Abstract
Perovskite Solar Cells are a promising solar energy harvesting technology due to their low cost and high-power conversion efficiency. A high-quality perovskite layer is fundamental for a highly efficient perovskite Solar Cell. Utilizing a gas quenching process (GQP) can eliminate the need for toxic, flammable, and expensive anti-solvents in the preparation of perovskite layers. It is a promising candidate technology for large scale preparation of perovskite layers, as it can be easily integrated in a production line by coupling up-scalable techniques. The GQP removes the need for polar solvents in the precursor solution layer by using nitrogen flow, rather than extracting them with non-polar solvents. The crystallization dynamics in this process can be significantly different. In this study, we found that the quality of perovskite crystal from GQP is much more sensitive to Lewis base molecules (LBMs) in the precursor solution than it is in anti-solvents technology. Thus, the processing parameters of the LBMs in anti-solvents technology cannot be directly transferred to the GQP. An XRD and 1H NMR study explains the origin of the S-shaped J–V curves and how these LBMs hinder the reaction between PbI2 and monovelent cations.
1. Introduction
The development of clean energy is considered to be one of the most important means of reducing the release of carbon dioxide from human activities [1,2]. Among clean energy technologies, solar cells have attracted much attention due to their wide distribution and renewable nature [3,4,5]. In less than 10 years of development, perovskite solar cells (PSCs) have achieved remarkable progress in power conversion efficiency (PCEs), which has increased to 25.8% in 2021 from 3.8% in 2009 [6,7,8,9,10]. Beside its compatible efficiency value compared to that of commercial solar cells based on monocrystalline silicon films, the low cost of production due to the abundant raw materials and low temperature coating process is another major advantage [11]. The outstanding performance of PSCs is mainly attributed to the photoactive layer made with perovskite materials, of which the generic formula can be described as ABX3, where A and B are monovalent cation and divalent cations, respectively, and X is a monovalent anion. Because of the fundamental role of the perovskite layer in PSCs, performance strongly depends on the quality of the perovskite layer, i.e., the coverage, thickness, and crystal grain size, etc. These properties are influenced by the composition of precursor solution and preparation processes [12,13,14,15]. Recently, the gas quenching process (GQP) has attracted more and more attention [16,17]. In the GQP, a nitrogen flow is used to remove the solvent in the perovskite precursor solution (PPS), rather than utilize anti-solvent as used in anti-solvent technology (AST). Compared to AST, a GQP is not only much more environmentally friendly and commercial, but also can be integrated in a production line by coupling up-scalable techniques such as slot-die coating or doctor blading [18,19,20,21]. In AST, it has been widely accepted that the Lewis base type molecules (LBMs) in the PPS strongly influence the quality of perovskite crystal layers [22,23]. Although LBMs also play similar important roles in a GQP, it is found that the formation of perovskite is quite different, and the processing parameters of the LBMs in AST cannot be simply transferred to the GQP. The anomalous unexpected S-shaped J–V curve, which reduces the fill factor (FF) significantly, can be easily formed in GQP using the same LBMs as in AST. Thus, the performance of p-i-n type PSCs from the GQP lags far behind (PCE < 17%), due to the poor understanding of the formation of perovskite in GQP. Considering the superior advantage of GQP, there is an urgent demand for the systematic study on how LBMs influence perovskite crystallization in the GQP [24,25,26].
In this study, two types of Lewis base type solvents (LBSs) and two types of Lewis base additives were investigated in a GQP. We found that too high of an amount or excessive alkalinity of the LBMs can hinder the reaction between PbX2 and FAI. It is also the cause of anomalous S-shaped J–V curves. Through investigation of the hydrogen bond with proton nuclear magnetic resonance (1H NMR), the way in which LBMs hinder the formation of perovskite crystal in GQP is determined. Based on this understanding of the function of the LBMs, we carefully controlled all the LBMs, and p-i-n devices having PCE over 18% without hysteresis were obtained by GQP.
2. Materials and Methods
Chemicals and Reagents: Lead iodide (PbI2, 99.9985%, ultra-dry and non-ultra-dry type), lead bromide (PbBr2, metal bias, ultra-dry) and thiourea (99%) were purchased from Alfa Aesar. The non-ultra-dry type lead iodide was further aged in the ambient environment (relative humidity was about 60%) for one week. Cesium iodide (CsI, metal bias), l-α-phosphatidylcholine (98%), poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA, average Mn 7,000~10,000), and all other solvents including N,N-dimethyl formamide (DMF, ultra-dry), dimethyl sulfoxide (DMSO, ultra-dry), N-methyl-2-pyrrolidone (NMP, ultra-dry) and sec-butanol (99%, ultra-dry) were sourced from Sigma-Aldrich(Shanghai, China). Formamidinium iodide (FAI) and methylamonium iodide (MAI) were purchased from Dyesol Ltd. (New South Wales, Australia). [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) is the product of American Dye Source Inc(Quebec, Canada). Aluminum-doped zinc oxide (AZO) has been deposited from a commercial nanoparticle dispersion (2.5 w.t.% in isopropanol, Prod. No. 8046, Avantama).
Solar cells fabrication: PTAA was deposited on the clean indium tin oxide (ITO) glass by spin-coating the PTAA solution (4.0 mg mL−1) at 6000 rpm for 20 s, then annealed at 100 °C for 5–10 min. For trication of the perovskite layer, Thiourea (6.6 mg), PbI2 (532.3 mg), PbBr2 (98.5 mg), MAI (31 mg) and FAI (190 mg) were dissolved in DMF containing 0.2 wt.% of l-α-phosphatidylcholine. The required amount of CsI was added as DMF or DMSO solution. Then the proper amount of DMSO or NMP was added to make sure that the total amount of LBS in the solution was the specific amount which had been designed before. The volume of all solvents should be 1.0 mL. The precursor solution was finally obtained by sonicating the mixture to dissolve all solids and filtrating through a polytetrafluoroethylene microfiltration membrane (caution: do not heat the precursor solution). 45 µL of precursor solution was spin-coated on the top of the PTAA coated ITO substrate at spin rate of 6000 rpm for 20 s (with extra 8 s of ramp time). The nitrogen purging should be started in the final 15 s and stopped immediately upon color-change of the intermediate layer. The distance between the nozzle and intermediate perovskite layer is within 3.0 cm. The intermediate layers were annealed for 10 min at 110 °C to get the perovskite layer. 50.0 mg of PCBM was mixed into 1.0 mL chlorobenzene and filtered through a polytetrafluoroethylene microfiltration membrane (pore size: 0.2 μm) to remove the insoluble solids. 20 μL of the PCBM solution and 12 μL of AZO nanoparticle dispersion were spin-coated on the perovskite layer in sequence. Further annealing at 85 °C for 30 min was carried out to remove the solvents and ligands in AZO. Finally, 100 nm of Al was evaporated under high vacuum (<1 × 10−6 Pa). The device area was defined by the shadow mask as 0.06 cm2.
