Structural Aspects of “Memory Effect” for MgGa LDHs: New Data Obtained by Simulation of XRD Patterns for 1D Disordered Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. TG-DTG-DTA
2.3. Powder X-ray Diffraction
2.4. In Situ XRD
3. Results and Discussion
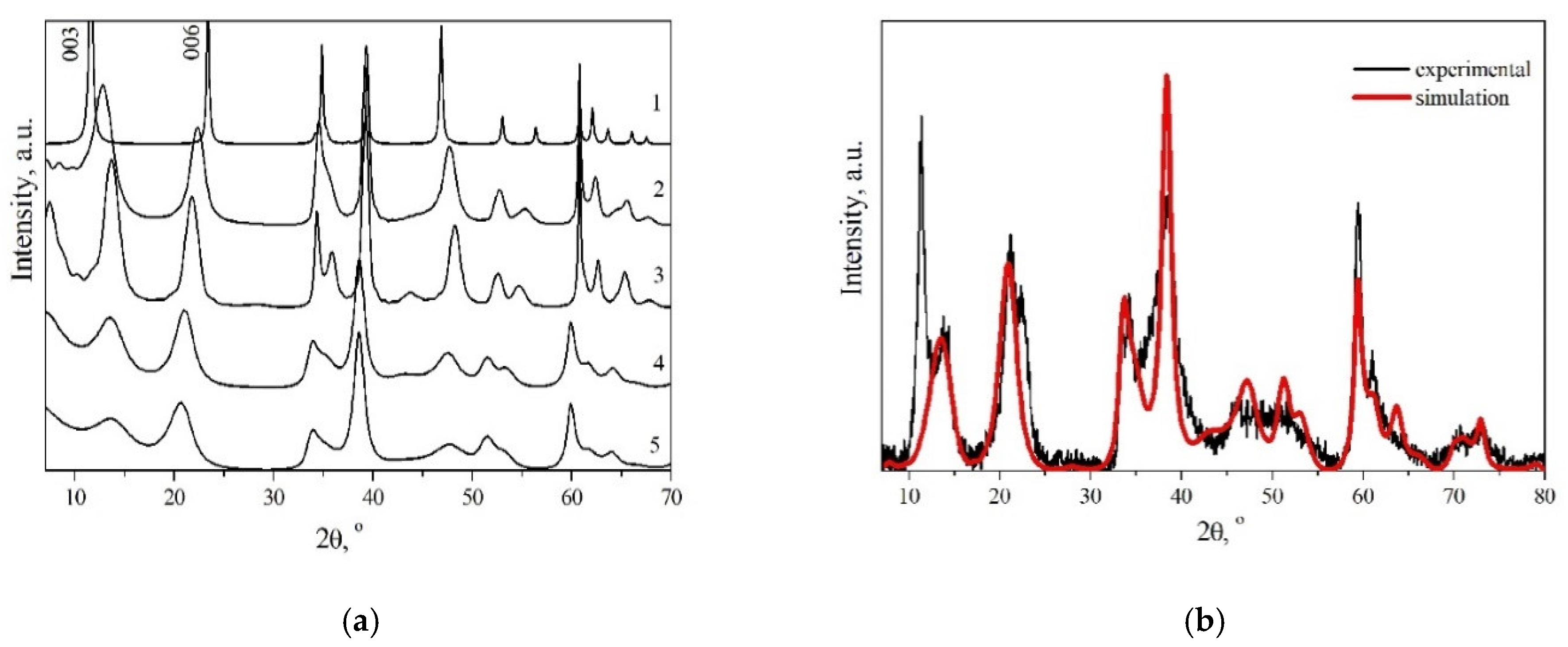
3.1. Structure of the Synthesized MgGa-CO3 LDHs
3.2. Thermal Decomposition of MgGa-CO3 LDHs
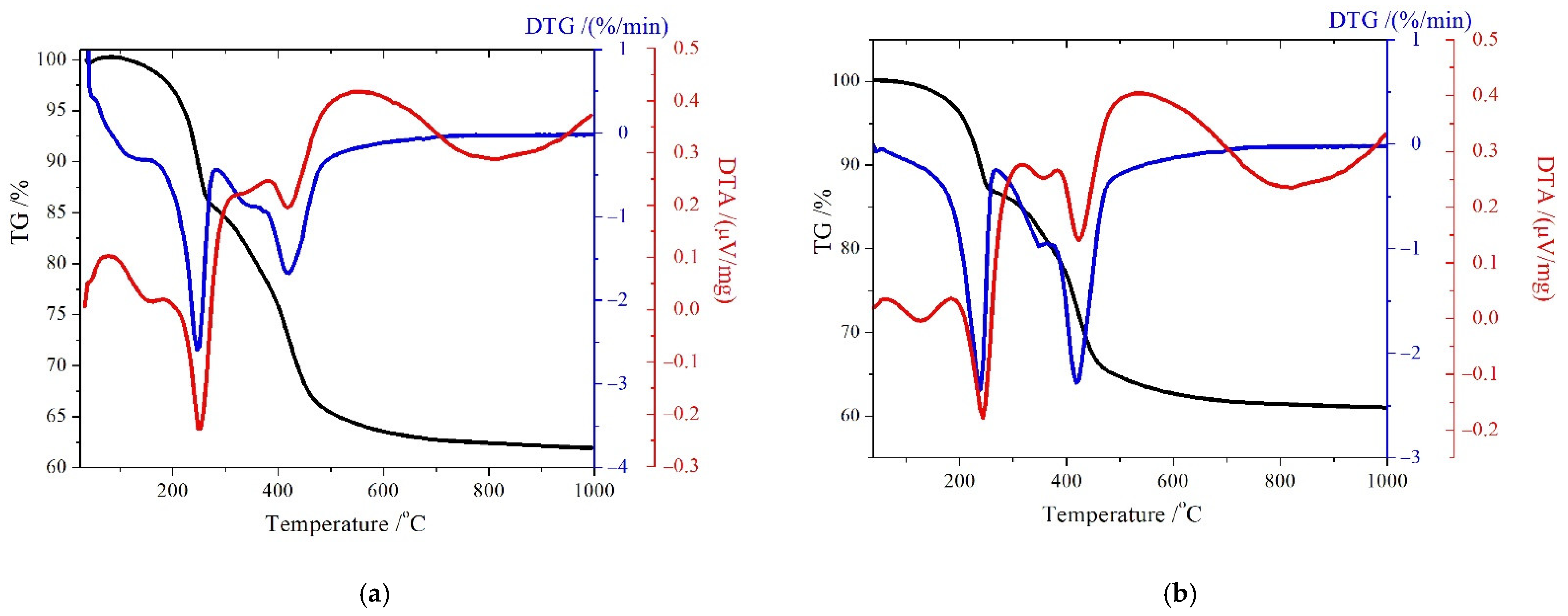
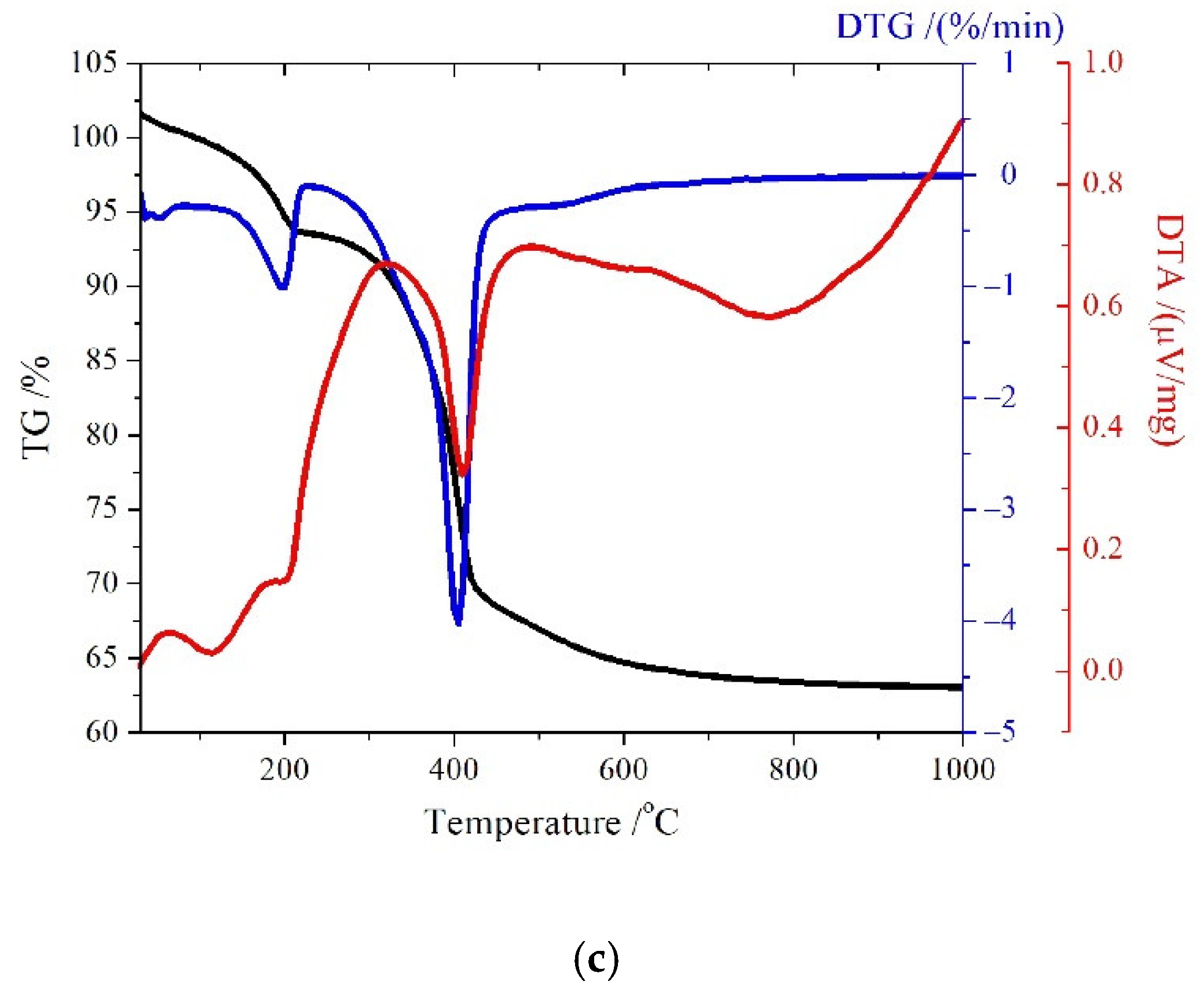
3.2.1. TG/DTG/DTA analysis
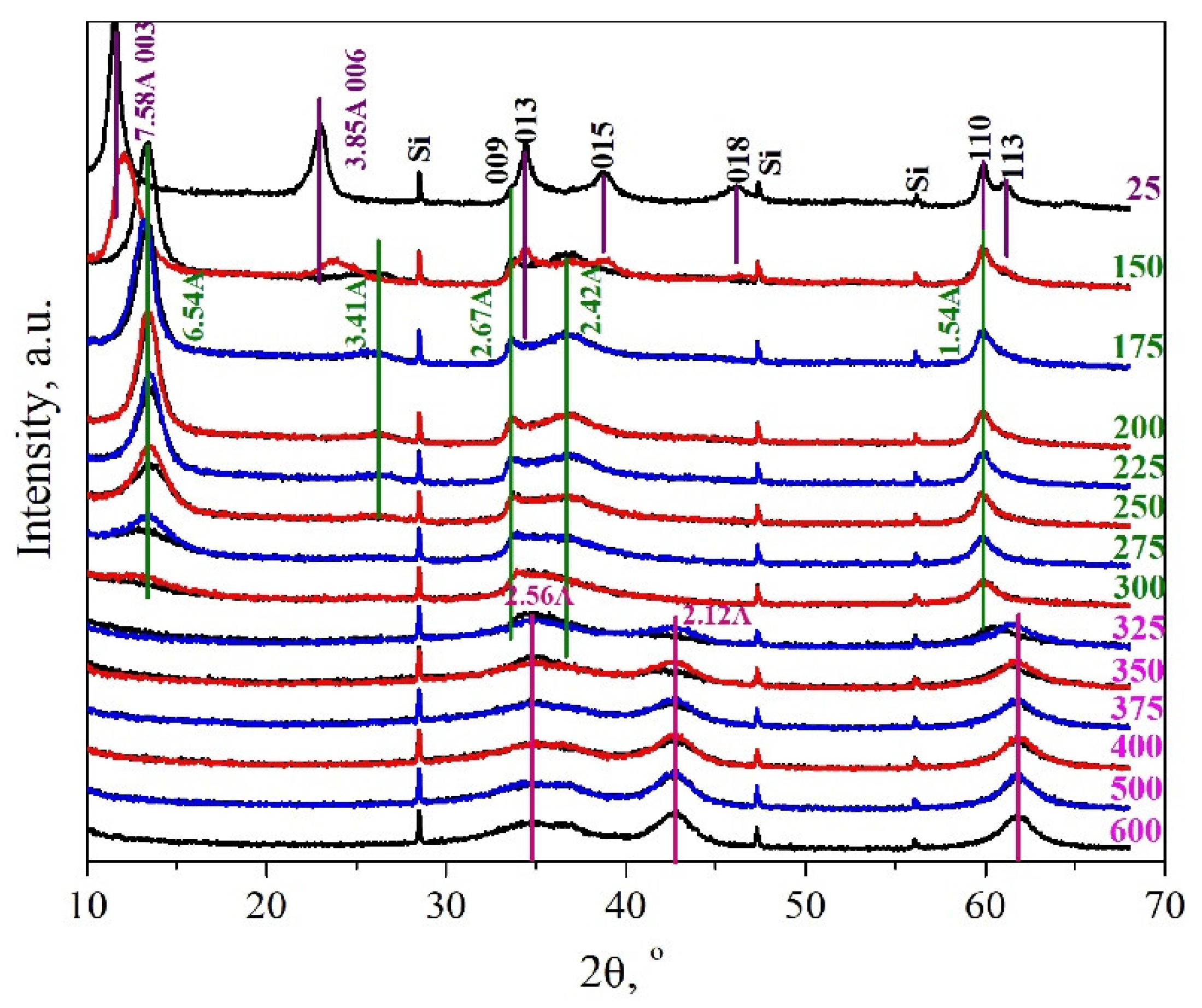
3.2.2. In Situ XRD Analysis
3.2.3. The Structure of Dehydrated MgGa LDH
3.2.4. Structure of Calcined MgGa LDHs
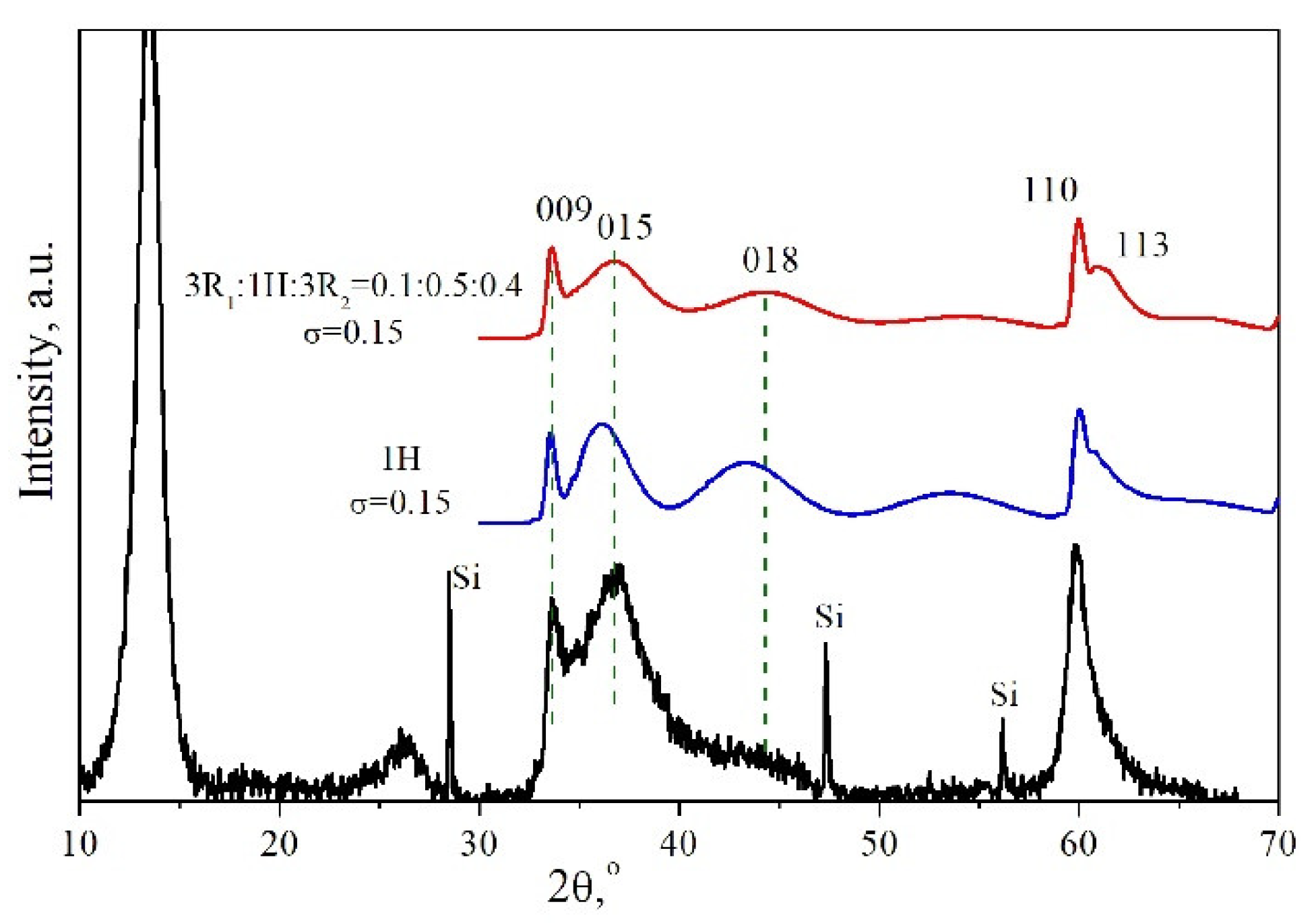
3.3. “Memory Effect” of the Heating Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryltsova, I.G.; Nestroinaya, O.V.; Lebedeva, O.E.; Schroeter, F.; Roessner, F. Synthesis and characterization of layered double hydroxides containing nickel in unstable oxidation state +3 in cationic sites. J. Solid State Chem. 2018, 265, 332–338. [Google Scholar] [CrossRef]
- Song, Y.; Beaumont, S.K.; Zhang, X.; Wilson, K.; Lee, A.F. Catalytic applications of layered double hydroxides in biomass valorization. Curr. Opin. Green Sustain. Chem. 2020, 22, 29–38. [Google Scholar] [CrossRef]
- Mittal, J. Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: A review. J. Environ. Manag. 2021, 295, 113017. [Google Scholar] [CrossRef] [PubMed]
- Prinetto, F.; Tichit, D.; Teissier, R.; Coq, B. Mg- and Ni-containing layered double hydroxides as soda substitutes in the aldol condensation of acetone. Catal. Today 2000, 55, 103–116. [Google Scholar] [CrossRef]
