Band Gap and Topology of 1D Perovskite-Derived Hybrid Lead Halide Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Topological Analysis of Crystal Structures
2.2. DFT Calculations
2.3. Analysis of the Distortions of Structures
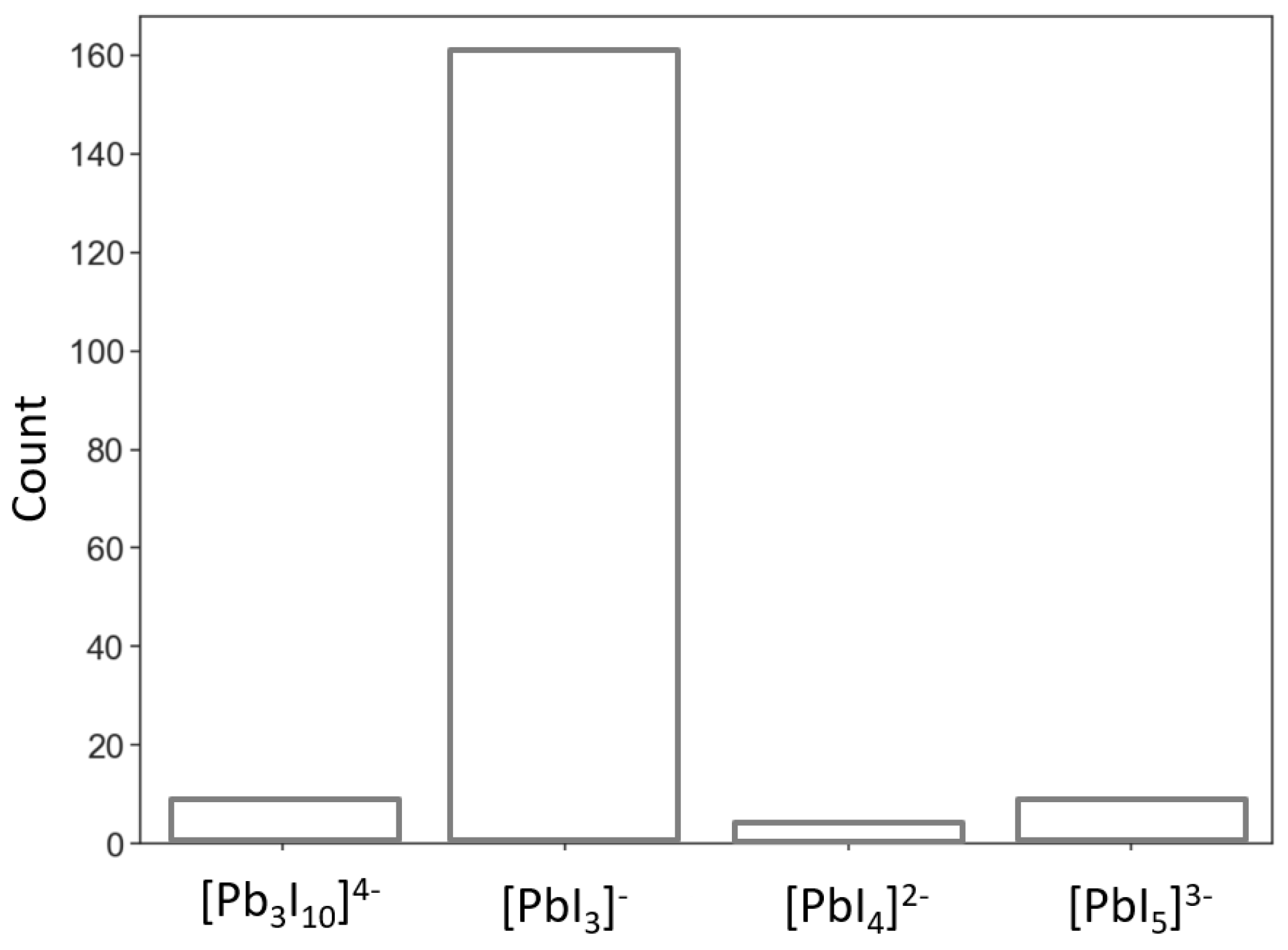
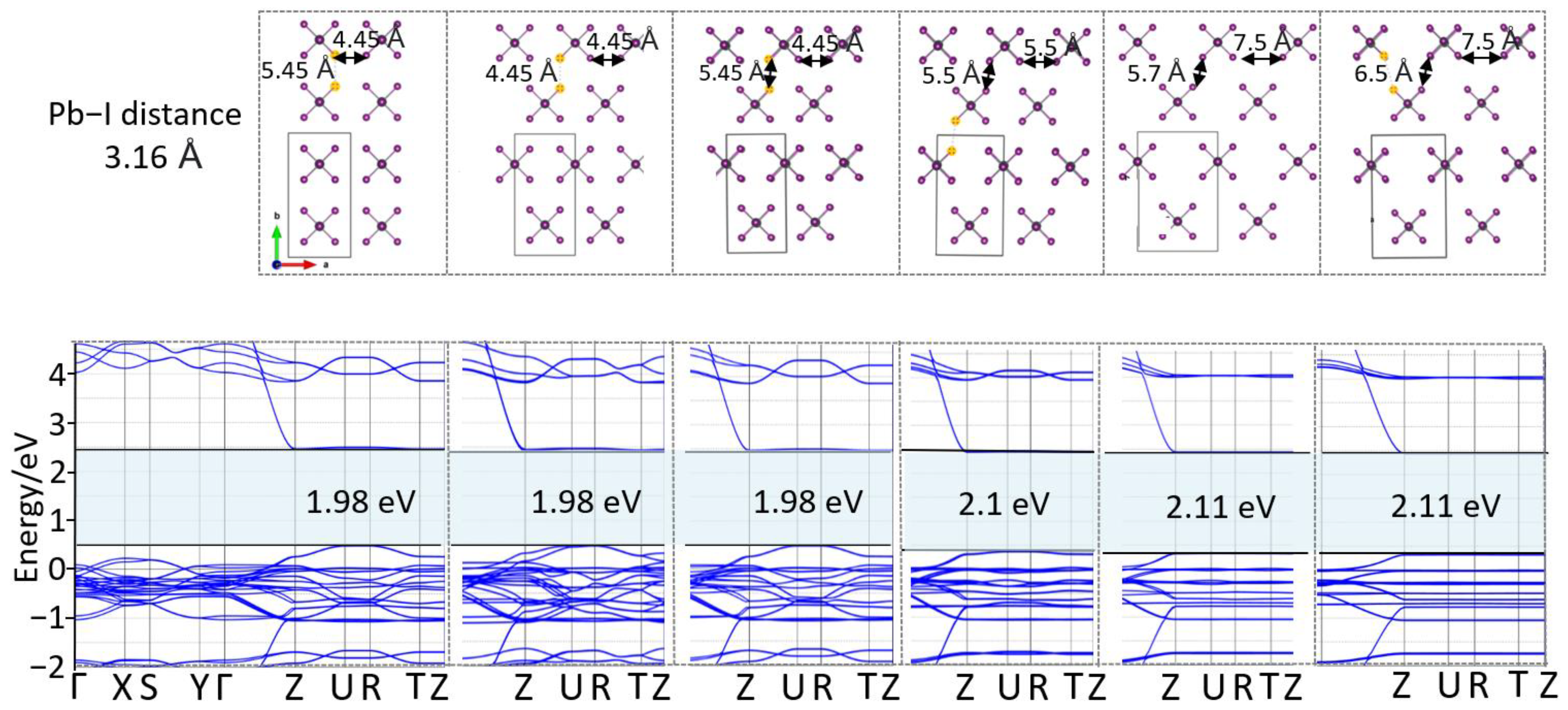
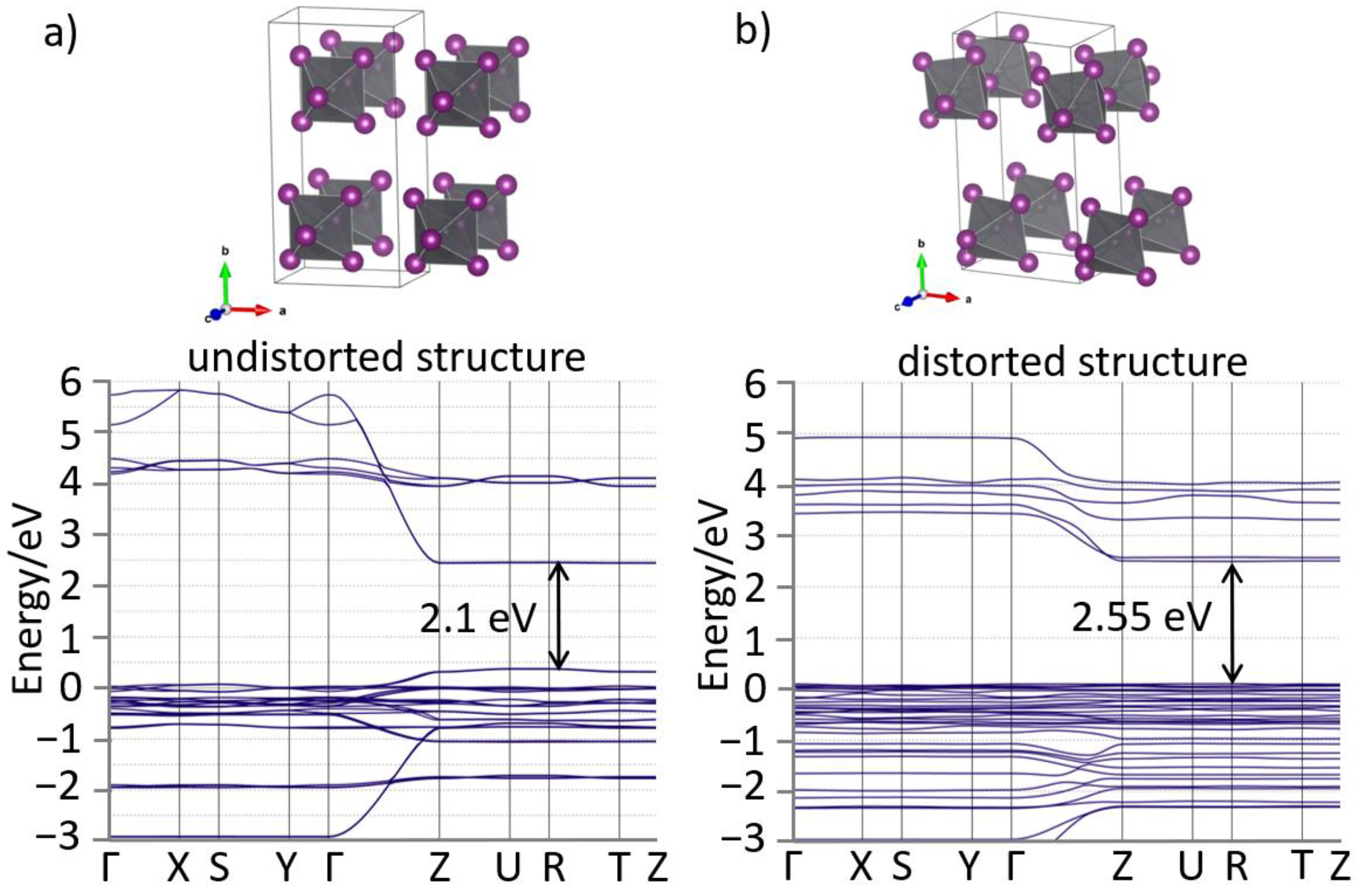
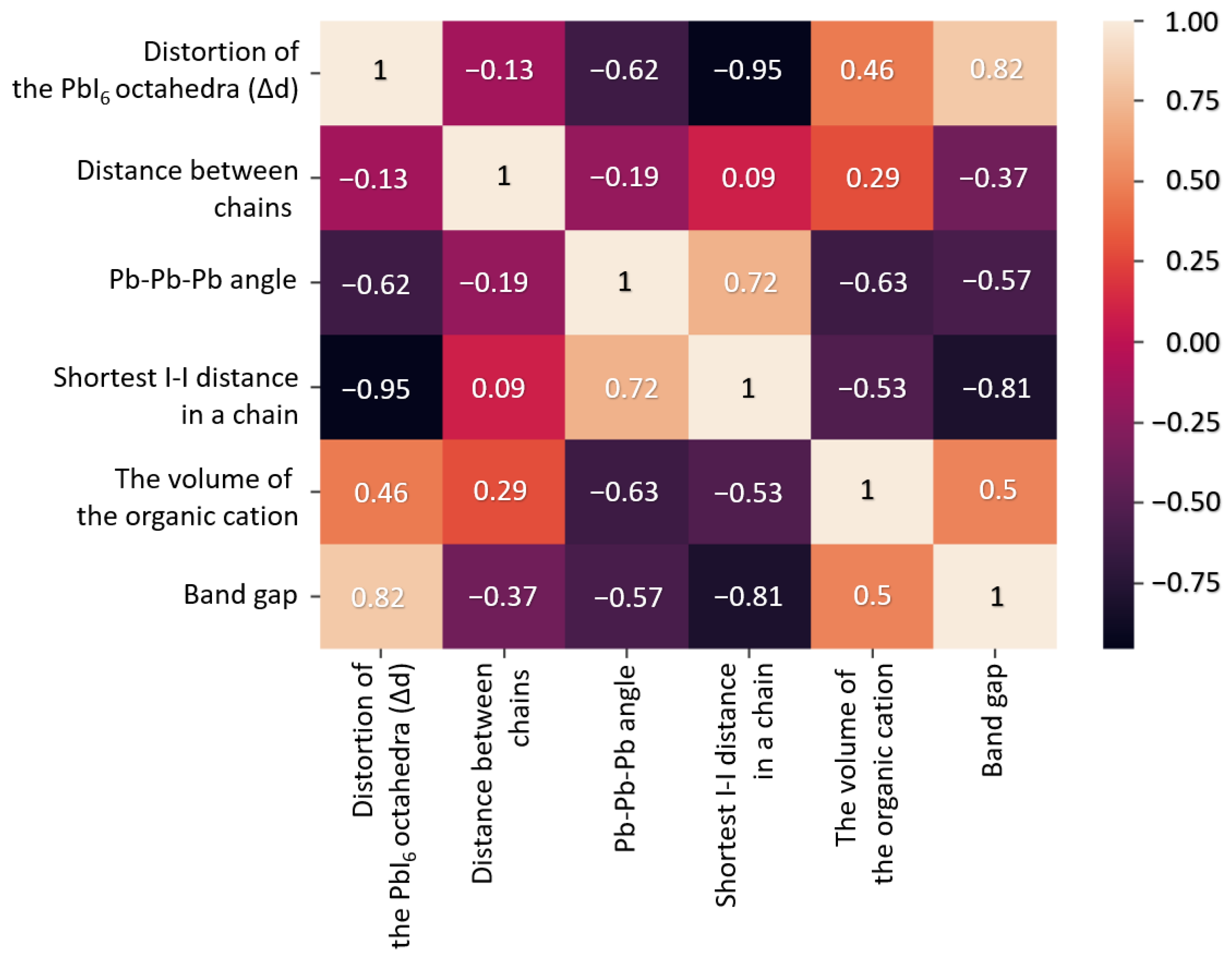
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| CSD Ref Code | Type of Chain | Topology of the Net * |
|---|---|---|
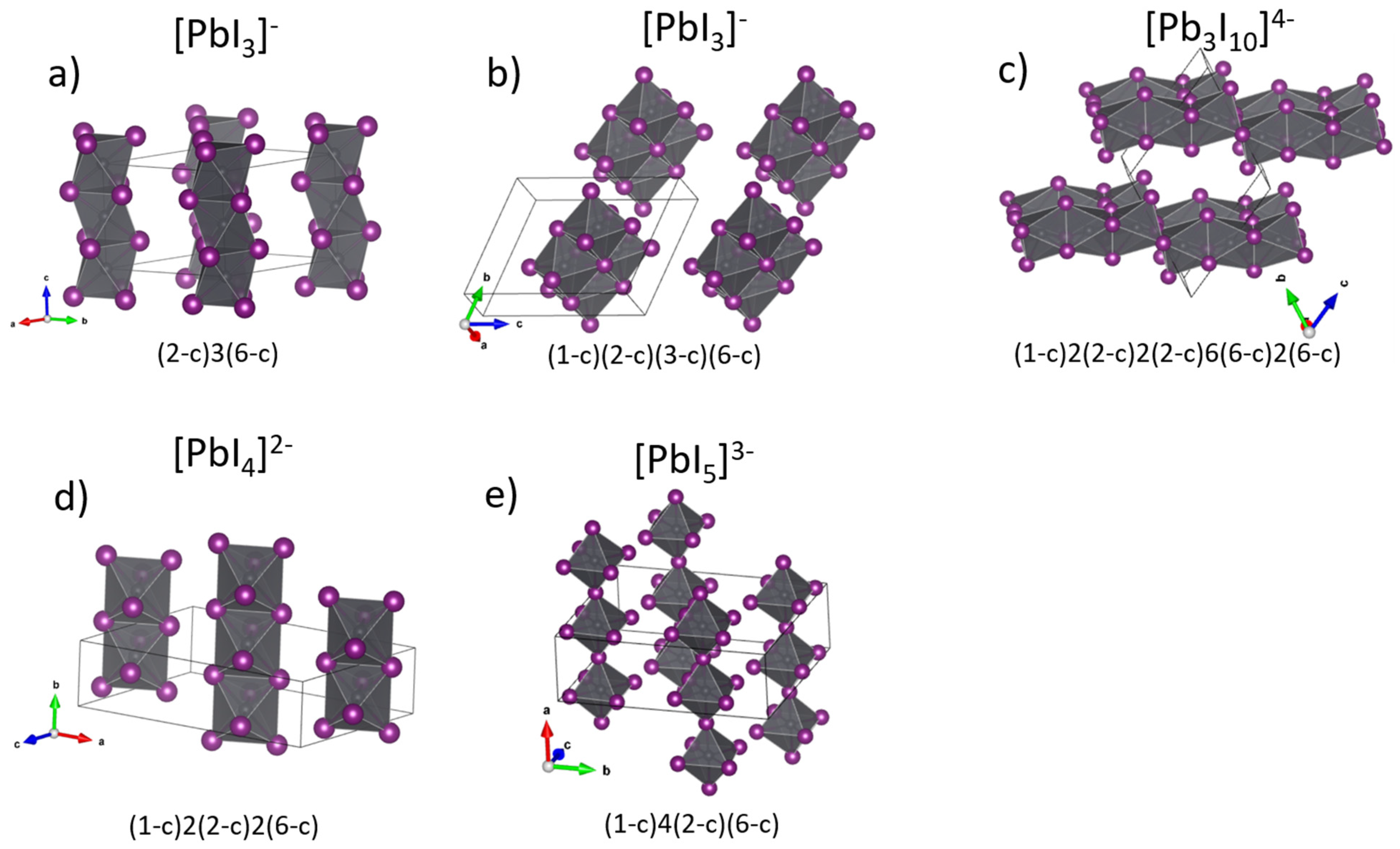
| 214790 | chains [0 1 0] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)6(2-c)2(6-c)(6-c)2; 5-nodal net |
| 104219 | chains [1 0 1] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)6(2-c)2(6-c)(6-c)2; 5-nodal net |
| 82074 | chains [0 0 1] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)2(2-c)6(6-c)2(6-c); 5-nodal net |
| 836347 | chains [0 1 −1] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)2(2-c)6(6-c)2(6-c); 5-nodal net |
| 1119690 | chains [1 0 0] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)2(2-c)6(6-c)2(6-c); 5-nodal net |
| 1515524-957318 | chains [1 0 1] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)2(2-c)6(6-c)2(6-c); 5-nodal net |
| 1515524-957318 | chains [1 0 1] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)2(2-c)6(6-c)2(6-c); 5-nodal net |
| 1945576 | chains [1 0 1] with [Pb3I10]4− | 1,2,2,6,6-c net with stoichiometry (1-c)2(2-c)2(2-c)6(6-c)2(6-c); 5-nodal net |
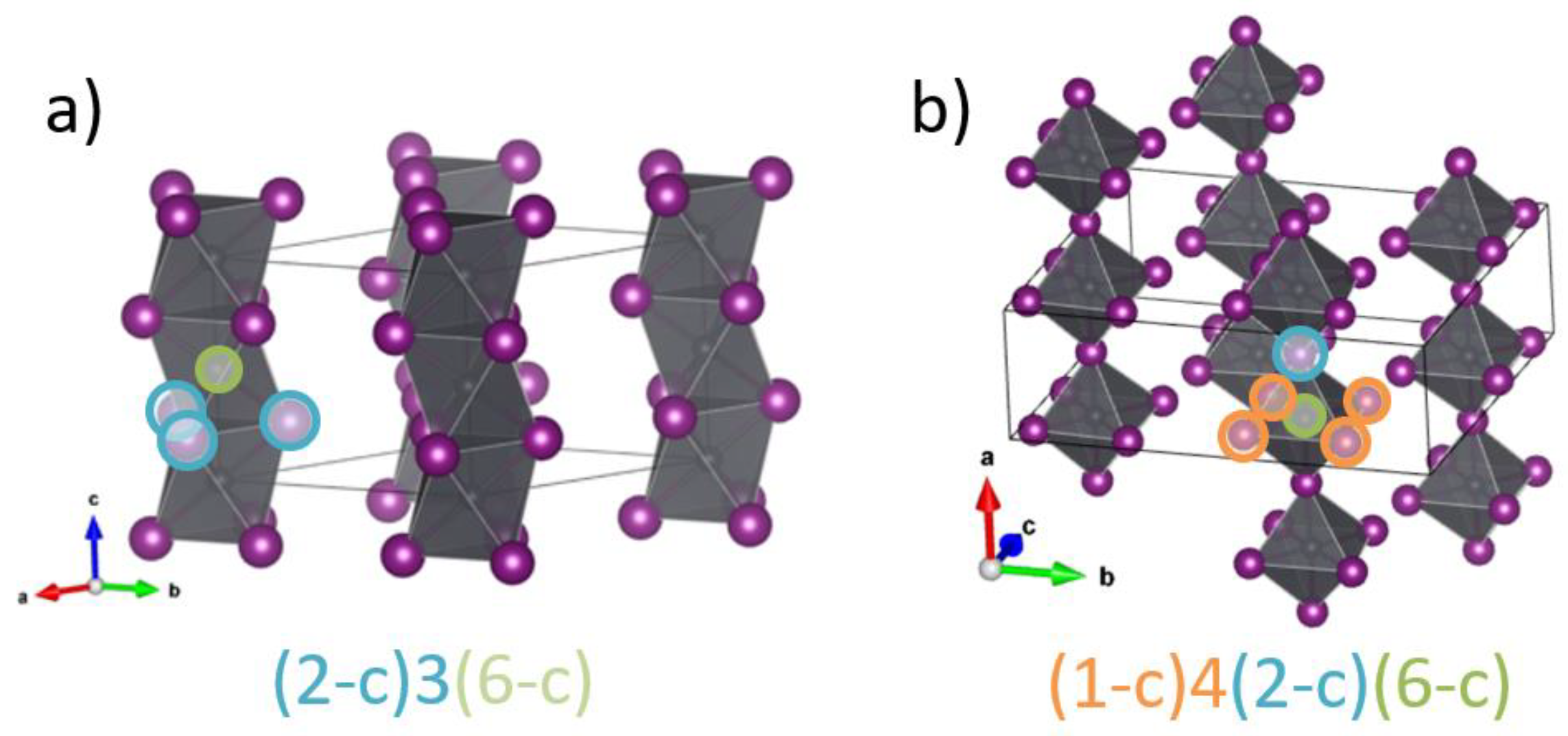
| 160842 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 210812 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 254879 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 277224 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 790923 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 291886 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 298933 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 604996 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 609997 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 632026 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 636241 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 722539 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 776897 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780403 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780404 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780405 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780408 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780409 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 