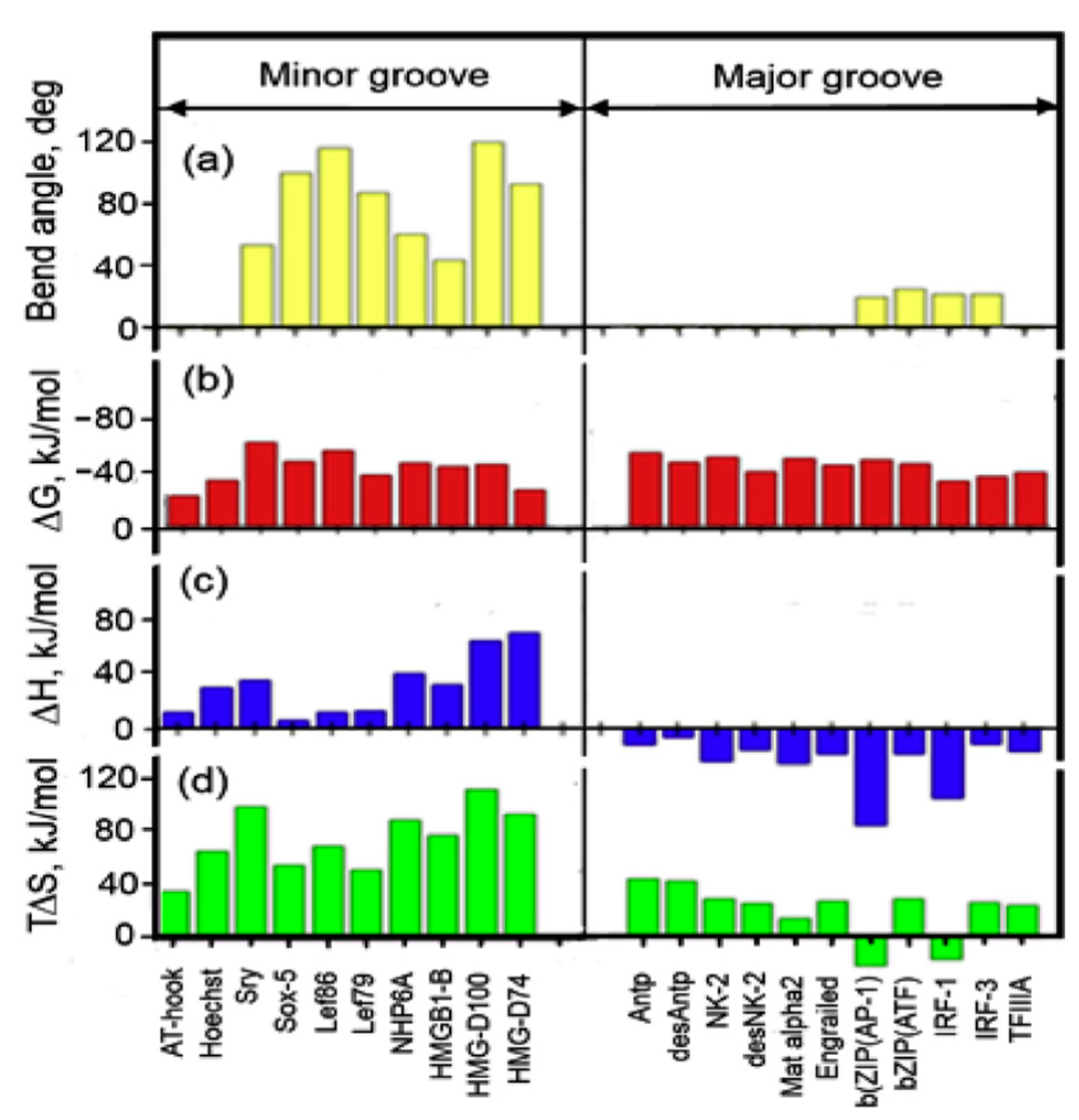
4. Thermodynamics of Binding Proteins to DNA
5. Heat Capacity Changes upon DBD Association with DNA
6. DNA Bending
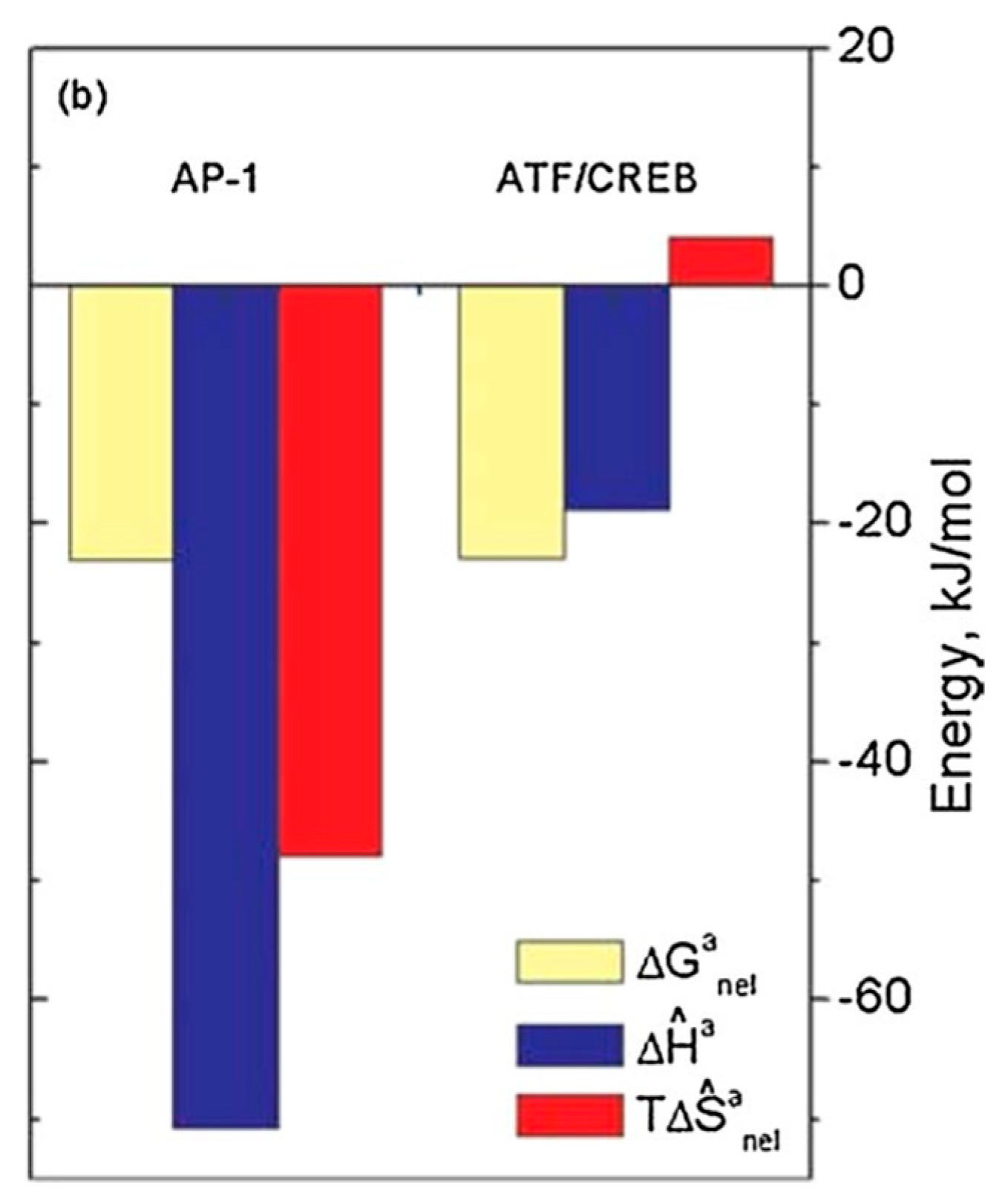
7. The Role of Hydration in Enthalpy/Entropy Compensation (EEC)
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneider, B.; Berman, H.M. Hydration of the DNA bases is local. Biophys. J. 1995, 69, 2661–2669. [Google Scholar] [CrossRef] [Green Version]
- Auffinger, P.; Hashem, Y. SwS: A solvation web service for nucleic acids. Bioinformatics 2007, 23, 1035–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drew, H.R.; Dickerson, R.E. Structure of a B-DNA dodecamer: III. Geometry of hydration. J. Mol. Biol. 1981, 151, 535–556. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [Green Version]
- Kopka, M.L.; Fratini, A.V.; Drew, H.R.; Dickerson, R.E. Ordered water structure around a B-DNA dodecamer. J. Mol. Biol. 1983, 163, 129–146. [Google Scholar] [CrossRef]
- Privé, G.G.; Heinemann, U.; Chandrasegaran, S.; Kan, L.-S.; Kopka, M.L.; Dickerson, R.E. Helix Geometry, Hydration, and G.A Mismatch in a B-DNA Decamer. Science 1987, 238, 498–504. [Google Scholar] [CrossRef]
- Liepinsh, E.; Otting, G.; Wüthrich, K. NMR observation of individual molecules of hydration water bound to DNA duplexes: Direct evidence for a spine of hydration water present in aqueous solution. Nucleic Acids Res. 1992, 20, 6549–6553. [Google Scholar] [CrossRef] [Green Version]
- Jóhannesson, H.; Halle, B. Minor Groove Hydration of DNA in Solution: Dependence on Base Composition and Sequence. J. Am. Chem. Soc. 1998, 120, 6859–6870. [Google Scholar] [CrossRef]
