Gemology, Mineralogy, and Coloration Mechanism of Pinkish-Purple Cobaltoan Dolomite from the Democratic Republic of Congo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Standard Gemological Analyses
2.2.2. Microstructure and Spectroscopy Characteristics of Cobaltoan Dolomite
2.2.3. Chemical Composition Characteristic of Cobaltoan Dolomite
2.2.4. Coloration Mechanism of Cobaltoan Dolomite
3. Results and Discussion
3.1. Sample Appearance and Gemological Properties
3.2. Microstructure and Spectroscopy Characteristics
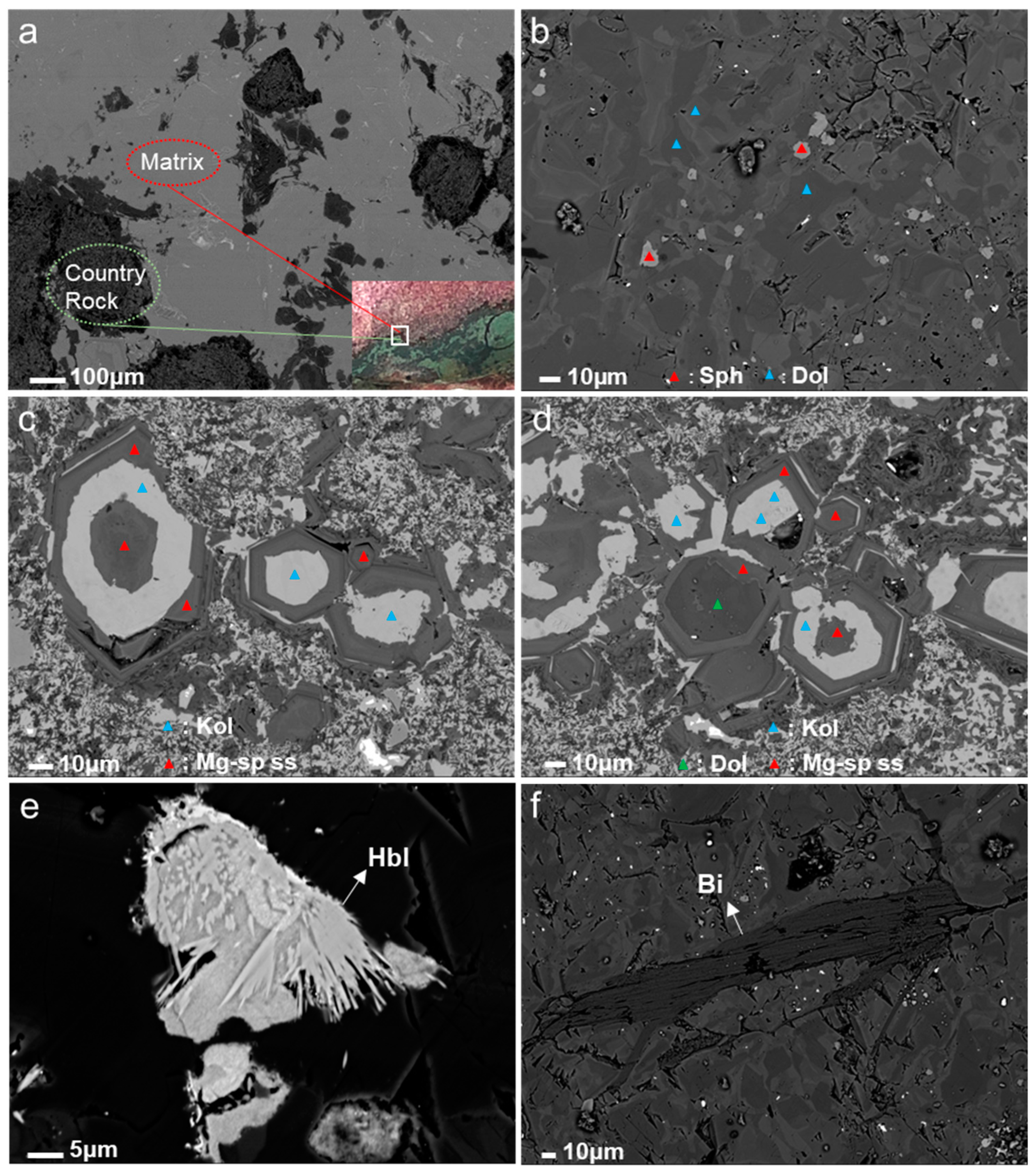
3.2.1. Microstructure Characteristics
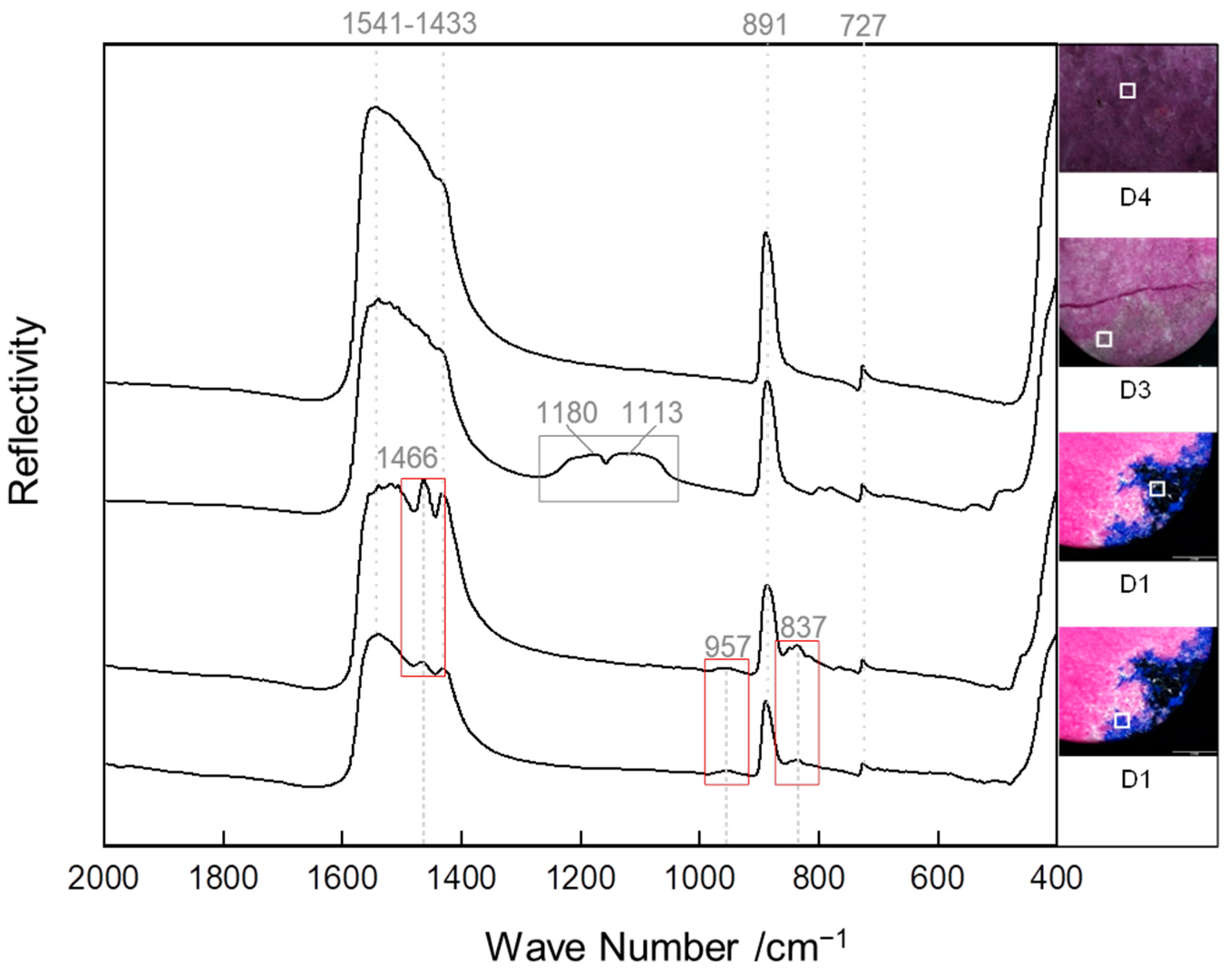
3.2.2. FTIR Spectra
3.3. Electron Microprobe Analyses
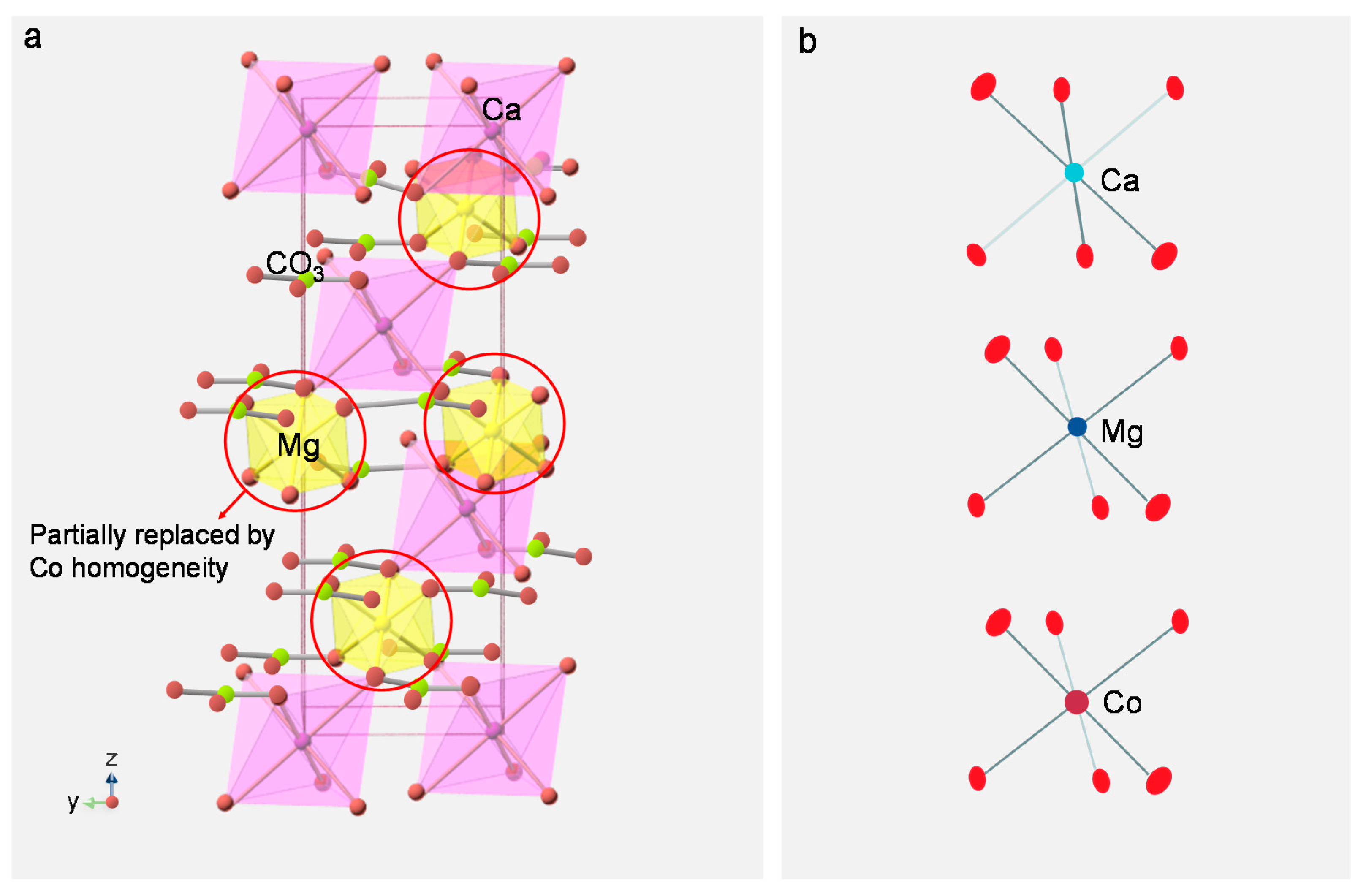
3.4. Analysis of Coloration Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siritheerakul, P.; Sangsawong, S. Pink and Reddish Purple COBALTOCALCITE. Gems Gemol. 2015, 51, 58–59. [Google Scholar]
- Zeng, L.; Wan, M.X.; Peng, Y. Order degree of dolomite and its application in petroleum geology. Nat. Ctas Explor. Dev. 2004, 4, 64–66. (In Chinese) [Google Scholar]
- Milton, C.; Axelrod, J.M.; Grimaldi, F.S. New minerals reedmergnerite (Na2O·B2O3·6SiO2) and eitelite (Na2O·MgO·CO2) associated with leucosphenite, shortite, searlesite, and crocidolite in the Green River Formation, Utah. Geol. Soc. Am. Bull. 1954, 65, 1286–1287. [Google Scholar]
- Li, S.R. Crystallography and Mineralogy; Geological Publishing House: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Hurlbut, C.S. Zincian and plumbian dolomite from Tsumeb, south-west Africa. Am. Mineral. 1957, 42, 798–803. [Google Scholar]