921641 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780410 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 785767 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 785768 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 785769 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 818548 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 834146 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 836348 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 917236 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1012805 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1123333 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869662 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869662 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869663 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1135285 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1962916 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1183349 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1308385 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400319 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400321 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400323 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400324-15701131 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400324-15701131 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400324-15701131 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400324-15701131 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400324-15701131 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1432458-1822500 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1432458-1822500 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1495871 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1526831 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1532918-968126 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1547867 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1570129 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1590157-1858276 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1590177 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1819979 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1828821 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1828823 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1853250 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869657-1869658 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869657-1869658 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869657-1869658 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1905762 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1909463 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1923364-1923365 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1934893-1934898 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1934893-1934898 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1934900 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1944788 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1969340 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1992695 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1992696 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 2072691-994664 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 2072691-994664 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 2072691-994664 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 2072691-994664 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 2072691-994664 | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 219758.cif. | chains [0 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 708406 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 780407 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 797634 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 861679 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1048276 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1135285-1962916 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1169102 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1871034 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400322 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1495872 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1495874 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1502217 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1504241 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1524688-1944787 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1524688-1944787 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1524689 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1562186 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1577162-1577163 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1828819 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1871034 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1901048-612444 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1901048-612444 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1915775 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1923364-1923365 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1944787 