- Stanimirova, T.; Balek, V. Characterization of layered double hydroxide Mg-Al-CO3 prepared by re-hydration of Mg-Al mixed oxide. J. Therm. Anal. Calorim. 2008, 94, 477–481. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Frost, R.L.; Musumeci, A.W. Nitrate absorption through hydrotalcite reformation. J. Colloid Interface Sci. 2006, 302, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Theiss, F.L.; Palmer, S.J.; Ayoko, G.A.; Frost, R.L. Sulfate intercalated layered double hydroxides prepeared by the reformation effect. J. Therm. Anal. Calorim. 2012, 107, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDHs) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Leont’eva, N.N.; Likholobov, V.A. The effect of Mg/Al ratio in layered double hydroxides on the sorption of Pt(IV) chloride complexes. J. Sib. Fed. Univ. Chem. 2012, 4, 361–375. [Google Scholar]
- Kovanda, F.; Maryšková, Z.; Kovář, P. Intercalation of paracetamol into the hydrotalcite-like host. J. Solid State Chem. 2011, 184, 3329–3335. [Google Scholar] [CrossRef]
- Shirin, V.K.A.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Bharadiya, P.; Hansora, D. Layered double hydroxide based bionanocompo-sites. Appl. Clay Sci. 2019, 177, 19–36. [Google Scholar] [CrossRef]
- Yan, L.; Gonca, S.; Zhu, G.; Zhang, W.; Chen, X. Layered double hydroxide nanostructures and nanocomposites for biomedical applications. J. Mater. Chem. B 2019, 7, 5583–5601. [Google Scholar] [CrossRef] [Green Version]
- Allou, N.; Yadav, A.; Pal, M.; Goswamee, R.L. Biocompatible nanocomposite of carboxymethyl cellulose and functionalized carbon-norfloxacin intercalated layered double hydroxides. Carbohydr. Polym. 2018, 186, 282–289. [Google Scholar] [CrossRef]
- Li, F.; Duan, X. Structure and bonding. In Applications of Layered Double Hydroxides; Evans, D.G., Ed.; Springer: New York, NY, USA, 2006; Volume 119, pp. 193–223. [Google Scholar]