- Fritsch, E.; Rossman, G.R. An update on color in gems. Part 1: Introduction and colors caused by dispersed metal ions. Gems Gemol. 1987, 23, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Du, J.M.; Zhao, X.Z. Geological characteristics and distribution of copper-cobalt deposits in Congo. Geol. Prospect. 2010, 46, 165–174. (In Chinese) [Google Scholar]
- Deliens, M.; Piret, P. La kolwesite, un hydrocarbonate de cuivre et de cobalt analogue a la glaukosphaerite et a la rosasite. Bull. Mineral. 1980, 103, 179–184. [Google Scholar] [CrossRef]
- Gauthier, G.; Deliens, M. Cobalt minerals of the Katanga Crescent, Congo. Mineral. Rec. 1999, 30, 255–273. [Google Scholar]
- Boni, M.; Mondillo, N.; Balassone, G.; Joachimski, M. Zincian dolomite related to supergene alteration in the Iglesias mining district (SW Sardinia). Int. J. Earth Sci. 2013, 102, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.X.; Hao, Y.J.; Dong, S.B. Geological characteristics and genesis of the Kamoya Copper-cobalt deposit in Congo (DRC). Miner. Explor. 2014, 2, 350–355. (In Chinese) [Google Scholar]
- El Desouky, H.A.; Muchez, P.; Dewaele, S. Postorogenic Origin of the Stratiform Cu Mineralization at Lufukwe, Lufilian Foreland, Democratic Republic of Congo. Econ. Geol. 2008, 103, 555–582. [Google Scholar] [CrossRef]
- Li, X.Q.; Mao, J.W.; Yan, Y.L. Regional geology and characteristics of ore deposits in Katangan copper-cobalt belt within Congo (Kinshasa), Central Africa. Miner. Depos. 2009, 28, 373–376. (In Chinese) [Google Scholar]
- Van Langendonck, S.; Muchez, P.; Dewaele, S.; Kaputo Kalubi, A.; Cailteux, J. Petrographic and mineralogical study of the sediment hosted Cu-Co ore deposit at Kambove West in the central part of the Katanga Copperbelt (DRC). Geol. Belg. 2013, 16, 91–104. [Google Scholar]