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1962916 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1977726 | chains [0 1 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1024096 | chains [0 1 −1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 221315 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 248812 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 776896 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 776898 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 776899 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 900606 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 958061 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 967300 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 998856 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1015245 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1047834 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1251540 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1274099 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1447264 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1447265 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1447266 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1483105-871217 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1483105-871217 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1495875 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1523553-1523557 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1523553-1523557 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1523553-1523557 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1523553-1523557 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1533556 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1535129-1535132 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1535129-1535132 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1535129-1535132 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1535129-1535132 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1577162-1577163 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1590186 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1828826 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1846735 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1874395 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1877051 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1877051-607736 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1877051-607736 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1877055-607737 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1877055-607737 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1899647 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1902819 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1934897-1934902 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1934897-1934902 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1934902 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1944781 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1944784 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1944785 | chains [1 0 0] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 776895 | chains [1 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1483104 | chains [1 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869657-1869658 | chains [1 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1869657-1869658 | chains [1 0 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 114129 | chains [1 1 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 734814 | chains [1 1 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 735413 | chains [1 1 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 745950 | chains [1 1 1] with [PbI3]− | 2,6-c net with stoichiometry (2-c)3(6-c); 2-nodal net |
| 1400320 | chains [1 0 0] with [PbI3]− | 1,2,3,6-c net with stoichiometry (1-c)(2-c)(3-c)(6-c); 4-nodal net |
| 1894399 | chains [1 0 0] with [PbI3]− | 1,2,3,6-c net with stoichiometry (1-c)(2-c)(3-c)(6-c); 4-nodal net |
| 1968137 | chains [0 0 1] with [PbI4]2− | 1,2,6-c net with stoichiometry (1-c)2(2-c)2(6-c); 3-nodal net |
| 1432454 | chains [1 0 0] with [PbI4]2− | 1,2,6-c net with stoichiometry (1-c)2(2-c)2(6-c); 3-nodal net |
| 1846083 | chains [0 1 0] with [PbI4]2− | 1,2,6-c net with stoichiometry (1-c)2(2-c)2(6-c); 3-nodal net |
| 1938185 | chains [0 0 1] with [PbI4]2− | 1,2,6-c net with stoichiometry (1-c)2(2-c)2(6-c); 3-nodal net |
| 1307516 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1429047 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1910573 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1860735-1861695 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1860735-1861695 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1860735-1861695 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1860735-1861695 | chains [0 0 1] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1048274 | chains [0 1 0] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
| 1505390 | chains [0 1 0] with [PbI5]3− | 1,2,6-c net with stoichiometry (1-c)4(2-c)(6-c); 3-nodal net |
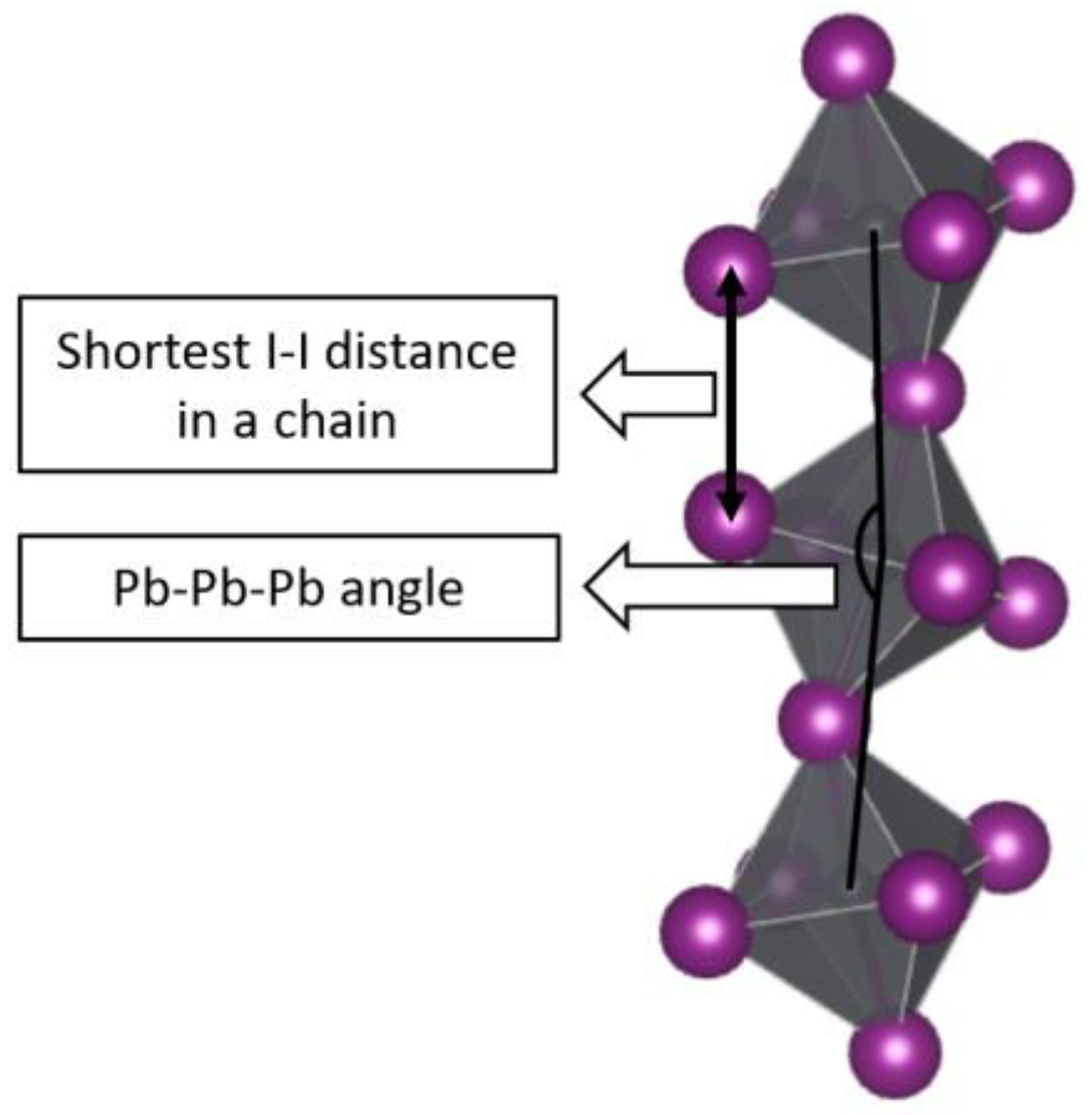