Measurement and Characterization: X-ray diffraction (XRD) patterns of the films were measured with a Bruker QUANTAX 200 diffractometer. 1H NMR of all acetonitrile-D3 solutions containing 10 mg AI salts and equivalent related LBM were recorded by Bruker BioSpin3.5. The 1H NMR spectra of the solutions were recorded under same conditions. The SEM images were obtained with Neon 40 (Zeiss). J–V curves were measured with Keithley 2400-C source meter and a solar simulator (300 W Newport, model91160, AM1.5G, 100 mW cm−2). EQE has been measured with a home-built tunable light source, calibrated with a power meter (Thorlabs). The spectral mismatch factor was 1.163.
3. Results and Discussion
Figure 1a,b shows the related composition in the PPS of GQP and the p-i-n device architecture in this study. First, we studied how the LBSs influence the device performances. Cs0.05(FA0.85MA0.15)0.95Pb(I0.85Br0.15)3 PPS containing DMF and LBSs. The molar ratio between LBS and A cation is noted as X here. In addition, thiourea additive (molar ratio is 0.06) is applied in all groups if no more declaration is given.
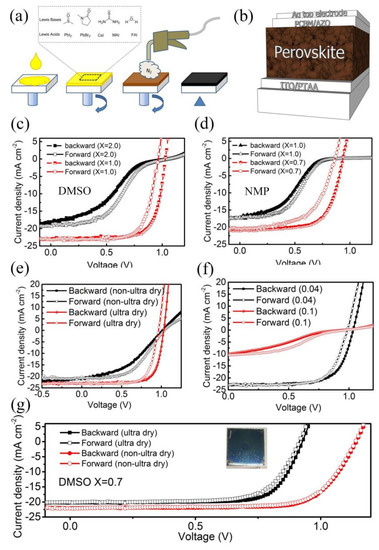
Figure 1.
(a) The gas quenching process for preparation of perovskite layer. The insets show the related chemicals in the PPSs. (b) The device architecture studied in this work. (c,d) The J–V curves of devices based on perovskite from different PPSs where X is the molar ratio between LBS and A cation. (e) J–V curves of devices prepared with ultra-dry and non-ultra-dry lead halides (DMSO/A = 1.0). (f) Shows how the thiourea additive influences the J–V curves. The number is the molar ratio between thiourea and A cation. (g) J–V curves of devices prepared with ultra-dry and non-ultra-dry lead halides (DMSO/A = 0.7). Ultra-dry type lead source, molar ratio of DMSO/A = 1.0, or 0.06 molar ratio of thiourea were applied to all PPSs except additional explanation.
The summary of the device performance is given in Table 1. When DMSO was used as the LBS in trication of perovskite based solar cells, typical J–V curves were obtained with X = 1, but S-shaped J–V curves were observed when X = 2, which is widely applied in preparation of FA based perovskite with AST [27,28,29], and no anomalous S-shaped J–V curve has been found [Figure S1]. Recent reports even suggest increasing the X to 3 to obtain an outstanding power conversion efficiency from AST [30]. The performance of the devices when X equals 1.0 is similar to previous reports [21]. Although equivalent ratio of NMP to A cations was applied, we found strong S-shaped J–V curves in GQP. The NMP group gave S-shaped J–V curves when X = 1 [see Figure 1d]. Only when the molar ratio of NMP and A cation decreased lower than 0.7, the S-shape of the J–V curve was prevented [see Figure 1d and Figure S2]. It is obvious that the formation of perovskite layer in the GQP is different from that in AST. Interestingly, when NMP was used as LBS for preparation of Cs0.5MA0.95PbI3 type perovskite with a GQP, the NMP/A molar ratio can be increased to 2.0 without observation of S-shaped J–V curves (see Figure S3). Compared to trication perovskite material studied here, the only difference is the monovalent organic cations (FA+) and minor bromide anions were replaced by MA+ and iodide anions, respectively. Because Pb2+ is the same for both cases, and anions do not bind to LBS molecules, it indicates that the S-shaped J–V curves of trication of perovskite-based devices mainly arises from the interaction between LBS and monovalent organic cations rather than that between LBS and Pb halides, or LBS and Cs+.

Table 1.
J–V properties of the champion devices with forward and backward scan directions.
Beside the Lewis base solvents, water and Lewis base additives have been also considered. Water has lone pair electrons which can combine with the Lewis acid. Water can be introduced into the PPS from the raw materials and environment during the preparation process. Figure 1e shows the J–V curves of solar cells prepared with different Pb sources, which are ultra-dry type or non-ultra-dry reagents at the same purity level. As claimed by the supplier, the only difference between them is that they are packed in either an inert or ambient environment. Strong S-shaped J–V curves were observed when a non-ultra-dry Pb source was used, while the other one gave good J–V curves. It indicates that the minor water absorbed by raw material can strongly influence the crystallization process. But we realize that the reportedly highly efficient solar cells have not necessarily used an ultra-dry Pb source. It should be attributed to their devices being based on methylammonium halide type perovskite, higher annealing temperature, or solvent anealling was applied in these processes. The relatively weak S-shaped J–V curves of devices with trication perovskite can be inhibited by a higher annealing temperature, or post solvent annealing technology [see Figures S4 and S5]. The raw materials can further react after the post treatments. Lewis additives have been successfully developed to tune the crystallization process [24,25,26,31]. Here, thiourea as a representative additive was studied. When the thiourea is less than 0.06, the short circuit current will increase with the increase of thiourea. It can be easily found out in Figure 1c,e,f. But if it is increased to 0.09 (10 mg), the strong S-shaped J–V curve is found, and the current density drops a lot at the same time.