- Perchiazzi, N.; Dragone, R.; Demitri, N.; Vignola, P. Incorporation of Co in the rosasitemalachite carbonate group of minerals: Crystal structure studies of kolwezite and of synthetic cobaltoan malachites. Eur. J. Mineral. 2017, 30, 609–620. [Google Scholar] [CrossRef]
- Chen, N.X.; Chen, C.; Li, G.G.; Cao, S.Q.; Lu, Y.; Zhang, H. Study on gemological characteristics of pink-purplish red dolomite. Superhard Mater. Eng. 2021, 33, 47–52. (In Chinese) [Google Scholar]
- Douglass, D.L. Cobaltoan Calcite ¼ Cobaltoan Dolomite. Mineral. Rec. 1992, 23, 445. [Google Scholar]
- Perchiazzi, N. Crystal structure study of a cobaltoan dolomite from Kolwezi, Democratic Republic of Congo (Article). Acta Crystallogr. Sect. E Struct. Rep. Online 2015, E71, i3. [Google Scholar] [CrossRef]
- Mincevastefanova, J. First finding of high miscibility in the system CaMg(CO3)2–CaCo(CO3)2 in nature. Geochem. Mineral. Petrol. 1997, 32, 5–16. [Google Scholar]
- Barton, I.F.; Yang, H.; Barton, M.D. The mineralogy, geochemistry, and metallurgy of cobalt in the rhombohedral carbonates. Can. Mineral. 2014, 52, 653–670. [Google Scholar] [CrossRef]
- Perchiazzi, N.; Barton, I.F.; Vignola, P. Incorporation of Co in the Dolomite Structure: Coupled Epma and Single Crystal Structural Studies of Co-rich Dolomite from the Tenke-fungurume District, Democratic Republic of Congo. Can. Mineral. 2018, 56, 151–158. [Google Scholar] [CrossRef]
- Ye, X.; Bai, F.; Li, M.; Sun, H. Gemology, Mineralogy, and Spectroscopy of an Attractive Tremolitized Diopside Anorthosite Gem Material from the Philippines: A New Type of Material with Similarities to Dushan Jade. Minerals 2021, 11, 152. [Google Scholar] [CrossRef]
- Bai, F.; Luo, F.; Yang, L.; Zheng, S. Study on gem mineralogy of suji, a new kind of precious jade material. J. China Non-Met. Miner. Ind. 2007, 3, 71–73. (In Chinese) [Google Scholar]