| Reference | Organic Cation | Distortion of Octahedra (Δd) | Distance between Chains, Å | Pb-Pb-Pb Angle, ° | Shortest I-I Distance in a Chain of Octahedra, Å | The Volume of the Organic Cation in the Structure, Å3 | Calculated Band Gap, eV |
| [28] | piperazine-1,4-diium | 7.27 × 10−4 | 7.08 | 83.127 | 4.16 | 179.94 | 2.15 |
| [29] | methylammonium, DMSO | 5.72 × 10−4 | 4.334 | 173.73 | 4.614 | 120.36 | 2.26 |
| [30] | guanidinium | 2.48 × 10−5 | 6.65 | 180 | 6.36 | 112.99 | 2.01 |
| [30] | guanidinium | 7.39 × 10−5 | 6.9 | 180 | 6.44 | 93.39 | 2.04 |
| [30] | guanidinium | 2.53 × 10−5 | 6.65 | 180 | 6.36 | 123.6 | 2.01 |
| [30] | guanidinium | 5.17 × 10−5 | 4.914 | 180 | 6.48 | 142.14 | 1.99 |
| [31] | iso-propylammonium | 3.35 × 10−4 | 4.7 | 89.75 | 5.06 | 115.53 | 2.25 |
| [32] | iodoformamidimium | 2.62 × 10−4 | 5.167 | 180 | 6.42 | 104.49 | 2.18 |
| [33] | tetraethylenepentamine | 5.94 × 10−4 | 6.538 | 84.53 | 4.25 | 399.43 | 2.33 |
References
- Wang, G.E.; Xu, G.; Wang, M.S.; Cai, L.Z.; Li, W.H.; Guo, G.C. Semiconductive 3-D haloplumbate framework hybrids with high color rendering index white-light emission. Chem. Sci. 2015, 6, 7222–7226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.E.; Jiang, X.M.; Zhang, M.J.; Chen, H.F.; Liu, B.W.; Wang, M.S.; Guo, G.C. Crystal structures and optical properties of iodoplumbates hybrids templated by in situ synthesized 1,4-diazabicyclo [2.2.2]octane derivatives. CrystEngComm 2013, 15, 10399–10404. [Google Scholar] [CrossRef]
- Liu, G.N.; Zhao, R.Y.; Xu, B.; Sun, Y.; Jiang, X.M.; Hu, X.; Li, C. Design, Synthesis, and Photocatalytic Application of Moisture-Stable Hybrid Lead-Free Perovskite. ACS Appl. Mater. Interfaces 2020, 12, 54694–54702. [Google Scholar] [CrossRef] [PubMed]
- Harwell, J.R.; Glackin, J.M.E.; Davis, N.J.L.K.; Gillanders, R.N.; Credgington, D.; Turnbull, G.A.; Samuel, I.D.W. Sensing of explosive vapor by hybrid perovskites: Effect of dimensionality. APL Mater. 2020, 8, 071106. [Google Scholar] [CrossRef]
- Chen, X.G.; Song, X.J.; Zhang, Z.X.; Zhang, H.Y.; Pan, Q.; Yao, J.; You, Y.M.; Xiong, R.G. Confinement-Driven Ferroelectricity in a Two-Dimensional Hybrid Lead Iodide Perovskite. J. Am. Chem. Soc. 2020, 142, 10212–10218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Song, X.J.; Cheng, H.; Zeng, Y.L.; Zhang, Y.; Li, P.F.; Liao, W.Q.; Xiong, R.G. A Three-Dimensional Lead Halide Perovskite-Related Ferroelectric. J. Am. Chem. Soc. 2020, 142, 4604–4608. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Terrón, S.; Jodlowski, A.D.; Verdugo-Escamilla, C.; Camacho, L.; De Miguel, G. Relaxing the Goldschmidt Tolerance Factor: Sizable Incorporation of the Guanidinium Cation into a Two-Dimensional Ruddlesden-Popper Perovskite. Chem. Mater. 2020, 32, 4024–4037. [Google Scholar] [CrossRef]
