Kirkendall Effect in Twin-Roll Cast AA 3003 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
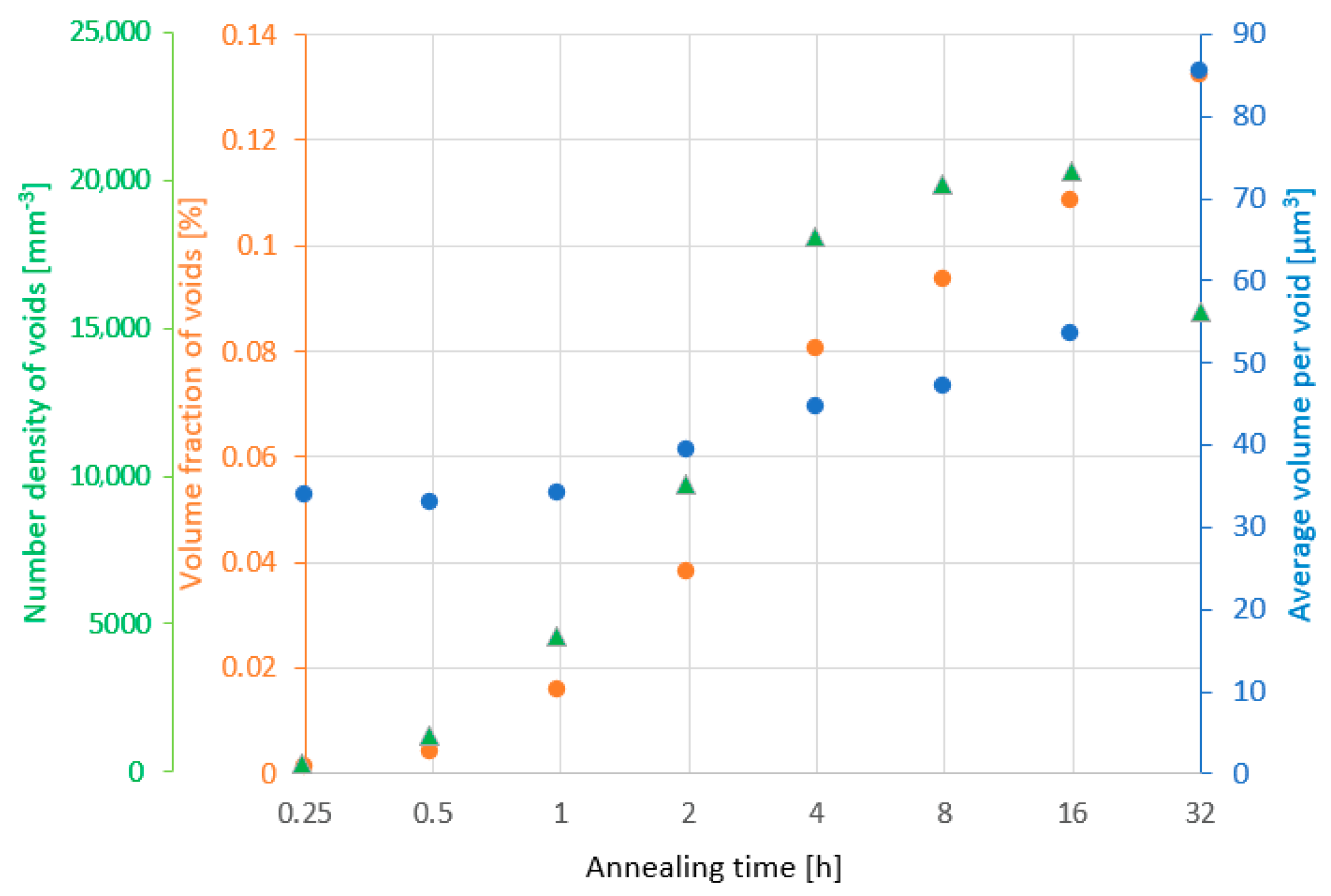
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, K.; Li, Y.J.; Marthinsen, K. Effect of heterogeneously distributed pre-existing dispersoids on the recrystallization behavior of a cold-rolled Al–Mn–Fe–Si alloy. Mater. Charact. 2015, 102, 92–97. [Google Scholar] [CrossRef]
- Huang, L.; Huang, G.; Cao, L.; Wu, X. Influence of pre-recovery on the subsequent recrystallization and mechanical properties of a twin-roll cast Al-Mn alloy. Mater. Sci. Eng. A 2017, 682, 63–72. [Google Scholar] [CrossRef]
- Birol, Y. Homogenization of a twin-roll cast thin Al–Mn strip. J. Alloys Compd. 2009, 471, 122–127. [Google Scholar] [CrossRef]
- Birol, Y. Analysis of macro segregation in twin-roll cast aluminium strips via solidification curves. J. Alloys Compd. 2009, 486, 168–172. [Google Scholar] [CrossRef]
- Šlapáková, M.; Zimina, M.; Zaunschirm, S.; Kastner, J.; Bajer, J.; Cieslar, M. 3D analysis of macrosegregation in twin-roll cast AA3003 alloy. Mater. Charact. 2016, 118, 44–49. [Google Scholar] [CrossRef]
- Kamat, R.G. AA3104 can-body stock ingot: Characterization and homogenization. J. Mater. 1996, 48, 34–38. [Google Scholar] [CrossRef]
- Lv, Z.; Du, F.; An, Z.; Huang, H.; Xu, Z.; Sun, J. Centerline segregation mechanism of twin-roll cast A3003 strip. J. Alloys Compd. 2015, 643, 270–274. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Luo, X.; Jiang, H.; Chen, M.; Wang, Z. Formation of Si nanoparticle in Al matrix for Al-7wt.%Si alloy during complex shear flow casting. J. Alloys Compd. 2018, 739, 30–34. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Sato, T. Formulation of initial artificial age hardening response in an Al-Mg-Si alloy based on the cluster classification using a high-detection-efficiency atom probe. J. Alloys Compd. 2018, 739, 1115–1123. [Google Scholar] [CrossRef]
- Aruga, Y.; Kim, S.N.; Kozuka, M.; Kobayashi, E.; Sato, T. Effects of cluster characteristics on two-step aging behavior in Al-Mg-Si alloys with different Mg/Si ratios and natural aging periods. Mater. Sci. Eng. A 2018, 718, 371–376. [Google Scholar] [CrossRef]
- Dehmas, M.; Valdés, R.; Lanfont, M.-C.; Lacaze, J. Identification of intermetallic precipitates formed during resolidification of brazed aluminium alloys. Scr. Mater. 2006, 55, 191–194. [Google Scholar] [CrossRef][Green Version]
- Xin, S.; Tan, L.; Guangming, X. Composition Homogenization Evolution of Twin-Roll Cast 7075 Aluminum Alloy Using Electromagnetic Field. Rare Metal Mat. Eng. 2015, 44, 581–586. [Google Scholar] [CrossRef]
- Kirkendall, E.O. Diffusion of zinc in alpha brass. Tran. Metall. AIME 1942, 147, 104–110. [Google Scholar]