- Chang, L.H.; Chen, M.Y.; Jin, W. Handbook for Identification of Transparent Mineral Flakes; Geological Publishing House: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Frost, R.L.; Martens, W.N.; Rintoul, L.; Mahmutagic, E.; Kloprogge, J.T. Raman spectroscopic study of azurite and malachite at 298 and 77 K. J. Raman Spectrosc. 2002, 33, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Fang, X.; Ju, J.W. Application of Spectrometry on Analysis of Blue and Green Pigments in the Thangka. J. Light Scatt. 2020, 32, 280–287. (In Chinese) [Google Scholar]
- Lu, Z.; He, X.; Lin, C.; Jin, X.; Pan, Y. Identification of Beihong Agate and Nanhong Agate from China Based on Chromaticity and Raman Spectra. Spectrosc. Spectr. Anal. 2019, 39, 2153–2159. [Google Scholar]
- Kingma, K.J.; Hemley, R.J. High-pressure crystalline transformations and amorphization in alpha-quartz. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 1994; Volume 309, pp. 39–42. [Google Scholar]
- Shi, X.L. Study on Surface Topography Formation Mechanism and Process in Lapping and Polishing of Tungsten Alloy. Master’s Thesis, Dalian University of Technology, Dalian, China, 2020. (In Chinese). [Google Scholar]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Xu, H.Y.; Yu, X.Y. Pressed Gibbsite and Calcite as A Rhodochrosite Imitation. Gems Gemol. 2019, 55, 406–415. [Google Scholar] [CrossRef]
- Moenke, H.H.W. Vibrational spectra and the crystal-chemical classification of minerals. In The Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society of Great Britain and Ireland: London, UK, 1974; pp. 111–118. [Google Scholar]