- Narayana, N.; Weiss, M.A. Crystallographic Analysis of a Sex-Specific Enhancer Element: Sequence-Dependent DNA Structure, Hydration, and Dynamics. J. Mol. Biol. 2009, 385, 469–490. [Google Scholar] [CrossRef] [Green Version]
- Privalov, P.L.; Dragan, A.I.; Crane-Robinson, C.; Breslauer, K.J.; Remeta, D.P.; Minetti, S.A. What drives proteins into the major or minor grooves of DNA? J. Mol. Biol. 2007, 365, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jelesarov, I.; Crane-Robinson, C.; Privalov, P.L. The energetics of HMG box interactions with DNA: Thermodynamic description of the target DNA duplexes. J. Mol. Biol. 1999, 294, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Privalov, P.L.; Crane-Robinson, C. Translational Entropy and DNA Duplex Stability. Biophys. J. 2018, 114, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, A.I.; Privalov, P.L.; Crane-Robinson, C. Thermodynamics of DNA: Heat capacity changes on duplex unfolding. Eur. Biophys. J. 2019, 48, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmur, J.; Doty, P. Determination of the base composition of deoxyribonucleic acid from its thermal melting temperature. J. Mol. Biol. 1962, 5, 109–118. [Google Scholar] [CrossRef]
- Vaitiekunas, P.; Crane-Robinson, C.; Privalov, P.L. The energetic basis of the DNA double helix: A combined microcalorimetric approach. Nucleic Acids Res. 2015, 43, 8577–8589. [Google Scholar] [CrossRef] [Green Version]
- Privalov, P.L.; Crane-Robinson, C. Forces maintaining the DNA double helix. Eur. Biophys. J. 2020, 49, 315–321. [Google Scholar] [CrossRef]