We presumed that water and thiourea hinder the formation of perovskite in a similar mechanism like LBSs, and the S-shape of J–V curves in devices prepared from non-ultra-dry lead source can be prevented by decreasing the total amount of LBMs. Thus, we decreased the amount of DMSO and eliminated the thiourea additive to decrease the total amount of LBMs. As expected, when we eliminated the thiourea additive and decreased the molar ratio of DMSO and A cation to 0.7, the strong S-shape of J–V curve was prevented completely [see Figure 1g], verifying the hypothesis. It is surprising that the hysteresis also disappeared in the non-ultra-dry lead source group, and was strongly inhibited in the ultra-dry lead source group [see the inset of Figure 1g]. Here, the open circuit voltage of the ultra-dry lead source group is much lower than that of the non-ultra-dry lead source group, due to the charge recombination from the poor coverage of PCBM on the former perovskite layer, and defects in the perovskite. For this low DMSO containing group, although S-shaped J–V curves and strong hysteresis were not found when the molar ratio of thiourea was increased to 0.1, the current density decreased a lot (see Figure S6). This trend is consistent with that of the low NMP molar ratio group [Figure S3b]. The thiourea additive in PPS made from a non-ultra-dry lead source could not further improve the device performance either, when only 0.7 mole equivalent of DMSO is applied (see Figure S8). The J–V curves and historical results for devices from the non-ultra-dry lead source with and without thiourea additive were given in Figure S7. When another bottle of non-ultra-dry PbI2 opened in the glove box was used, the best DMSO mole ratio changed to 0.9 (see Figure S8). The SEM images of the perovskite layers from ultra-dry and non-ultra-dry PbI2 using DMSO as LBM (X = 0.9) are presented in Figure S9. These results mean there is a synergistic effect from LBS and LBM additives, which jointly influence the device performance. The detailed mechanism of S-shape and hysteresis of J–V curves will be discussed below. The Rs and Rsh of devices with different perovskite layers were calculated from the related J–V curves and listed in Table S1. The devices having S-shaped J–V curves gave the abnormally high series resistance and the lowest short resistance. It indicates that some unwanted insulative materials are formed in these layers.
To reveal the origin of the of S-shaped J–V curves, SEM and XRD measurement were carried out to analyze the typical perovskite layers from different PPSs. When DMSO was used as LBS and X = 1.0, the perovskite layer is uniform and the crystal grain size is about 1.0 μm [Figure 2a]. But if the lead source is the non-ultra-dry type and same amount of thiourea additive is used, some obvious different materials (whitish particles) are found on the perovskite surface [Figure 2b]. When NMP was used as the LBS [Figure 2c,d], only a few whitish grains were found on the perovskite surface in the SEM images, but the crystal sizes decrease to smaller than 500 nm. This illustrates why the current density of the device built by perovskite from PPS with lower molar ratio is lower [Figure S2b], because of the perovskite layer with bigger crystals usually gives higher current density [32,33,34]. Solvent annealing is a very effective technology for preparation of large grain sized perovskite layers [35,36,37,38]. Although the application of DMSO vapor annealing did increase the crystal size to near 2.0 μm [Figure 2e], there were some unexpected rod-like materials different from perovskite formed. Similarly, too much thiourea in PPS can lead to the formation of unwanted phase. XRD is an efficient and powerful tool to investigate the phase purity of the materials. In Figure 2g, diffraction peaks at 12.6° and 38.6° belonging to PbI2 were not observed, when the perovskite layer prepared from a PPS with DMSO/A molar ratio of 1.0 and ultra-dry lead source. But those intense peaks assigned to PbI2 are observed in the diffractogram of the perovskite layer prepared from a PPS with too high of an NMP/A molar ratio or non-ultra-dry lead source. For the group with DMSO/A molar ratio = 2.0 or molar ratio of thiourea additive up to 0.1, the diffraction signal of pure PbI2 was not observed, but a signal around 10.0° assigned to PbI2(DMSO)2 complex phase was found (Figure S10) [26,39,40,41]. The strong peaks from PbI2 or its complex indicate the PbI2 could not react properly in these cases, the molar ratio of monovalent salts and PbI2 are same for them though. It is easy to notice that all of the devices giving S-shaped J–V curves are built with perovskite layers containing much unconsumed PbI2 or the PbI2(DMSO)2 impurity. For NMP, the peak at 12.6° was observed but very weak when X decreased to 0.35, and the related devices gave normal J–V curves. It means a few unreacted PbI2 cannot lead to S-shaped J–V curves, which is consistent with the previous reports [13].
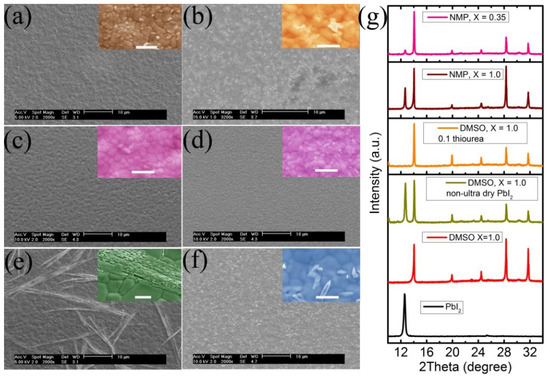
Figure 2.
SEM images of perovskite obtained from PPS with (a) DMSO, X = 1.0, (b) DMSO, X = 1.0 (non-ultra-dry lead source). (c) NMP, X = 0.35, (d) NMP, X = 1.0, (e) NMP, X = 1.0, solvent annealing (f) DMSO, X = 1.0, thiourea molar ratio = 0.1. All scale bars for insets represent 1.0 μm. (g) X-ray diffractograms of PbI2, and Cs0.05(FA0.85MA0.15)0.95Pb(I0.85Br0.15)3 from PPSs with different LBM/A molar ratio and lead sources.