- Grancini, G.; Nazeeruddin, M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2019, 4, 4–22. [Google Scholar] [CrossRef]
- Lyu, R.; Moore, C.E.; Liu, T.; Yu, Y.; Wu, Y. Predictive Design Model for Low-Dimensional Organic-Inorganic Halide Perovskites Assisted by Machine Learning. J. Am. Chem. Soc. 2021, 143, 12766–12776. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Liu, N.; Han, Y.; Zou, M.; Song, T. 1D perovskitoid as absorbing material for stable solar cells. Crystals 2021, 11, 241. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Chen, S.; Qi, L.; Zhao, M.; Zhao, C.; Tang, J.; Cai, X.; Lu, F.; Jiu, T. Growth of 1D Nanorod Perovskite for Surface Passivation in FAPbI3 Perovskite Solar Cells. Small 2022, 18, 1–9. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Korolev, V.V.; Mitrofanov, A.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Layer Shift Factor in Layered Hybrid Perovskites: Univocal Quantitative Descriptor of Composition-Structure-Property Relationships. Chem. Mater. 2021, 33, 1213–1217. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Korolev, V.V.; Fateev, S.A.; Mitrofanov, A.; Eremin, N.N.; Goodilin, E.A.; Tarasov, A.B. Relationships between Distortions of Inorganic Framework and Band Gap of Layered Hybrid Halide Perovskites. Chem. Mater. 2021, 33, 7518–7526. [Google Scholar] [CrossRef]
- Wong, W.P.D.; Hanna, J.V.; Grimsdale, A.C. The classification of 1D ‘perovskites’. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 307–308. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Blatov, V.A.; Proserpio, D.M. Topological relations between three-periodic nets. II. Binodal nets. Acta Crystallogr. A. 2009, 65, 202–212. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: Recommended terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; De Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihara, S. Burai 1.3, A GUI of Quantum ESPRESSO. Available online: https://burai.readthedocs.io/en/latest/ (accessed on 27 March 2022).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.S.; Qteish, A.; Payne, M.C.; Heine, V. Optimized and transferable nonlocal separable ab initio pseudopotentials. Phys. Rev. B 1993, 47, 4174–4180. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Marchenko, E.I.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Aksenov, S.M.; Goodilin, E.A.; Tarasov, A.B. FA2PbBr4: Synthesis, Structure, and Unusual Optical Properties of Two Polymorphs of Formamidinium-Based Layered (110) Hybrid Perovskite. Chem. Mater. 2021, 33, 1900–1907. [Google Scholar] [CrossRef]