- Gaffey, S.J. Spectral reflectance of carbonate minerals in the visible and near-infrared (0.35~2.55 microns); Calcite, aragonite, and dolomite. Am. Mineral. 1986, 71, 151–162. [Google Scholar]
- Huang, C.K.; Kerr, P.F. Infrared study of the carbonate minerals. Am. Mineral. 1960, 45, 311–324. [Google Scholar]
- Peng, W.S.; Liu, G.K. Mineral Infrared Spectrum Atlas; Science Press: Beijing, China, 1982. (In Chinese) [Google Scholar]
- Goldsmith, J.R.; Northrop, D.A. Sub solidus phase relations in the systems CaCO3–MgCO3–CoCO3 and CaCO3–MgCO3–NiCO3. J. Geol. 1965, 73, 817–829. [Google Scholar] [CrossRef]
- Zhang, Y. Ore-bearing strata and mineralization characteristics of the Copper-cobalt deposit in Kambvo region, Upper Katanga Province, Congo Technology. Innov. Appl. 2017, 26, 191–193. (In Chinese) [Google Scholar]
- You, X.L.; Jia, W.Q.; Xu, F.; Liu, Y. Mineralogical Characteristics of Ankerite and Mechanisms of Primary and Secondary Origins. Geoscience 2018, 43, 4046–4055. (In Chinese) [Google Scholar]
- Wan, S.Q. New color reactions of cobalt divalent. Chem. World 1957, 9, 404. (In Chinese) [Google Scholar]
- Luo, H.Y.; Wang, C.S.; Liao, S.Y. Theoretical explanation of transition metal ions in gemstones. Acta Petrol. Mineral. 2004, 2, 177–185. (In Chinese) [Google Scholar]

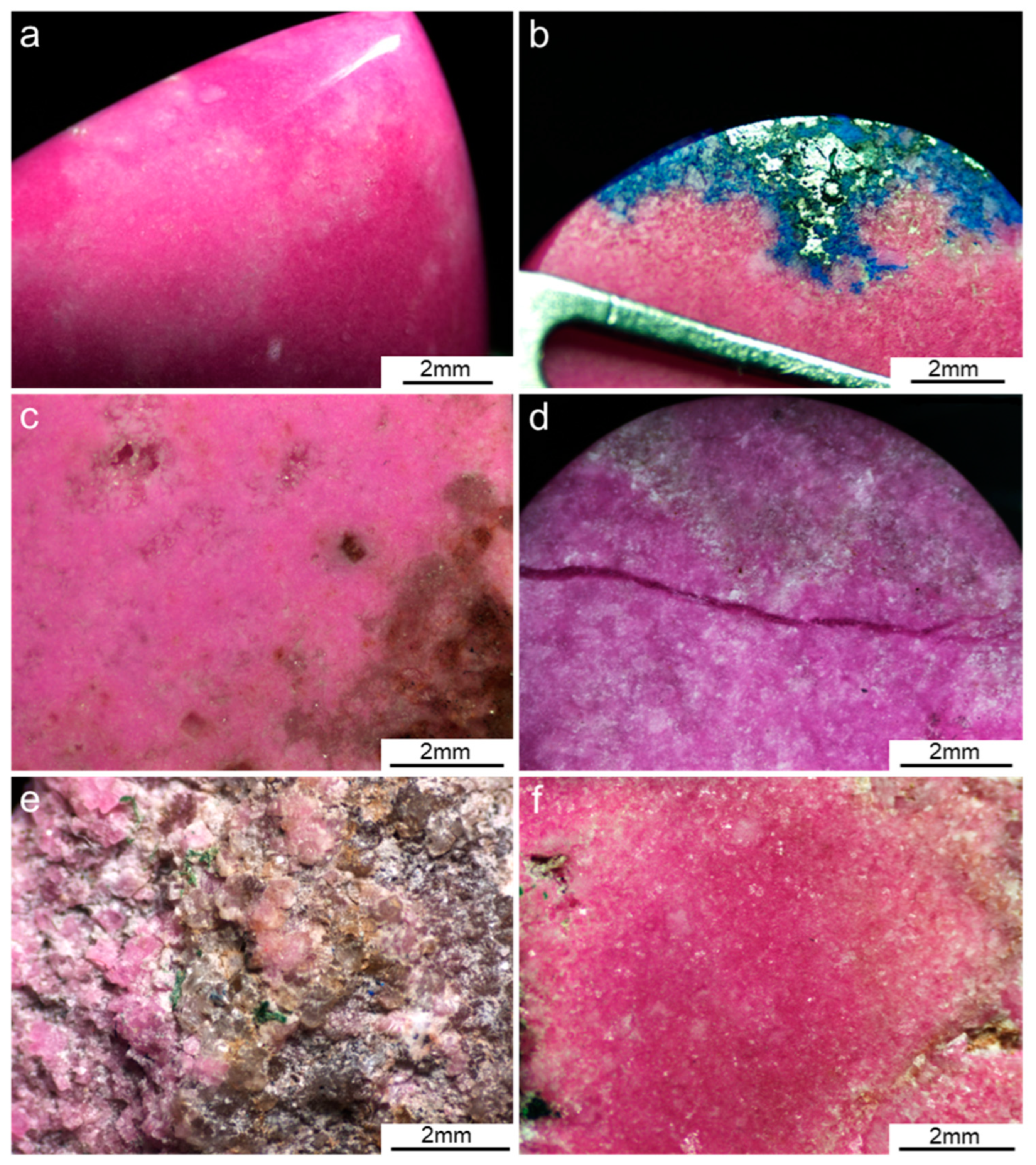
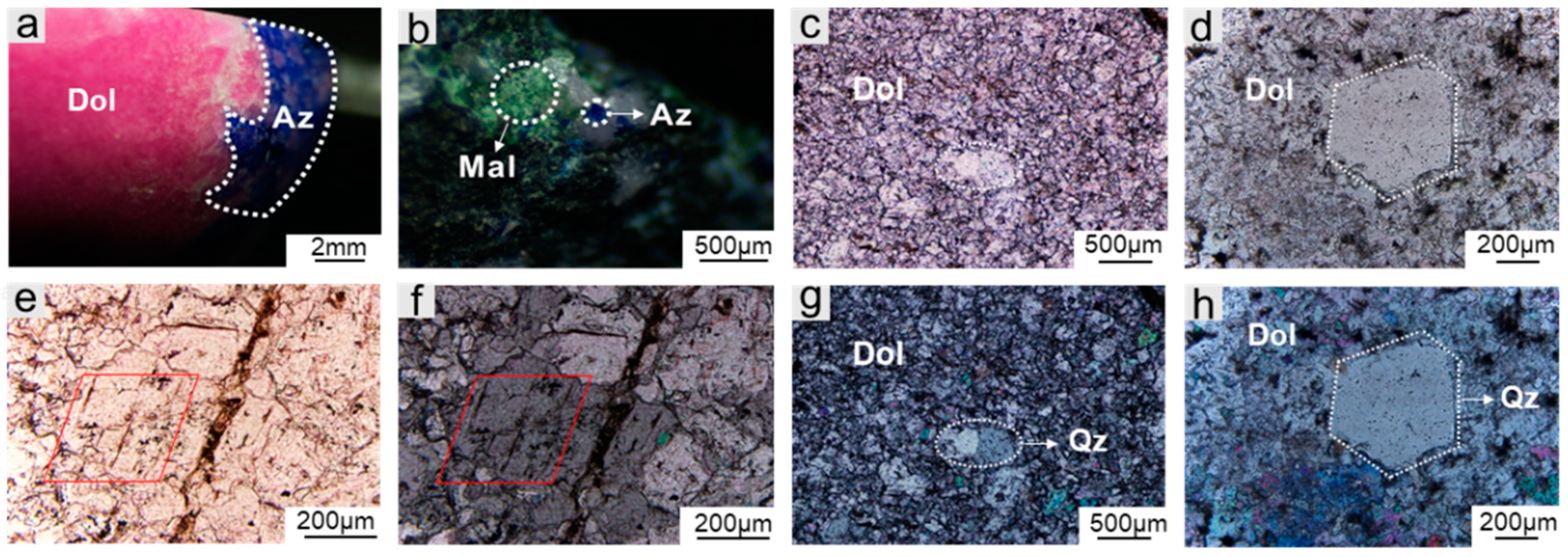
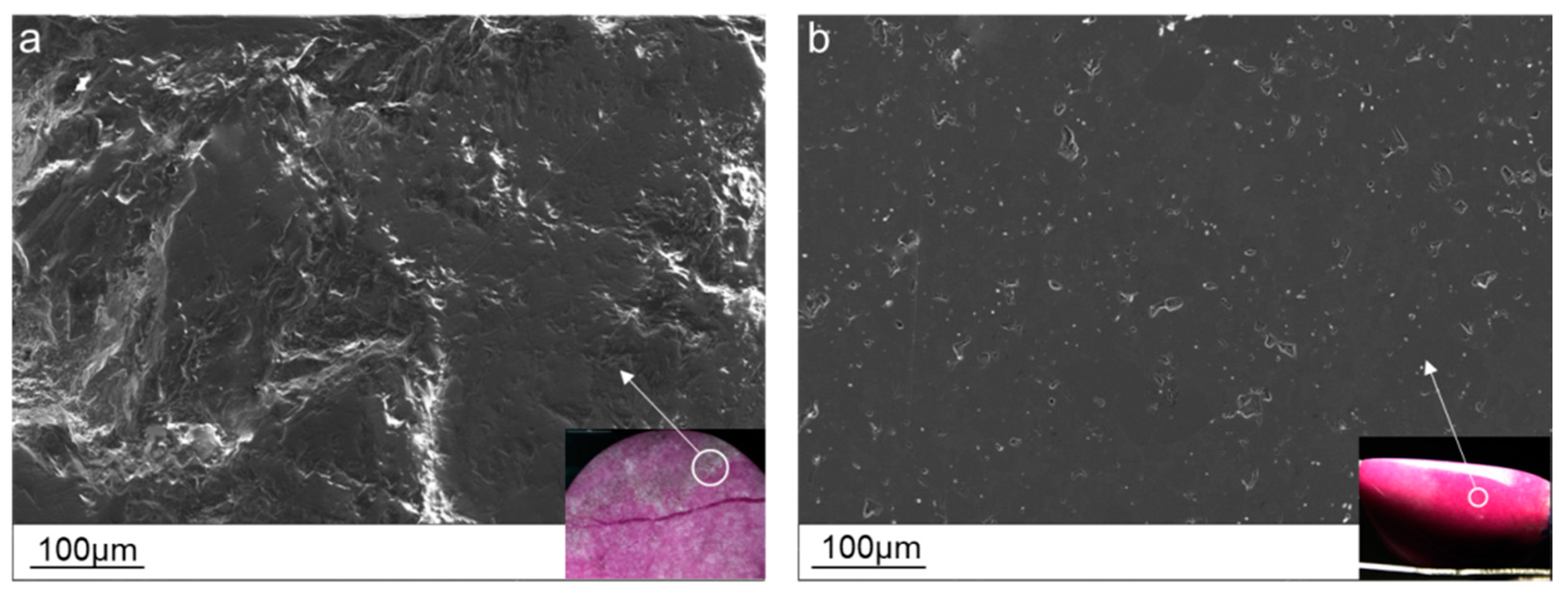

| Sample Number | Color | Luster | Transparency | Refractive Index | Fluorescence | Specific Gravity |
|---|---|---|---|---|---|---|
| D1 | pink; blue | vitreous luster | opaque | 1.55 | None | 2.85 |
| D2 | pink | subvitreous luster | opaque | 1.51 | None | 2.81 |
| D3 | reddish-purple | oil-subvitreous luster | opaque | 1.52 | None | 2.84 |
| D4 | deep purple | vitreous luster | opaque | 1.57 | None | 2.86 |