- Lukashin, A.V.; Chernysheva, M.V.; Vertegel, A.A.; Tretyakov, Y.D. The synthesis of Pt/LDH nanocomposites by chemical modification of layered double hydroxides. Dokl. Chem. 2003, 388, 200–204. [Google Scholar] [CrossRef]
- Garcés-Polo, S.I.; Villaroel-Rocha, J.; Sapag, K.; Korili, S.A.; Gil, A. Adsorption of CO2 on mixed oxides derived from hydrotalcites at several temperatures and high pressures. Chem. Eng. J. 2018, 332, 24–32. [Google Scholar] [CrossRef]
- Cao, Y.; Li, G.; Li, X. Graphene/layered double hydroxide nanocomposite: Properties, synthesis, and applications. Chem. Eng. J. 2016, 292, 207–223. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Suzuki, K.; Takehira, K.; Hayakawa, T. Combined partial oxidation and dry reforming of methane to synthesis gas over noble metals supported on Mg-Al mixed oxide. Appl. Catal. A Gen. 2004, 275, 149–155. [Google Scholar] [CrossRef]
- Abelló, S.; Verboekend, D.; Bridier, B.; Pérez- Ramírez, J. Activated takovite catalysts for partial hydrogenation of ethyne, propyne, and propadiene. J. Catal. 2008, 259, 85–95. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Leont’eva, N.N.; Gulyaeva, T.I.; Drozdov, V.A.; Doronin, V.P.; Zaikovskii, V.I.; Likholobov, V.A. A study on the formation of platinum sites over basic supports of the layered double hydroxide (LDH) type. 2. The effect of the nature of interlayer anion in aluminum-magnesium layered hydroxides on the anchoring of platinum and formation of Pt/MgAlOx. Kinet. Catal. 2011, 52, 899–909. [Google Scholar]