To further confirm that the S-shaped J–V curves originate from the insulative FA salts and PdI2, three groups of devices were built with perovskite layers from PPS with different PbI2. In Figure S11, regardless of whether too much more or less PbI2 was used, S-shaped J–V curves were observed. However, less PbI2 (−1.5%) was used (equals to excess unreacted FAI left in perovskite layer), only the Rs increased to 14.6 Ω·cm−2, which is 10 times higher than that of the control device. To confirm that the unreacted FA salts in the perovskite layer can give S-shaped J–V curves, the perovskite layer was treated with an FA salts isopropanol solution to introduce excess FA salts. The S-shaped J–V curves appeared again as expected (see Figure S12).
In the anti-solvent process [40,42], for FAPbI3 only or CsFAPbI3 type of perovskite solar cell, no S-shaped curve was observed when the NMP/A molar ratio was 1.0. This should be attributed to the different crystallization dynamic and higher annealing temperature applied. The increased annealing temperature facilitates the further reaction of A+ cation and PdI2 in solid state. But for Cs0.05(FA0.85MA0.15)0.95Pb(I0.85Br0.15)3 perovskite, increasing the annealing temperature is not a good option to solve the problem. In Figure S5b, when the increased annealing temperature was applied, the relative intensity of diffraction peaks at 12.6°, and 38.6° of PbI2 caused a significant decrease, but could not be eliminated completely. It means that there is much unreacted PbI2 remaining in the perovskite layer. The PbI2 and A cations can react further under the increased annealing temperature, but the methylammonium salt is not thermally stable enough. Part of MA based perovskite can transform into PbI2 in our operation environment at high temperature. This is consistent with the previous reports on perovskite containing methylammonium constituents [43,44,45]. It is worth noting that the MAI based perovskite is stable at 200 °C under TGA analysis condition [46,47,48]. The discrepancy should mainly be attributed to the fact that the annealing atmosphere environment of the preparation process for the perovskite layer is different from the inert gas flow in the TGA measurement. For device preparation, a perovskite layer was obtained and annealed in a more complicated environment containing polar solvent vapor, such as DMSO, DMF, and water, in glove box rather than the relatively ideal inert environment of TGA. The exposure of MAI based perovskite to these polar solvent vapors will lead to partial decomposition when the annealing temperature is higher than 110 °C. Figure S5a shows the J–V curves of solar cells with perovskite layers obtained under different annealing temperatures. Obviously, the strong S-shaped of J–V curves disappeared, and the Rs decreased to 1.19 kΩ·cm−2 from 11.5 Ω·cm−2 when the annealing temperature increased from 110 to 150 °C. However, the Rs is still much higher than that of the control devices from DMSO group in Table S1. The higher annealing temperature decreased the short circuit current intensity, the FF increased to more than 65% from less than 30% of lower annealing temperature group though. Based on the discussion above, we attribute these results to the following reasons: (1) higher temperature decreased the amount of unreacted PbI2 and A cations; (2) the decomposition of MAI leads to that the composition of photoactive layer deviates from the state-of-the-art composition. Thus, a higher efficiency device can hardly be prepared using a perovskite layer with too much unreacted PbI2 and A cations through increasing annealing temperature in this case.
Based on discussion above, the origin of S-shaped J–V curves can be illustrated in Figure 3. Here, in the present cases where excess LBMs are used, lots of unreacted PdI2, PdI2(DMSO)2 complex, and monovalent salt remain in the perovskite layer [Figure 3a]. As the conduction band energy level of PbI2 is much higher than that of perovskite, an unwanted diode is formed between perovskite and PdI2 [Figure 3b]. Excitons cannot transport through these areas, due to the charge transport barrier between perovskite and PdI2. Further, the unreacted AI is insulative and dramatically increases the charge transport resistance. Theoretical investigation suggests that the high injection barrier in the device could be one of the main reasons for the unwanted S-shaped J–V curves [49]. Based upon the discussion above, the S-shaped J–V curves originate from the incomplete reaction between Pb source and A halides. According to previous research, in a one-step method for preparing perovskite, (A+)3Pb3I93-·S2, where S is solvent, is the key intermediate. Perovskite cannot be formed if this intermediate is not prepared properly [50,51]. However, how the chemical environment influencing the formation of this key intermediate have not been well understood.
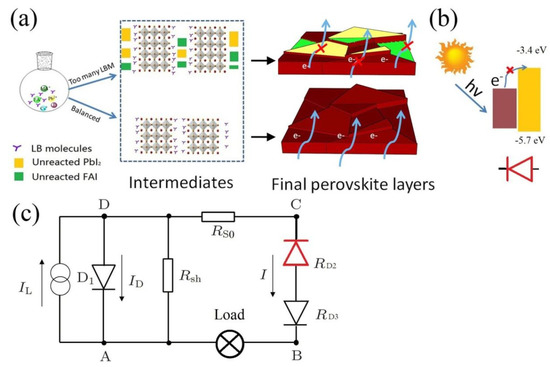
Figure 3.
(a) Schematic illustration of how Lewis base molecules influence the formation of perovskite. (b) Band energy level alignment between Perovskite layer and PbI2. (c) Equivalent circuit of solar cell.
As discussed above, the device performance is mainly influenced by the interaction between LBM and A cations in PPS. We carried out study on this interaction. As illustrated in route (a) Figure 4, no matter the formation of INT1 or INT2, the involvement of a specific amount of A+ and I− is necessary. Obviously, when the concentration of A+ and I− is too low, these intermediates cannot be formed properly. A+ cations are typical Lewis acids which can strongly interact with Lewis bases. Thus, there is a dynamic equilibrium between free A+ cations and Lewis base complex [A·LBM]+. Dissociation constant (Kd) can be used to describe the relationship between the concentration of free ions and complex.