| R1 | pink; green | vitreous luster | opaque | - | None | 2.85 |
| R2 | pink | vitreous luster | opaque | - | None | 2.83 |
| R3 | light pink | vitreous luster | opaque | - | None | 2.78 |
| R4 | pink | vitreous luster | opaque | - | None | 2.83 |
| R5 | pink | vitreous luster | opaque | - | None | 2.82 |
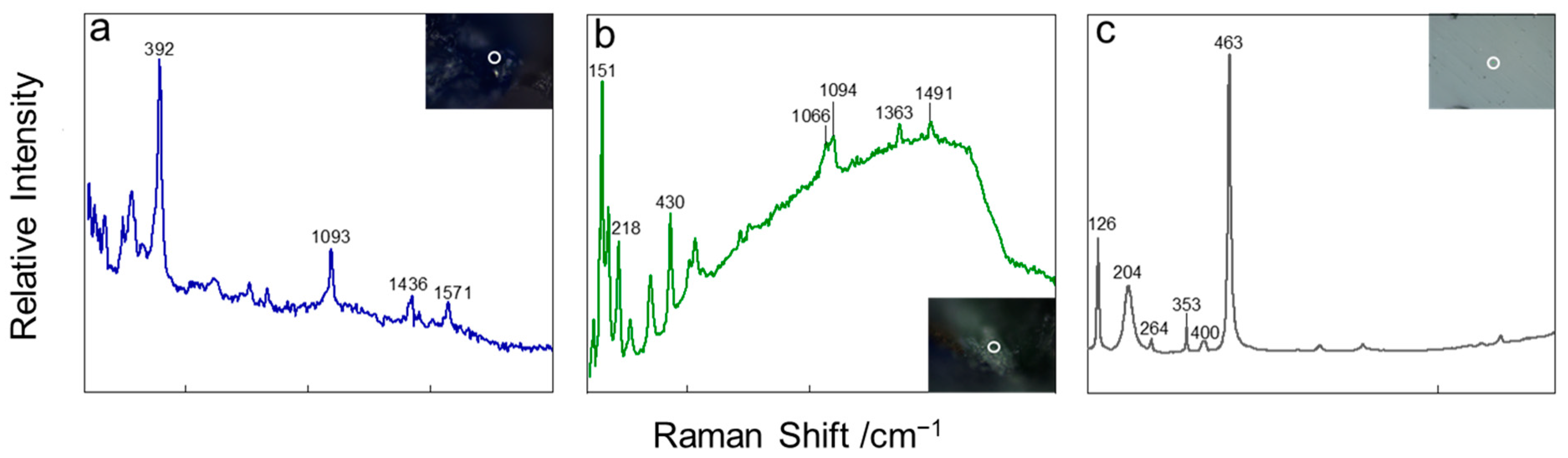
| Minerals | Raman Shift, cm−1 | Assignment | Reference |
|---|---|---|---|
| Azurite | 1093 | V1 (C–O) | [25] |
| 1436 | V3 (C–O) | ||
| 1571 | Vas (CO32−) | ||
| 392 | Vs (Cu–O) | ||
| Malachite | 1066, 1094 | Vs (C–O) | [26] |
| 1491 | V3 (CO32−) | ||
| 430 | Vs (Cu–OH) | ||
| 218 | Bending vibration of O–Cu–OH | ||
| 151 | Bending vibration of O–Cu–O/OH | ||
| Quartz | 126, 204 | [SiO4]R/[SiO4]T | [27,28] |
| 463 | Vs (T–O–T) | ||
| 264, 353, 400 | Vs (Si–O) |
| Minerals | Wave Number, cm−1 | Assignment | Reference |
|---|---|---|---|
| Dolomite | 1541, 1433 | V (C–O) | [32,33,34] |
| 891 | V (CO32−) | ||
| 727 | V (O–C–O) | ||
| Quartz | 1180, 1113 | Vs (Si–O–Si) | [35] |
| Azurite | 1466 | Asymmetric bending vibration of C–O | [26] |
| 957, 837 | V2 (CO32−) |
| Sample; Mineral 1 | R2-a-1; Dol | R2-a-2; Dol | R2-a-3; Dol | R2-b-1; Sph | R2-b-2; Sph |
|---|---|---|---|---|---|
| NaO | 0.035 | 0.029 | 0.037 | 0.086 | 0.090 |
| MgO | 17.769 | 20.057 | 16.346 | 15.297 | 15.229 |
| CaO | 29.209 | 29.934 | 28.529 | 1.64 | 2.224 |
| TiO2 | 0.047 | 0.071 | 0.000 | 0.061 | 0.051 |
| MnO | 0.119 | 0.152 | 0.106 | 0.217 | 0.148 |
| FeO | 1.574 | 0.426 | 0.062 | 0.072 | 0.147 |
| CoO | 2.532 | 0.966 | 6.111 | 37.449 | 37.348 |
| total | 51.285 | 51.635 | 51.191 | 54.822 | 55.237 |
| Sample; Mineral 1 | R1-a-1; Mg-sp ss | R1-a-2; Mg-sp ss | R1-a-3; Mg-sp ss | R1-a-4; Mg-sp ss | R1-a-5; Kol | R1-a-6; Kol | R1-a-7; Kol | R1-b-1; Dol |