- Kim, I.-T.; Ahn, K.-H.; Jung, J.; Jeong, Y.; Shin, D.-C.; Lee, Y.-E. Removal of Tar Contents Derived from Lignocellulosic Biomass Gasification Facilities Using MgAl-LDH@clinoptilolite. Catalysts 2021, 11, 1111. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, S.; Asif, M.; In, S.-I. Layered Double Hydroxide (LDH) Based Photocatalysts: An Outstanding Strategy for Efficient Photocatalytic CO2 conversion. Catalysts 2020, 10, 1185. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Biochem. Pharmacol. 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Zhang, W.; Li, N.; Xie, Z.; Liu, Z.; Huang, Q. ScienceDirect Defective layered double hydroxide formed by H2O2 treatment act as highly efficient electrocatalytic for oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 21858–21864. [Google Scholar] [CrossRef]
- Hou, L.; Du, Q.; Su, L.; Di, S.; Ma, Z.; Chen, L.; Shao, G. Ni-Co layered double hydroxide with self-assembled urchin like morphology for asymmetric supercapacitors. Mater. Lett. 2019, 237, 262–265. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Qin, Q.; Zul, S.; Shah, S.; Bahadur, I. An overview of semiconductors/layered double hydroxides composites: Properties, synthesis, photocatalytic and photoelectrochemical applications. J. Mol. Liq. 2019, 289, 111114. [Google Scholar] [CrossRef]
- Bookin, A.S.; Drits, V.A. Polytype diversity of the hydrotalcite-like minerals; I. Possible polytypes and their diffraction features. Clays Clay Miner. 1993, 41, 551–557. [Google Scholar] [CrossRef]
- Bookin, A.S.; Cherkashin, V.I.; Drits, V.A. Polytype diversity of the hydrotalcite-like minerals; II. Determination of the polytypes of experimentally studied varieties. Clays Clay Miner. 1993, 41, 558–564. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Lukashin, A.V.; Vertegel, A.A.; Tarasov, V.P.; Tretyakov, Y.D. A study on crystallization of Mg-Al layered double hydroxides. Dokl. Chem. 2002, 387, 777–781. [Google Scholar] [CrossRef]