- Fan, H.J.; Gösele, U.; Zacharias, M. Formation of Nanotubes and Hollow Nanoparticles Based on Kirkendall and Diffusion Processes: A Review. Small 2007, 3, 1660–1671. [Google Scholar] [CrossRef]
- Aldinger, F. Controlled porosity by an extreme Kirkendall effect. Acta Metall. 1974, 22, 923–928. [Google Scholar] [CrossRef]
- Paz y Puente, A.E.; Dunand, D.C. Effect of Cr content on interdiffusion and Kirkendall pore formation during homogenization of pack-aluminized Ni and Ni-Cr wires. Intermetallics 2018, 101, 108–115. [Google Scholar] [CrossRef]
- Fan, H.J.; Knez, M.; Scholz, R.; Hesse, D.; Nielsch, K.; Zacharias, M.; Gösele, U. Influence of Surface Diffusion on the Formation of Hollow Nanostructures Induced by the Kirkendall Effect: The Basic Concept. Nano Lett. 2007, 7, 993–997. [Google Scholar] [CrossRef]
- Chen, M.R.; Jiang, Y.; He, Y.H.; Lin, L.W.; Huang, B.Y.; Liu, C.T. Pore evolution regulation in synthesis of open pore structured Ti–Al intermetallic compounds by solid diffusion. J. Alloys Compd. 2012, 521, 12–15. [Google Scholar] [CrossRef]
- Fujikawa, S.; Hirano, K.; Fukushima, Y. Diffusion of silicon in aluminum. Metall. Trans. A 1978, 9, 1811–1815. [Google Scholar] [CrossRef]
- Urrejola, E.; Peter, K.; Plagwitz, H.; Schubert, G. Distribution of Silicon in the Aluminum Matrix for Rear Passivated Solar Cells. Energy Procedia 2011, 8, 331–336. [Google Scholar] [CrossRef][Green Version]
- Kranz, C.; Baumann, U.; Wolpensinger, B.; Lottspeich, F.; Müller, M.; Palinginis, P.; Brendel, R.; Dullweber, T. Void formation in screen-printed local aluminum contacts modeled by surface energy minimization. Sol. Energy Mater. Sol. Cells 2016, 158, 11–18. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Y.; Su, X.; Wang, J.; Tu, H.; Huang, J. Diffusion mobilities in the fcc A1 Cu–Si, Al–Si and Al–Cu–Si alloys. J. Alloys Compd. 2013, 551, 155–163. [Google Scholar] [CrossRef]
- Haag, S.T.; Richard, M.-I.; Welzel, U.; Favre—Nicolin, V.; Balmes, O.; Richter, G.; Mittemeijer, E.J.; Thomas, O. Concentration and Strain Fields inside a Ag/Au Core−Shell Nanowire Studied by Coherent X-ray Diffraction. Nano Lett. 2013, 13, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Klinger, L.; Rabkin, E. On the nucleation of pores during the nanoscale Kirkendall effect. Mater. Lett. 2015, 161, 508–510. [Google Scholar] [CrossRef]
- Gusak, A.M.; Hodaj, F.; Zaporozhets, T.V. Thermodynamics of void nucleation in nanoparticles. Philos. Mag. Lett. 2011, 91, 741–750. [Google Scholar] [CrossRef]