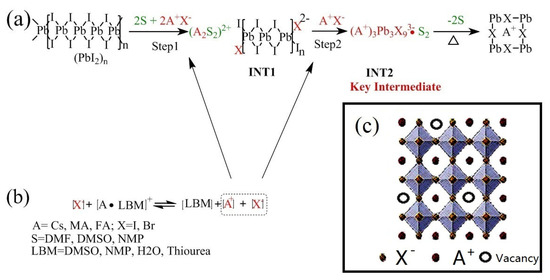
Figure 4.
Schematic illustration of how LBMs in the PPS influence the formation of perovskite. (a) is the route of the formation of intermediates and perovskite. (b) Equilibrium concentrations of the related species in PPS. (c) The vacancies of monovalent cation in the perovskite due to the incomplete reaction.
The concentration of free A+ cations depends on the binding energy of [A·LBM]+. Stronger binding energy of the complex in the PPS will provide lower concentrations of free A+ cations. When the concentration of free A+ cations is too low in the PPS, there will be, of course, a lot of unreacted lead iodide left in the perovskite layer, and unexpected inversed diodes will form in the final devices to give S-shaped J–V curves. When excessive LBMs are used in Figure 1f, even though there is not too much lead iodide left to form inversed diodes, the incomplete reaction of A+ cations will form lots of cation vacancies in the perovskite [see Figure 3c], which is one of the reasons for hysteresis and decreased open circuit voltage [52]. Here, the complexes [A·LBM]+ were formed by hydrogen bond, which can be easily studied by proton nuclear magnetic resonance (1H NMR). Thus 1H NMR measurement was carried out to confirm the illustration above.
The acetonitrile-D3 was used as the solvent for 1H NMR measurement due to its high polarity to solve AI salts weak Lewis basicity. The 1H NMR of pure FAI and MAI are presented in Figure 5a,b, respectively. All peaks in 1H NMR have been assigned to the related protons on the molecules. The protons labeled as Ha are the active protons, which form the hydrogen bonds with other LBMs. The related peaks of Ha in 1H NMR are broad peaks located around 7.96 ppm and 6.11 ppm for FAI and MAI, respectively. To investigate the interaction between AI and various LBMs, one equivalent of related LBM was added to the AI solution. Although the solution containing water was also measured, the signals of Ha in 1H NMR disappeared because of the speed of proton exchange between AI and H2O [see Figure S20]. The changes of the peaks of Ha in 1H NMR can be used to estimate the concentration changes of free AI, due to the observed chemical shift δobs can be described below:
where XA and XA•LBM are mole fraction of A+ and A•LBM complex in the equilibrium mixture, δA and δA•LBM are chemical shifts of free A+ and A•LBM complex in the solution, respectively [53]. As acetonitrile is the weakest Lewis base among them, the solution has the highest mole fraction of A+. Here AI salt acetonitrile solutions are noted as ‘free’, there is a weak hydrogen bond between A+ and acetonitrile though. After adding other LBMs, the A•LBM complex is formed and the chemical shift in 1H NMR will move to the direction of A•LBM complex. This process can be illustrated in Figure 5c. The δobs can move to lower field or higher field after adding LBMs compared to pure AI. The direction of the movements depends upon the type of the hydrogen bond. Short hydrogen bonds (HB-S) are correlated with low-field shifts, larger bond lengths (HB-L) with shifts to higher field [54]. The distance between δobs and δA notes as △δ. No matter what direction δobs moves, |△δ| is proportional to the binding energy, Ka and concentration of A•LBM [55]. Because
δobs = XAδA + XA•LBMδA•LBM
XA + XA•LBM = 1
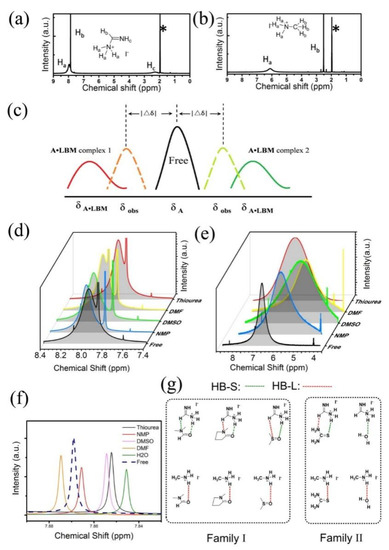
Figure 5.
(a,b) The 1H NMR of pure FAI and MAI in acetonitrile-D3, respectively. The different peaks are also assigned to the labeled hydrogen atoms of the related molecules. The peaks marked with * are attributed to deuterium solvent. (c,d) Changes of chemical shift of active hydrogen atoms (Ha) on FAI and MAI with equivalent various LBM in acetonitrile-D3, respectively. Note: due to the overlapping of signals from active hydrogen atoms of MAI and thiourea, the peak in (e) for adding the thiourea group is separated from normalized results [see Figure S13]. (f) Change of chemical shift of Hb on FAI with equivalent various LBM in acetonitrile-D3, respectively. (g) Different hydrogen bonds between AI and various LBM. Different type of hydrogen bonds were noted with different colors: HB-S (green) and HB-L (red).
Formula (2) can be changed into:
δobs = XA(δA − δA•LBM) + δA•LBM
As the δA•LBM mainly depends upon the hydrogen donor [54], δA•LBM is similar for same kind of AI. It is obvious that the smaller XA is, the father the δobs deviates from the δA, thus:
|△δ| ∝ bingding energy ∝ [A•LBM] ∝ 1/Kd ∝ 1/[A]
In Figure 5d, the peaks of Ha of FAI in the solutions containing DMF, DMSO and NMP move to the lower field, but that of the solutions containing thiourea moves to a higher field. The shifts of these peaks for both FAI and MAI are listed in Table 2. It means the hydrogen bond between FAI and thiourea is different from those between FAI and other LBMs. For FAI, there is such an order: |△δ|NMP > |△δ|DMSO > |△δ|DMF > |△δ|thiourea. According to formula (5), the binding energy between FAI and NMP is stronger than the remaining molecule pairs. The solution using NMP has the lowest concentration of A+, which is critical for the formation of the key intermediate as discussed above. It explains why NMP can strongly hinder the reaction between FAI and PbI2. It also indicates that hysteresis depends upon the amount of LBMs in the PPS. Excess LBMs in the PPS can prevent the reactivity of A cations and leave some vacancies in the final perovskite layer, which has proved to be the origin of hysteresis [52].