- Evance, D.G.; Slade, R.T. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; Volume 119, pp. 1–874. [Google Scholar]
- Ryltsova, I.G.; Lebedeva, O.E. The synthesis and investigation of layered hydroxides containing cobalt(III). Belgorod State University Scientific Bulletin. Nat. Sci. 2008, 7, 96–100. [Google Scholar]
- Stanimirova, T.S.; Kirov, G.; Dinolova, E. Mechanism of hydrotalcite regeneration. J. Mater. Sci. Lett. 2001, 20, 453–455. [Google Scholar] [CrossRef]
- Prikhodko, R.V.; Sychev, M.V.; Astrelin, I.M.; Erdmann, K.; Mangel, A.; van Santen, R.A. Synthesis and Structural Transformations of Hydrotalcite-like Materials Mg-Al and Zn-Al. Russ. J. Appl. Chem. 2001, 74, 1621–1626. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Abelló, S.; van der Pers, N.M. Influence of the Divalent Cation on the Thermal Activation and Reconstruction of Hydrotalcite-like Compounds. J. Phys. Chem. C 2007, 111, 3642–3650. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Graffin, P.; Tichit, D. Synthesis and characterization of sol–gel Mg/Al and Ni/Al layered double hydroxides and comparison with co-precipitated samples. Microporous Mesoporous Mater. 2000, 39, 229–247. [Google Scholar] [CrossRef]
- López-Salinas, E.; García-Sánchez, M.; Ramón-García, M.L.; Schifter, I. New Gallium-substituted hydrotalcites: [Mg1-x Gax (OH)2](CO3) x/2 mH2O. J. Porous Mater. 1996, 3, 169–174. [Google Scholar] [CrossRef]
- Bedolla-Valdez, Z.I.; Ramirez-Solis, S.; Prince, J.; Lima, E.; Pfeiffer, H.; Valente, J.S. Dynamic water vapor sorption on Mg(Ga3+)O mixed oxides: Analysis of the LDH thermal regeneration process. Thermochim. Acta 2013, 553, 49–53. [Google Scholar] [CrossRef]
- Thomas, G.S.; Kamath, P.V. Reversible thermal behavior of the layered double hydroxides (LDHs) of Mg with Ga and In. Mater. Res. Bull. 2005, 40, 671–681. [Google Scholar] [CrossRef]