|---|---|---|---|---|---|---|---|---|
| NaO | 0.188 | 0.269 | 0.187 | 0.268 | 0.180 | 0.260 | 0.142 | 0.115 |
| MgO | 28.600 | 26.038 | 27.080 | 25.600 | 0.626 | 0.783 | 2.511 | 19.925 |
| SiO2 | 0.175 | 0.054 | 0.216 | 0.123 | 0.035 | 0.141 | 0.025 | 0.026 |
| CaO | 0.921 | 0.185 | 1.006 | 0.221 | 0.002 | 0.028 | 0.002 | 30.524 |
| TiO2 | 0.011 | 0.000 | 0.064 | 0.000 | 0.030 | 0.000 | 0.000 | 0.000 |
| MnO | 0.035 | 0.050 | 0.122 | 0.077 | 0.000 | 0.003 | 0.017 | 0.027 |
| FeO | 0.000 | 0.028 | 0.028 | 0.058 | 0.129 | 0.113 | 0.068 | 0.020 |
| CoO | 21.028 | 25.939 | 24.071 | 26.447 | 2.974 | 4.608 | 4.568 | 1.496 |
| NiO | 0.000 | 0.595 | 0.096 | 0.915 | 0.000 | 0.000 | 0.000 | 0.022 |
| CuO | 1.043 | 1.059 | 0.872 | 1.014 | 63.032 | 60.632 | 59.718 | 1.015 |
| total | 52.001 | 54.217 | 53.742 | 54.723 | 67.008 | 66.568 | 67.051 | 53.170 |
| NaO | 0.127 | 0.310 | 0.109 | 0.333 | 0.125 | 0.094 | 0.288 | 0.402 |
| MgO | 27.600 | 27.093 | 30.296 | 26.605 | 25.619 | 0.623 | 1.146 | 1.111 |
| SiO2 | 0.022 | 0.140 | 0.029 | 0.206 | 0.027 | 0.069 | 0.066 | 0.049 |
| CaO | 0.695 | 1.121 | 0.169 | 0.949 | 0.131 | 0.014 | 0.049 | 0.043 |
| TiO2 | 0.053 | 0.000 | 0.000 | 0.063 | 0.000 | 0.040 | 0.040 | 0.040 |
| MnO | 0.154 | 0.059 | 0.107 | 0.165 | 0.081 | 0.037 | 0.005 | 0.023 |
| FeO | 0.000 | 0.000 | 0.000 | 0.003 | 0.027 | 0.000 | 0.000 | 0.061 |
| CoO | 24.030 | 23.875 | 21.594 | 24.549 | 25.339 | 5.417 | 6.204 | 6.136 |
| NiO | 0.410 | 0.278 | 0.833 | 0.133 | 0.613 | 0.000 | 0.000 | 0.000 |
| CuO | 0.655 | 0.715 | 0.868 | 0.820 | 2.433 | 60.265 | 56.966 | 59.074 |
| total | 53.746 | 53.591 | 54.005 | 53.826 | 54.395 | 66.559 | 64.764 | 66.939 |