- Privalov, P.L.; Crane-Robinson, C. Forces maintaining the DNA double helix and its complexes with transcription factors. Prog. Biophys. Mol. Biol. 2018, 135, 30–48. [Google Scholar] [CrossRef]
- Dragan, A.I.; Privalov, P.L.; Crane-Robinson, C. Thermodynamic basis of the α-helix and DNA duplex. Eur. Biophys. J. 2021, 50, 787–792. [Google Scholar] [CrossRef]
- Privalov, P.L.; Crane-Robinson, C. Role of water in the formation of macromolecular structures. Eur. Biophys. J. 2017, 46, 203–224. [Google Scholar] [CrossRef] [Green Version]
- Dragan, A.I.; Read, C.M.; Makeyeva, E.N.; Milgotina, E.I.; Churchill, M.E.; Crane-Robinson, C.; Privalov, P.L. DNA Binding and Bending by HMG Boxes: Energetic Determinants of Specificity. J. Mol. Biol. 2004, 343, 371–393. [Google Scholar] [CrossRef]
- Makhatadze, G.I.; Privalov, P.L. Energetics of protein structure. Adv. Prot. Chem. 1995, 47, 307–425. [Google Scholar]
- Dragan, A.I.; Read, C.M.; Crane-Robinson, C. Hydration differences between the major and minor grooves of DNA revealed from heat capacity measurements. Eur. Biophys. J. 2019, 48, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, W.H.; Hagerman, P.J. Application of the method of phage T4 DNA ligase-catalyzed ring-closure to the study of DNA structure. J. Mol. Biol. 1990, 212, 363–376. [Google Scholar] [CrossRef]
- Privalov, P.L.; Dragan, A.I.; Crane-Robinson, C. The cost of DNA bending. Trends Biochem. Sci. 2009, 34, 464–470. [Google Scholar] [CrossRef]
- Dragan, A.I.; Frank, L.; Liu, Y.; Makeyeva, E.N.; Crane-Robinson, C.; Privalov, P.L. Thermodynamic Signature of GCN4-bZIP Binding to DNA Indicates the Role of Water in Discriminating Between the AP-1 and ATF/CREB Sites. J. Mol. Biol. 2004, 343, 865–878. [Google Scholar] [CrossRef]