- Rietveld, H.M. Profile Refinement Method for Nuclear and Magnetic Structures. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Newsam, J.M.; Deem, M.W. A General Recursion Method for Calculating Diffracted Intensities from Crystals Containing Planar Faults. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1991, 433, 499–520. [Google Scholar]
- Cherepanova, S.V.; Tsybulya, S.V. Simulation of X-ray powder diffraction patterns for one-dimensionally disordered crystals. Mater. Sci. Forum 2004, 443, 87–90. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Salanov, A.N.; Serkova, A.N.; Likholobov, V.A. SEM study of the surface morphology and chemical composition of the MgAl- and MgGa-layered hydroxides in different steps of platinum catalysts Pt/Mg(Al, Ga)Ox synthesis. Appl. Clay Sci. 2018, 157, 267–273. [Google Scholar] [CrossRef]
- Leont’eva, N.N.; Cherepanova, S.V.; Drozdov, V.A.; Belskaya, O.B.; Tsybulya, S.V.; Stepanova, L.N. The effect of Mg to Al ratio on restructuring of hydrotalcites. Theor. Exp. Chem. 2012, 48, 257–261. [Google Scholar] [CrossRef]
- Pfeiffer, H.; Martínez-dlCruz, L.; Lima, E.; Flores, J.; Vera, M.A.; Valente, J.S. Influence of Mg/Al Ratio on the Thermokinetic Rehydration of Calcined Mg−Al Layered Double Hydroxides. J. Phys. Chem. C 2010, 114, 8485–8492. [Google Scholar] [CrossRef]
- Gabrovska, M.; Evdreva-Kardjieva, R.; Crisan, D.; Tzvetkov, P.; Shopska, M.; Shtereva, I. Ni-Al layered double hydroxides as catalyst precursors for CO2 removal by methanation. React. Kinet. Mech. Catal. 2012, 105, 79–99. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Bezdička, P.; Jirátová, K.; Obalová, L.; Pacultová, K.; Bastl, Z.; Grygar, T. Effect of hydrothermal treatment on properties of Ni–Al layered double hydroxides and related mixed oxides. J. Solid State Chem. 2009, 182, 27–36. [Google Scholar] [CrossRef]