| Number | D1-1 | D1-2 | D1-3 | D1-4 | D2-1 | D3-1 | D3-2 | D4-1 |
|---|---|---|---|---|---|---|---|---|
| CaO | 55.54 | 54.93 | 3.06 | 3.13 | 55.40 | 52.24 | 53.96 | 54.05 |
| MgO | 39.15 | 37.58 | 0.00 | 0.00 | 35.67 | 32.95 | 33.59 | 31.93 |
| CoO | 1.07 | 3.97 | 0.42 | 0.32 | 5.48 | 7.89 | 7.69 | 8.70 |
| SiO2 | 1.40 | 1.60 | 2.92 | 2.04 | 1.30 | 4.32 | 1.76 | 1.11 |
| SO3 | 0.95 | 0.78 | 35.44 | 33.74 | 1.10 | 1.17 | 0.82 | 1.16 |
| Al2O3 | 0.00 | 1.59 | 3.83 | 2.04 | 0.00 | 1.22 | 1.76 | 1.96 |
| CuO | 0.72 | 0.03 | 52.25 | 57.78 | 0.31 | 0.22 | 0.02 | 0.08 |
| Fe2O3 | 0.64 | 0.28 | 0.97 | 0.43 | 0.02 | 0.39 | 0.21 | 0.55 |
| MnO | 0.27 | 0.13 | 0.00 | 0.00 | 0.61 | 0.05 | 0.09 | 0.27 |
| K2O | 0.04 | 0.06 | 0.20 | 0.08 | 0.09 | 0.07 | 0.02 | 0.06 |
| total | 99.78 | 100.95 | 99.09 | 99.56 | 99.98 | 100.52 | 99.92 | 99.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Yu, X.-Y. Gemology, Mineralogy, and Coloration Mechanism of Pinkish-Purple Cobaltoan Dolomite from the Democratic Republic of Congo. Crystals 2022, 12, 639. https://doi.org/10.3390/cryst12050639
Yan Y, Yu X-Y. Gemology, Mineralogy, and Coloration Mechanism of Pinkish-Purple Cobaltoan Dolomite from the Democratic Republic of Congo. Crystals. 2022; 12(5):639. https://doi.org/10.3390/cryst12050639
Chicago/Turabian StyleYan, Ying, and Xiao-Yan Yu. 2022. "Gemology, Mineralogy, and Coloration Mechanism of Pinkish-Purple Cobaltoan Dolomite from the Democratic Republic of Congo" Crystals 12, no. 5: 639. https://doi.org/10.3390/cryst12050639
APA StyleYan, Y., & Yu, X.-Y. (2022). Gemology, Mineralogy, and Coloration Mechanism of Pinkish-Purple Cobaltoan Dolomite from the Democratic Republic of Congo. Crystals, 12(5), 639. https://doi.org/10.3390/cryst12050639