Table 2.
Chemical shift of Ha of FAI and MAI in different solutions.
Surprisingly, the δobs of MAI upfield shifts when LBMs are added [Figure 5e]. It means the hydrogen bonds between MAI and LBMs are longer than those hydrogen bonds between FAI and LBMs. Besides, |△δ|DMSO is the largest and |△δ|NMP is the smallest in LBSs (even weaker than DMF, see Table 2). The order of |△δ| for FAI and MAI inversed. It means the binding energy between MAI and NMP is the weakest in all LBSs discussed here. Thus, the use of excess NMP in Cs0.5MA10PbI3 type PPS will not decrease the concentration of MA+ cations, and hinders the formation of key intermediates and perovskite (Figure S3). It also explained why the molar ratio of DMSO needs to be carefully controlled in MAI based perovskite [22]. Actually, theoretical study has found that the DMSO has a stronger interaction energy (−1.391 eV) with MA cations than NMP (−1.210 eV) [42]. The present experimental study shows the decreased binding energy in the MA·NMP complex should be attributed to the weak hydrogen bond between MA+ and NMP.
In Figure 5f, it is found that the 1H NMR signals assigned to Hb in various FAI acetonitrile solutions were also influenced by the different LBMs. This influence should not be attributed to an inductive effect from the hydrogen bond of Ha. Because the 1H NMR signals of Hb of MAI are not influenced at all, its Ha can form strong hydrogen bond though [See Figures S20–S26]. This means there is an extra hydrogen bond between Hb and LBMs for FA. We believe that this extra hydrogen bond also enhances the binding energy between FA and LBM. All of the hydrogen bonds interaction between AI and LBMs were presented in Figure 5g.
4. Conclusions
In conclusion, S-shaped J–V curves of PSCs originate from the high resistance and diodes formed by unreacted precursors. The incomplete reaction of precursors should be attributed to the binding between LBS and monovalent organic cations, rather than that between LBS and Pb halides, or LBS and Cs+. Then, incomplete reactions between lead halides and monovalent organic cations can also result in the vacancies of monovalent organic cations in the perovskite and strong hysteresis. Thus, the non-valent bond needs to be manipulated carefully to get S-shape free PSCs. The hydrogen bonds varied due to the different monovalent organic cations. The hydrogen bond in the complex MA·NMP is much weaker than that in FA·NMP and MA·DMSO. The chemical shift movement △δ of monovalent salt originates from solvents can be used to evaluate the interaction strength between LBS and monovalent organic cations. When the difference of △δ induced by the main solvent and the additive is larger than 0.03 ppm, controlling their ratio is necessary to avoid the S-shaped J–V curves. This work has important value for further understanding of perovskite crystal growth process and its influence on device performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12050610/s1.
Author Contributions
Conceptualization, L.Z. (Lei Zhao); Data curation, Y.C.; Investigation, L.Y. and J.L.; Project administration, H.Z.; Software, Y.S.; Writing—original draft, L.Z. (Lin Zhang); Writing—review & editing, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (12064050); grant from Key Laboratory for Crop Production and Smart Agriculture of Yunnan Province; the Major Project of Science and Technology of Yunnan Province under Grant (202002AE090010); Agricultural Union Youth Program of Yunnan Provincial Science and Technology Department (2018FG001-102).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (12064050); grant from Key Laboratory for Crop Production and Smart Agriculture of Yunnan Province; the Major Project of Science and Technology of Yunnan Province under Grant (202002AE090010); Agricultural Union Youth Program of Yunnan Provincial Science and Technology Department (2018FG001-102).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Renno, C.; Perone, A. Energy and economic analysis of a point-focus concentrating photovoltaic system when its installation site varies. Front. Energy 2021, 15, 384. [Google Scholar] [CrossRef]
- Rzetelska, D.; Combrinck, M. Fuel poverty and low carbon emissions: A comparative study of the feasibility of the hybrid renewable energy systems incorporating combined heat and power technology. Front. Energy 2021, 1–21. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, S.; Yuan, Y.; Li, G.; Wang, Q. A thermoelectric generator and water-cooling assisted high conversion efficiency polycrystalline silicon photovoltaic system. Front. Energy 2021, 15, 358. [Google Scholar] [CrossRef]
- Li, Q.; Yu, J.; Zang, Y.; Wang, N.; Jiang, Y. Enhancement of open circuit voltage in organic solar cells by doping a fluorescent red dye. Front. Energy 2012, 6, 179. [Google Scholar] [CrossRef]
- Meng, F.; Liu, J.; Shen, L.; Shi, J.; Han, A.; Zhang, L.; Liu, Y.; Yu, J.; Zhang, J.; Zhou, R.; et al. High-quality industrial n-type silicon wafers with an efficiency of over 23% for Si heterojunction solar cells. Front. Energy 2017, 11, 78. [Google Scholar] [CrossRef]
- NREL Best Research-Cell Effciences Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 16 March 2022).