- Mokhnache, O.; Boughzala, H. Crystal structure of a new hybrid compound based on an iodidoplumbate(II) anionic motif. Acta Crystallogr. Sect. E Crystallogr. Commun. 2016, 72, 56–59. [Google Scholar] [CrossRef]
- Cao, J.; Jing, X.; Yan, J.; Hu, C.; Chen, R.; Yin, J.; Li, J.; Zheng, N. Identifying the Molecular Structures of Intermediates for Optimizing the Fabrication of High-Quality Perovskite Films. J. Am. Chem. Soc. 2016, 138, 9919–9926. [Google Scholar] [CrossRef]
- Wilke, M.; Casati, N. Insight into the Mechanochemical Synthesis and Structural Evolution of Hybrid Organic-Inorganic Guanidinium Lead(II) Iodides. Chem. A Eur. J. 2018, 24, 17701–17711. [Google Scholar] [CrossRef]
- Hartono, N.T.P.; Sun, S.; Gélvez-Rueda, M.C.; Pierone, P.J.; Erodici, M.P.; Yoo, J.; Wei, F.; Bawendi, M.; Grozema, F.C.; Sher, M.-J.; et al. The effect of structural dimensionality on carrier mobility in lead-halide perovskites. J. Mater. Chem. A 2019, 7, 23949–23957. [Google Scholar] [CrossRef]
- Wang, S.; Mitzi, D.B.; Feild, C.A.; Guloy, A. Synthesis and Characterization of [NH2C(I):NH2]3MI5 (M = Sn, Pb): Stereochemical Activity in Divalent Tin and Lead Halides Containing Single.ltbbrac.110.rtbbrac. Perovskite Sheets. J. Am. Chem. Soc. 1995, 117, 5297–5302. [Google Scholar] [CrossRef]
- Liu, G.-N.; Shi, J.-R.; Han, X.-J.; Zhang, X.; Li, K.; Li, J.; Zhang, T.; Liu, Q.-S.; Zhang, Z.-W.; Li, C. A comparison study of aliphatic and aromatic structure directing agents influencing the crystal and electronic structures, and properties of iodoplumbate hybrids: Water induced structure conversion and visible light photocatalytic properties. Dalton Trans. 2015, 44, 12561–12575. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, E.I.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Band Gap and Topology of 1D Perovskite-Derived Hybrid Lead Halide Structures. Crystals 2022, 12, 657. https://doi.org/10.3390/cryst12050657
Marchenko EI, Fateev SA, Goodilin EA, Tarasov AB. Band Gap and Topology of 1D Perovskite-Derived Hybrid Lead Halide Structures. Crystals. 2022; 12(5):657. https://doi.org/10.3390/cryst12050657
Chicago/Turabian StyleMarchenko, Ekaterina I., Sergey A. Fateev, Eugene A. Goodilin, and Alexey B. Tarasov. 2022. "Band Gap and Topology of 1D Perovskite-Derived Hybrid Lead Halide Structures" Crystals 12, no. 5: 657. https://doi.org/10.3390/cryst12050657
APA StyleMarchenko, E. I., Fateev, S. A., Goodilin, E. A., & Tarasov, A. B. (2022). Band Gap and Topology of 1D Perovskite-Derived Hybrid Lead Halide Structures. Crystals, 12(5), 657. https://doi.org/10.3390/cryst12050657