- Rives, V. Characterisation of Layered Double Hydroxides and Their Decomposition Products. Mater. Chem. Phys. 2002, 75, 19–25. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. Hydrotalcite Decomposition Mechanism: A Clue to the Structure and Reactivity of Spinel-like Mixed Oxides. J. Phys. Chem. 1996, 100, 8535–8542. [Google Scholar] [CrossRef]
- Cherepanova, S.V.; Leontéva, N.N.; Drozdov, V.A.; Doronin, V.P. Thermal Evolution of Mg-Al and Ni-Al Layered Double Hydroxides: The Structure of the Dehydrated Phase. Acta Crystallogr. Sect. A Found. Adv. 2016, A72, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. XRD and 1H MAS NMR spectroscopic study of mixed oxides obtained by calcination of layered-double hydroxides. Mater. Lett. 2000, 46, 309–314. [Google Scholar] [CrossRef]
- Johnsen, R.E.; Wu, Q.; Sjåstad, A.O.; Vistad, O.B.; Krumeich, F.; Norby, P. Nanostructured Materials Produced by Mixing and Restacking of Delaminated Layered Double Hydroxides. J. Phys. Chem. C 2008, 112, 16733–16739. [Google Scholar] [CrossRef]

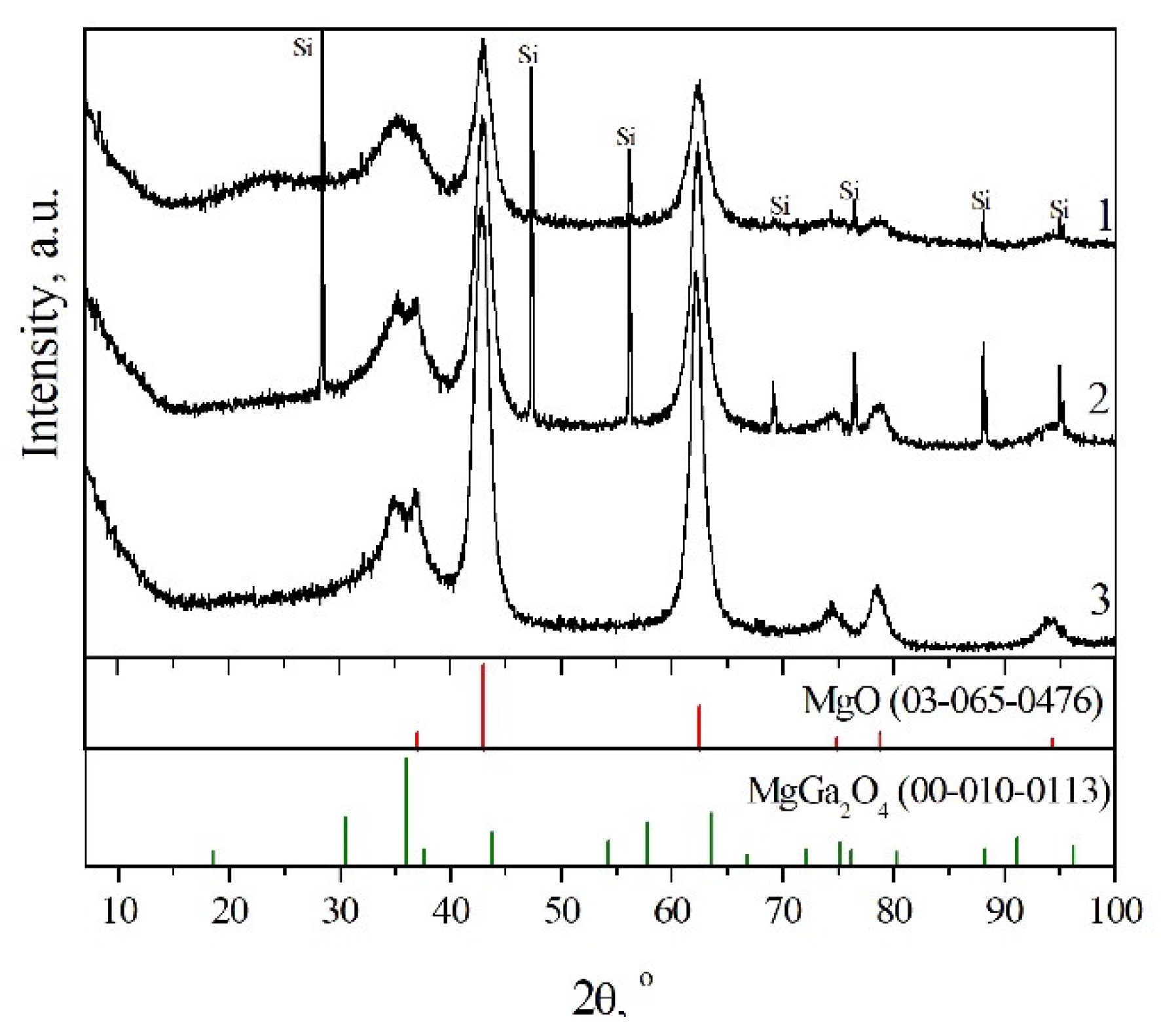
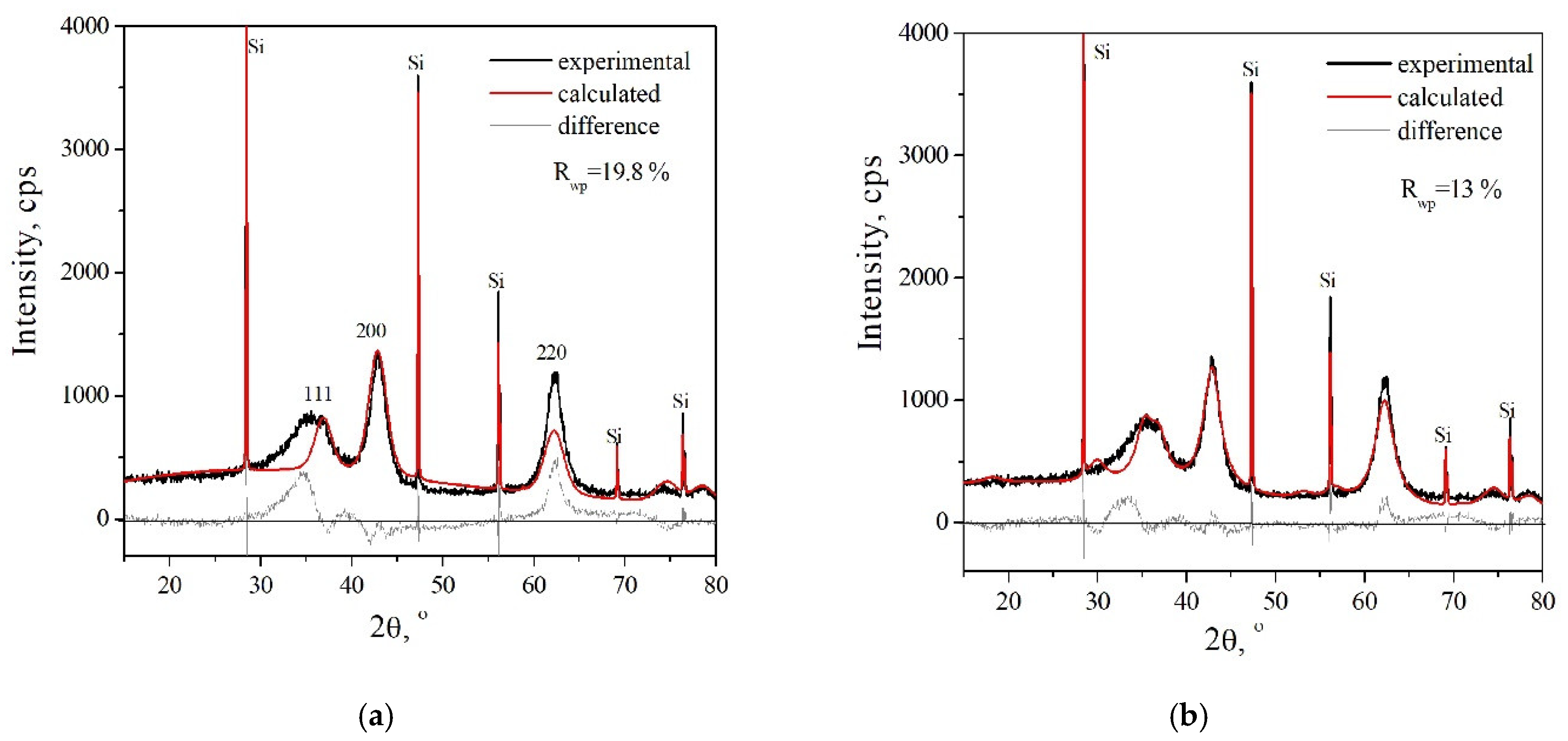
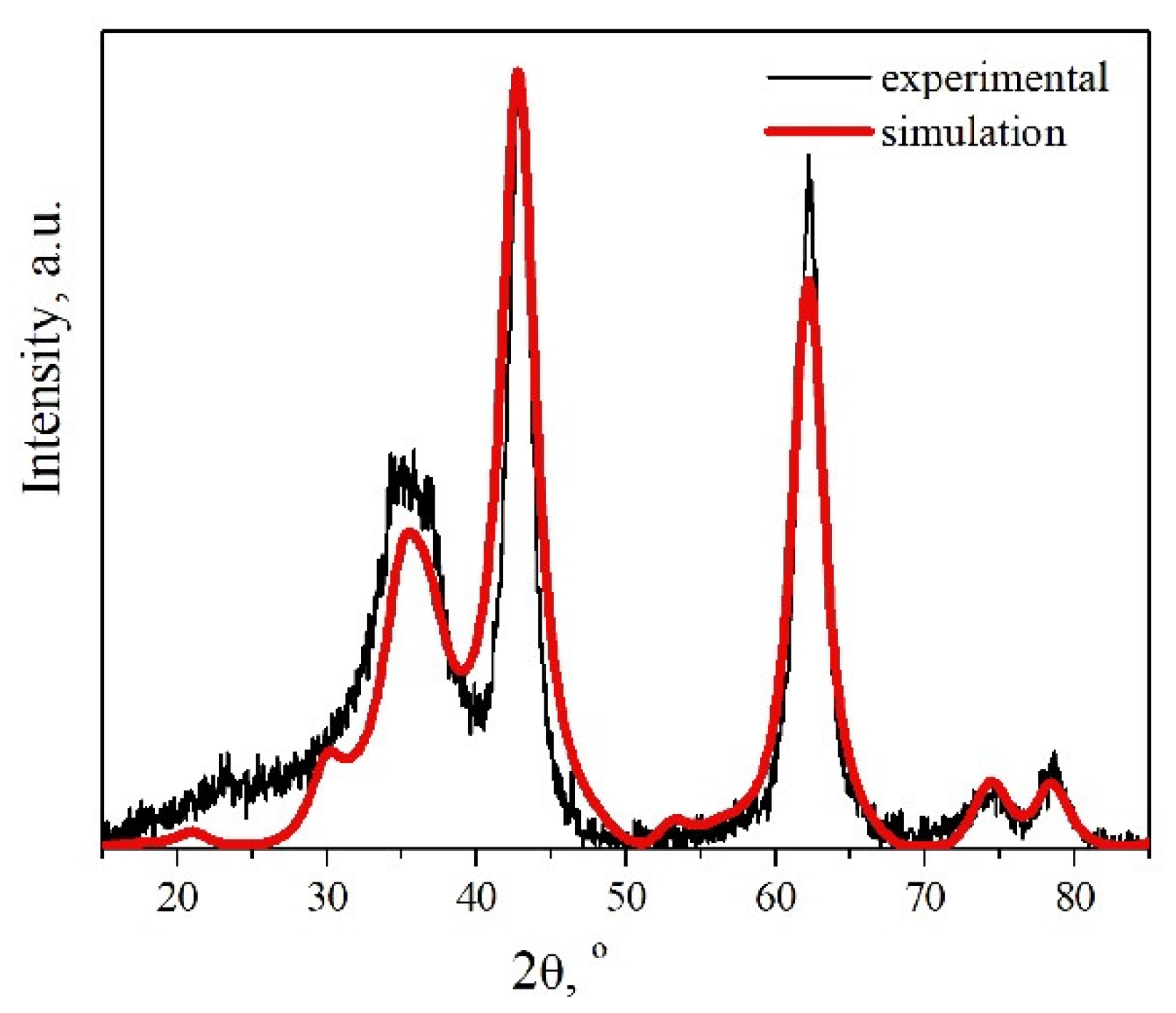
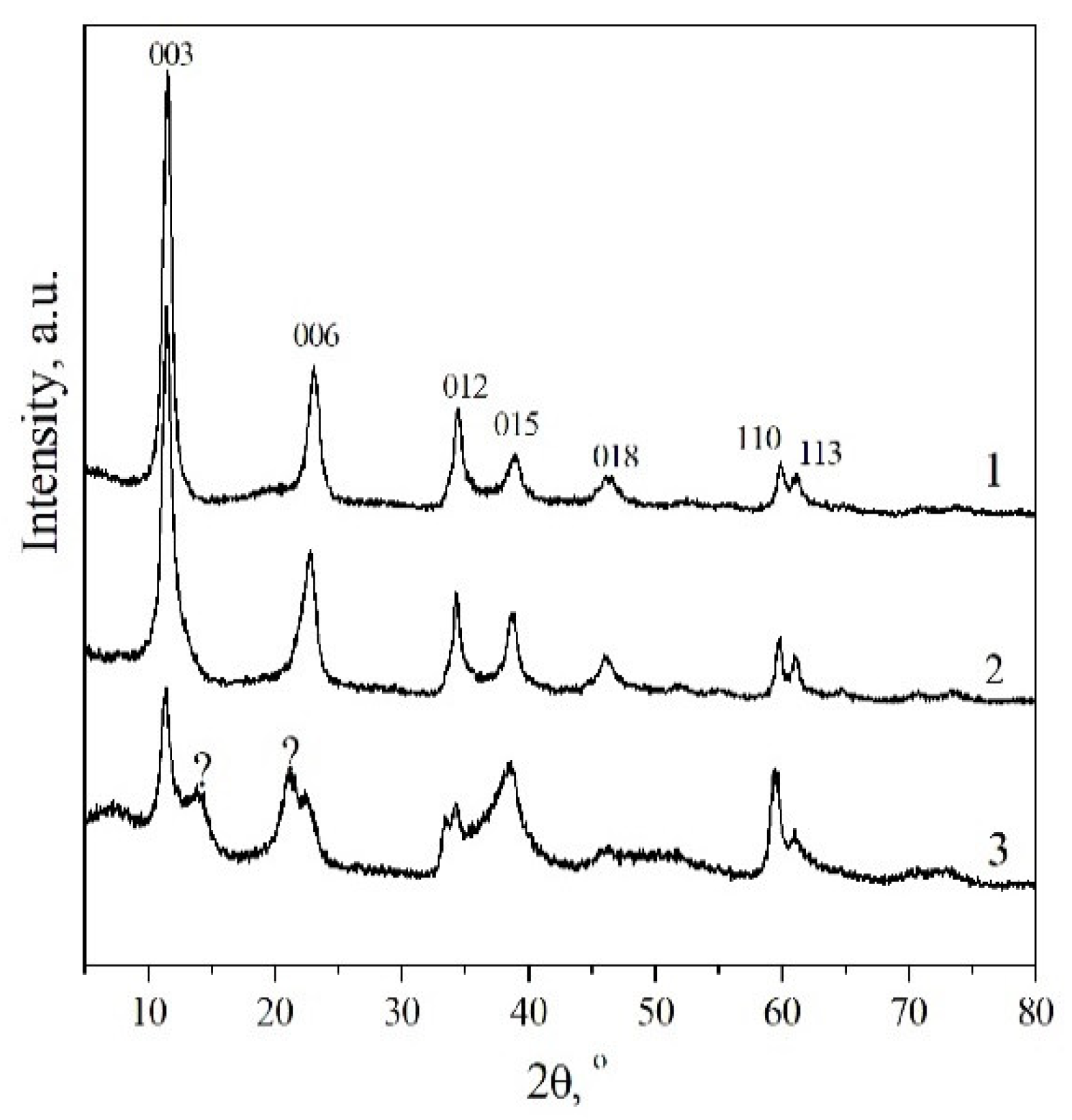
| Fraction of Mg2+ | Phase Composition | a, Å | c, Å | La, nm | Lc, nm |
|---|---|---|---|---|---|
| 0.67 | LDH | 3.087 | 22.88 | 37.1 | 56.5 |
| 0.75 | LDH | 3.093 | 23.33 | 51.5 | 91.3 |
| 0.80 | LDH | 3.104 | 23.81 | 26.6 | 24.1 |
| Fraction of Mg2+ | Position | Cation | Occupancy | Rwp, % |
|---|---|---|---|---|
| 0.67 | 8a | Ga3+ | 0.42 | 13 |
| 16c | Mg2+ | 0.93 | ||
| 16d | Mg2+ | 1.66 | ||