- Li, Y.; Wu, H.; Qi, W.; Zhou, X.; Li, J.; Cheng, J.; Zhao, Y.; Li, Y.; Zhang, X. Passivation of defects in perovskite solar cell: From a chemistry point of view. Nano Energy 2020, 77, 105237. [Google Scholar] [CrossRef]
- Chi, W.; Banerjee, S.K. Progress in Materials Development for the Rapid Efficiency Advancement of Perovskite Solar Cells. Small 2020, 16, 1907531. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Roy, A.; Mallick, T.K.; Sundaram, S. Impact of different light induced effect on organic hole-transporting layer in perovskite solar cells. Mater. Lett. 2020, 268, 127568. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Cost-Performance Analysis of Perovskite Solar Modules. Adv. Sci. 2017, 4, 1600269. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2017, 2, 17102. [Google Scholar] [CrossRef]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.-D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wu, C.-G. A Method for the Preparation of Highly Oriented MAPbI3 Crystallites for High-Efficiency Perovskite Solar Cells to Achieve an 86% Fill Factor. ACS Nano 2018, 12, 10355. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chu, Y.; Sun, Y.; Zhang, R.; Li, J.; Zhao, L.; Zhao, H.; Liu, P.; Li, S. Beyond the Limit of Goldschmidt Tolerance Factor: Crystal Surface Engineering to Boost the α-Phase Stability of Formamidinium-Only Hybrid Inorganic–Organic Perovskites. Sol. RRL 2021, 5, 2100188. [Google Scholar] [CrossRef]
- Brinkmann, K.O.; He, J.; Schubert, F.; Malerczyk, J.; Kreusel, C.; van gen Hassend, F.; Weber, S.; Song, J.; Qu, J.; Riedl, T. Extremely Robust Gas-Quenching Deposition of Halide Perovskites on Top of Hydrophobic Hole Transport Materials for Inverted (p–i–n) Solar Cells by Targeting the Precursor Wetting Issue. ACS Appl. Mater. Inter. 2019, 11, 40172. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, F.; Hou, T.; Sun, X.; Yu, H.; Zhang, M. A Review on Gas-Quenching Technique for Efficient Perovskite Solar Cells. Sol. RRL 2021, 5, 2100386. [Google Scholar] [CrossRef]
- Hwang, K.; Jung, Y.-S.; Heo, Y.-J.; Scholes, F.H.; Watkins, S.E.; Subbiah, J.; Jones, D.J.; Kim, D.-Y.; Vak, D. Toward Large Scale Roll-to-Roll Production of Fully Printed Perovskite Solar Cells. Adv. Mater. 2015, 27, 1241. [Google Scholar] [CrossRef]
- Babayigit, A.; D’Haen, J.; Boyen, H.-G.; Conings, B. Gas Quenching for Perovskite Thin Film Deposition. Joule 2018, 2, 1205. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-S.; Hwang, K.; Heo, Y.-J.; Kim, J.-E.; Lee, D.; Lee, C.-H.; Joh, H.-I.; Yeo, J.-S.; Kim, D.-Y. One-Step Printable Perovskite Films Fabricated under Ambient Conditions for Efficient and Reproducible Solar Cells. ACS Appl. Mater. Inter. 2017, 9, 27832. [Google Scholar] [CrossRef]
- Conings, B.; Babayigit, A.; Klug, M.T.; Bai, S.; Gauquelin, N.; Sakai, N.; Wang, J.T.-W.; Verbeeck, J.; Boyen, H.-G.; Snaith, H.J. A Universal Deposition Protocol for Planar Heterojunction Solar Cells with High Efficiency Based on Hybrid Lead Halide Perovskite Families. Adv. Mater. 2016, 28, 10701. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xu, W.; Ran, G.; Li, Y.; Qin, G.G. Enhancing performance of flexible perovskite solar cell by secondary methimazole treating. Mater. Lett. 2021, 290, 129461. [Google Scholar] [CrossRef]
- Fei, C.; Li, B.; Zhang, R.; Fu, H.; Tian, J.; Cao, G. Highly Efficient and Stable Perovskite Solar Cells Based on Monolithically Grained CH3NH3PbI3 Film. Adv. Energy Mater. 2017, 7, 1602017. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, F.; Liu, H.; Li, X.; Xiao, Y.; Wang, S. Tuning the crystal growth of perovskite thin-films by adding the 2-pyridylthiourea additive for highly efficient and stable solar cells prepared in ambient air. J. Mater. Chem. A 2017, 5, 13448. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Y.; Zhang, P.; Shi, J.; Zhao, Y.; Zhang, H.; Wu, J.; Luo, Y.; Li, D.; Meng, Q. Investigation on the role of Lewis bases in the ripening process of perovskite films for highly efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 20874. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhou, Y.; Wang, L.; Wu, Y.; Tu, B.; Yu, B.; Liu, F.; Tam, H.-W.; Wang, G.; Djurišić, A.B.; et al. Molecule-Doped Nickel Oxide: Verified Charge Transfer and Planar Inverted Mixed Cation Perovskite Solar Cell. Adv. Mater. 2018, 30, 1800515. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Huang, J.; Liu, Z.; Zhang, P.; Yun, J.S.; Soufiani, A.M.; Seidel, J.; Sun, K.; Hameiri, Z.; et al. Improvement of Cs-(FAPbI3)0.85(MAPbBr3)0.15 Quality Via DMSO-Molecule-Control to Increase the Efficiency and Boost the Long-Term Stability of 1 cm2 Sized Planar Perovskite Solar Cells. Sol. RRL 2018, 3, 1800338. [Google Scholar] [CrossRef]
- Zhang, F.; Bi, D.; Pellet, N.; Xiao, C.; Li, Z.; Berry, J.J.; Zakeeruddin, S.M.; Zhu, K.; Grätzel, M. Suppressing defects through the synergistic effect of a Lewis base and a Lewis acid for highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2018, 11, 3480–3490. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, C.; Grice, C.R.; Shrestha, N.; Zhao, D.; Liao, W.; Guan, L.; Awni, R.A.; Meng, W.; Cimaroli, A.J.; et al. Synergistic Effects of Lead Thiocyanate Additive and Solvent Annealing on the Performance of Wide-Bandgap Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1177. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.-H.; Oh, K.S.; Jo, Y.; Yoon, H.; Kim, K.-H.; Lee, H.; Kim, J.Y.; Kim, D.S. High-Temperature–Short-Time Annealing Process for High-Performance Large-Area Perovskite Solar Cells. ACS Nano 2017, 11, 6057. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liang, C.; Bao, B.; Li, Y.; Hu, X.; Wang, Y.; Zhang, Y.; Li, F.; Shao, G.; Song, Y. Inkjet manipulated homogeneous large size perovskite grains for efficient and large-area perovskite solar cells. Nano Energy 2018, 46, 203. [Google Scholar] [CrossRef]