- Dragan, A.I.; Read, C.M.; Crane-Robinson, C. Enthalpy–entropy compensation: The role of solvation. Eur. Biophys. J. 2017, 46, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Ellenberger, T.E.; Brandl, C.J.; Struhl, K.; Harrison, S.C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha Helices: Crystal structure of the protein-DNA complex. Cell 1992, 71, 1223–1237. [Google Scholar] [CrossRef]
- Keller, W.; Konig, P.; Richmond, T.J. Crystal structure of a bZIP/DNA complex at 2.2 Å: Determinants of DNA specific recognition. J. Mol. Biol. 1995, 254, 657–667. [Google Scholar] [CrossRef] [Green Version]




| Base Pair | ∆Hcoop (kJ/mol-bp) | ∆Scoop (J/K·mol-bp) | ∆Gcoop (kJ/mol-bp) | ∆Cp (kJ/K·mol-bp) |
|---|---|---|---|---|
| CG | 19.0 | 36.2 | 8.2 | 0.13 |
| AT | 28.0 | 73.5 | 6.1 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crane-Robinson, C. Role of Water in Defining the Structure and Properties of B-Form DNA. Crystals 2022, 12, 818. https://doi.org/10.3390/cryst12060818
Crane-Robinson C. Role of Water in Defining the Structure and Properties of B-Form DNA. Crystals. 2022; 12(6):818. https://doi.org/10.3390/cryst12060818
Chicago/Turabian StyleCrane-Robinson, Colyn. 2022. "Role of Water in Defining the Structure and Properties of B-Form DNA" Crystals 12, no. 6: 818. https://doi.org/10.3390/cryst12060818
APA StyleCrane-Robinson, C. (2022). Role of Water in Defining the Structure and Properties of B-Form DNA. Crystals, 12(6), 818. https://doi.org/10.3390/cryst12060818