| 0.75 | 8a | Ga3+ | 0.33 | 13 |
| 16c | Mg2+ | 0.91 | ||
| 16d | Mg2+ | 1.55 | ||
| 0.80 | 8a | Ga3+ | 0.27 | 15 |
| 16c | Mg2+ | 0.91 | ||
| 16d | Mg2+ | 1.46 |
| Initial Mole Fraction of Mg2+ | Calculated Mole Fraction of Mg2+ | Phase Composition | c, Å | Lc, nm | , Å | La, nm | Lh, nm | Lb, nm |
|---|---|---|---|---|---|---|---|---|
| 0.67 | 0.69 | LDH | 23.17 | 13 | 3.088 | 16.3 | - | - |
| 0.75 | 0.74 | LDH | 23.56 | 8.8 | 3.094 | 19.3 | - | - |
| 0.80 | 0.84 | LDH | 23.63 | 9 | 3.108 | 14.5 | - | - |
| IS-phase | - | - | 1.2 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leont’eva, N.N.; Cherepanova, S.V.; Stepanova, L.N.; Drozdov, V.A.; Lavrenov, A.V. Structural Aspects of “Memory Effect” for MgGa LDHs: New Data Obtained by Simulation of XRD Patterns for 1D Disordered Crystals. Crystals 2022, 12, 629. https://doi.org/10.3390/cryst12050629
Leont’eva NN, Cherepanova SV, Stepanova LN, Drozdov VA, Lavrenov AV. Structural Aspects of “Memory Effect” for MgGa LDHs: New Data Obtained by Simulation of XRD Patterns for 1D Disordered Crystals. Crystals. 2022; 12(5):629. https://doi.org/10.3390/cryst12050629
Chicago/Turabian StyleLeont’eva, Natalia N., Svetlana V. Cherepanova, Liudmila N. Stepanova, Vladimir A. Drozdov, and Aleksandr V. Lavrenov. 2022. "Structural Aspects of “Memory Effect” for MgGa LDHs: New Data Obtained by Simulation of XRD Patterns for 1D Disordered Crystals" Crystals 12, no. 5: 629. https://doi.org/10.3390/cryst12050629
APA StyleLeont’eva, N. N., Cherepanova, S. V., Stepanova, L. N., Drozdov, V. A., & Lavrenov, A. V. (2022). Structural Aspects of “Memory Effect” for MgGa LDHs: New Data Obtained by Simulation of XRD Patterns for 1D Disordered Crystals. Crystals, 12(5), 629. https://doi.org/10.3390/cryst12050629