- He, J.; Xiang, Y.; Zhang, F.; Lian, J.; Hu, R.; Zeng, P.; Song, J.; Qu, J. Improvement of red light harvesting ability and open circuit voltage of Cu:NiOx based p-i-n planar perovskite solar cells boosted by cysteine enhanced interface contact. Nano Energy 2018, 45, 471. [Google Scholar] [CrossRef]
- Zhang, F.; Song, J.; Hu, R.; Xiang, Y.; He, J.; Hao, Y.; Lian, J.; Zhang, B.; Zeng, P.; Qu, J. Interfacial Passivation of the p-Doped Hole-Transporting Layer Using General Insulating Polymers for High-Performance Inverted Perovskite Solar Cells. Small 2018, 14, 1704007. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dyck, O.; Poplawsky, J.; Keum, J.; Puretzky, A.; Das, S.; Ivanov, I.; Rouleau, C.; Duscher, G.; Geohegan, D.; et al. Perovskite Solar Cells with Near 100% Internal Quantum Efficiency Based on Large Single Crystalline Grains and Vertical Bulk Heterojunctions. J. Am. Chem. Soc. 2015, 137, 9210. [Google Scholar] [CrossRef]
- You, J.; Yang, Y.; Hong, Z.; Song, T.-B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.-H.; Li, G.; et al. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zheng, J.; Zheng, L.; Yan, X.; Lin, H.; Zhang, F. Crack-free CH3NH3PbI3 layer via continuous dripping method for high-performance mesoporous perovskite solar cells. Appl. Surf. Sci. 2017, 392, 960. [Google Scholar] [CrossRef]
- Jo, Y.; Oh, K.S.; Kim, M.; Kim, K.-H.; Lee, H.; Lee, C.-W.; Kim, D.S. High Performance of Planar Perovskite Solar Cells Produced from PbI2(DMSO) and PbI2(NMP) Complexes by Intramolecular Exchange. Adv. Mater. Interfaces 2016, 3, 1500768. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897. [Google Scholar] [CrossRef]
- Lee, J.-W.; Dai, Z.; Lee, C.; Lee, H.M.; Han, T.-H.; De Marco, N.; Lin, O.; Choi, C.S.; Dunn, B.; Koh, J.; et al. Tuning Molecular Interactions for Highly Reproducible and Efficient Formamidinium Perovskite Solar Cells via Adduct Approach. J. Am. Chem. Soc. 2018, 140, 6317. [Google Scholar] [CrossRef] [PubMed]
- Dualeh, A.; Tétreault, N.; Moehl, T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Effect of Annealing Temperature on Film Morphology of Organic–Inorganic Hybrid Pervoskite Solid-State Solar Cells. Adv. Funct. Mater. 2014, 24, 3250. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, S.; Li, M.; Xu, W.; Yin, G.; Wang, Z.; Sun, B.; Gao, X. Annealing Induced Re-crystallization in CH3NH3PbI3−xClx for High Performance Perovskite Solar Cells. Sci. Rep. 2017, 7, 46724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Zhang, J.; Zhang, C.; Chang, J.; Lin, Z.; Chen, D.; Xi, H.; Hao, Y. Effects of Annealing Conditions on Mixed Lead Halide Perovskite Solar Cells and Their Thermal Stability Investigation. Materials 2017, 10, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal Behavior of Methylammonium Lead-Trihalide Perovskite Photovoltaic Light Harvesters. Chem. Mater. 2014, 26, 6160. [Google Scholar] [CrossRef]
- Williams, A.E.; Holliman, P.J.; Carnie, M.J.; Davies, M.L.; Worsley, D.A.; Watson, T.M. Perovskite processing for photovoltaics: A spectro-thermal evaluation. J. Mater. Chem. A 2014, 2, 19338. [Google Scholar] [CrossRef]
- Padchasri, J.; Yimnirun, R. Effects of annealing temperature on stability of methylammonium lead iodide perovskite powders. J. Alloy. Compd. 2017, 720, 63. [Google Scholar] [CrossRef]
- Shi, L.X.; Wang, Z.S.; Huang, Z.; Sha, W.E.I.; Wang, H.; Zhou, Z. The effects of interfacial recombination and injection barrier on the electrical characteristics of perovskite solar cells. AIP Advances 2018, 8, 025312. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Jing, X.; Yan, J.; Hu, C.; Chen, R.; Yin, J.; Li, J.; Zheng, N. Identifying the Molecular Structures of Intermediates for Optimizing the Fabrication of High-Quality Perovskite Films. J. Am. Chem. Soc. 2016, 138, 9919. [Google Scholar] [CrossRef]
- Guo, Y.; Shoyama, K.; Sato, W.; Matsuo, Y.; Inoue, K.; Harano, K.; Liu, C.; Tanaka, H.; Nakamura, E. Chemical Pathways Connecting Lead(II) Iodide and Perovskite via Polymeric Plumbate(II) Fiber. J. Am. Chem. Soc. 2015, 137, 15907. [Google Scholar] [CrossRef]
- Long, M.; Zhang, T.; Liu, M.; Chen, Z.; Wang, C.; Xie, W.; Xie, F.; Chen, J.; Li, G.; Xu, J. Abnormal Synergetic Effect of Organic and Halide Ions on the Stability and Optoelectronic Properties of a Mixed Perovskite via In Situ Characterizations. Adv. Mater. 2018, 30, 1801562. [Google Scholar] [CrossRef] [PubMed]
- Fielding, L. Determination of Association Constants (Ka) from Solution NMR Data. Tetrahedron 2000, 56, 6151. [Google Scholar] [CrossRef]
- Wagner, G.; Pardi, A.; Wuethrich, K. Hydrogen bond length and proton NMR chemical shifts in proteins. J. Am. Chem. Soc. 1983, 105, 5948. [Google Scholar] [CrossRef]
- Wong, K.F.; Ng, S. On the use of the modified Benesi—Hildebrand equation to process NMR hydrogen bonding data. Spectrochim. Acta Pt. A Mol. Spectrosc. 1976, 32, 455. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).