- Kastner, J.; Harrer, B.; Degischer, H.P. High resolution cone beam X-ray computed tomography of 3D-microstructures of cast Al-alloys. Mater. Charact. 2011, 62, 99–107. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Kastner, J.; Zaunschirm, J.S.; Baumgartner, S.; Requena, G.; Pinto, H.; Garcés, G. 3D-Microstructure Characterization of Thermomechanically Treated Mg-Alloy by High Resolution X-ray Computed Tomography. In Proceedings of the 11th European Conference on Nondestructive Testing (ECNDT 2014), Prague, Czech Republic, 6–10 October 2014. [Google Scholar]
- Kastner, J.; Plank, B.; Requena, G. Non-destructive characterisation of polymers and Al-alloys by polychromatic cone-beam phase contrast tomography. Mater. Charact. 2012, 64, 79–87. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, T.; Yao, Z.; Zhu, M. Modeling of hydrogen porosity formation during solidification of dendrites and irregular eutectics in Al–Si alloys. Materialia 2018, 4, 211–220. [Google Scholar] [CrossRef]
- Lashkari, O.; Yao, L.; Cockcroft, S.; Maijer, D. X-Ray Microtomographic Characterization of Porosity in Aluminum Alloy A356. Metall Mater. Trans. A 2009, 40, 991–999. [Google Scholar] [CrossRef]
- Nicoletto, G.; Anzelotti, G.; Konečná, R. X-ray computed tomography vs. metallography for pore sizing and fatigue of cast Al-alloys. Procedia Eng. 2010, 2, 547–554. [Google Scholar] [CrossRef][Green Version]
- Bajer, J.; Zaunschirm, S.; Šlapáková Poková, M.; Zimina, M. 3D analysis of macrosegregation in twin-roll cast AA3003 alloy before and after heat treatment. In Proceedings of the METAL 2018—27th International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2018. [Google Scholar]
- Cieslar, M.; Slámová, M.; Hájek, M.; Veselý, J. Effect of Thermomechanical Pretreatment on Mechanical Properties of Modified Al-Mn-Fe-Si Based Alloys. Mater. Sci. Forum. 2008, 347, 130–135. [Google Scholar] [CrossRef]
- Li, Y.J.; Arnberg, L. Evolution of eutectic intermetallic particles in DC-cast AA3003 alloy during heating and homogenization. Mater. Sci. Eng. A 2003, 347, 130–135. [Google Scholar] [CrossRef]
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.-Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Eggersmann, M.; Mehrer, H. Diffusion in intermetallic phases of the Fe-Al system. Philos. Mag. A 2000, 80, 1219–1244. [Google Scholar] [CrossRef]
- Sivadasan, V.; Rhead, S.; Leadley, D.; Myronov, M. Kirkendall void formation in reverse step graded Si1−xGex/Ge/Si(001) virtual substrates. Semicond. Sci. Technol. 2018, 33, 024002. [Google Scholar] [CrossRef]
- Müller, W.H.; Weinberg, K.; Böhme, T. On the effect of Kirkendall voids on solder joint reliability. PAMM 2008, 7, 4030035–4030036. [Google Scholar] [CrossRef]
- Weinberg, K.; Boehme, T. Condensation and growth of Kirkendall voids in intermetallic compounds. IEEE Trans. Compon. Packag. Manuf. Technol. 2009, 32, 684–692. [Google Scholar] [CrossRef]
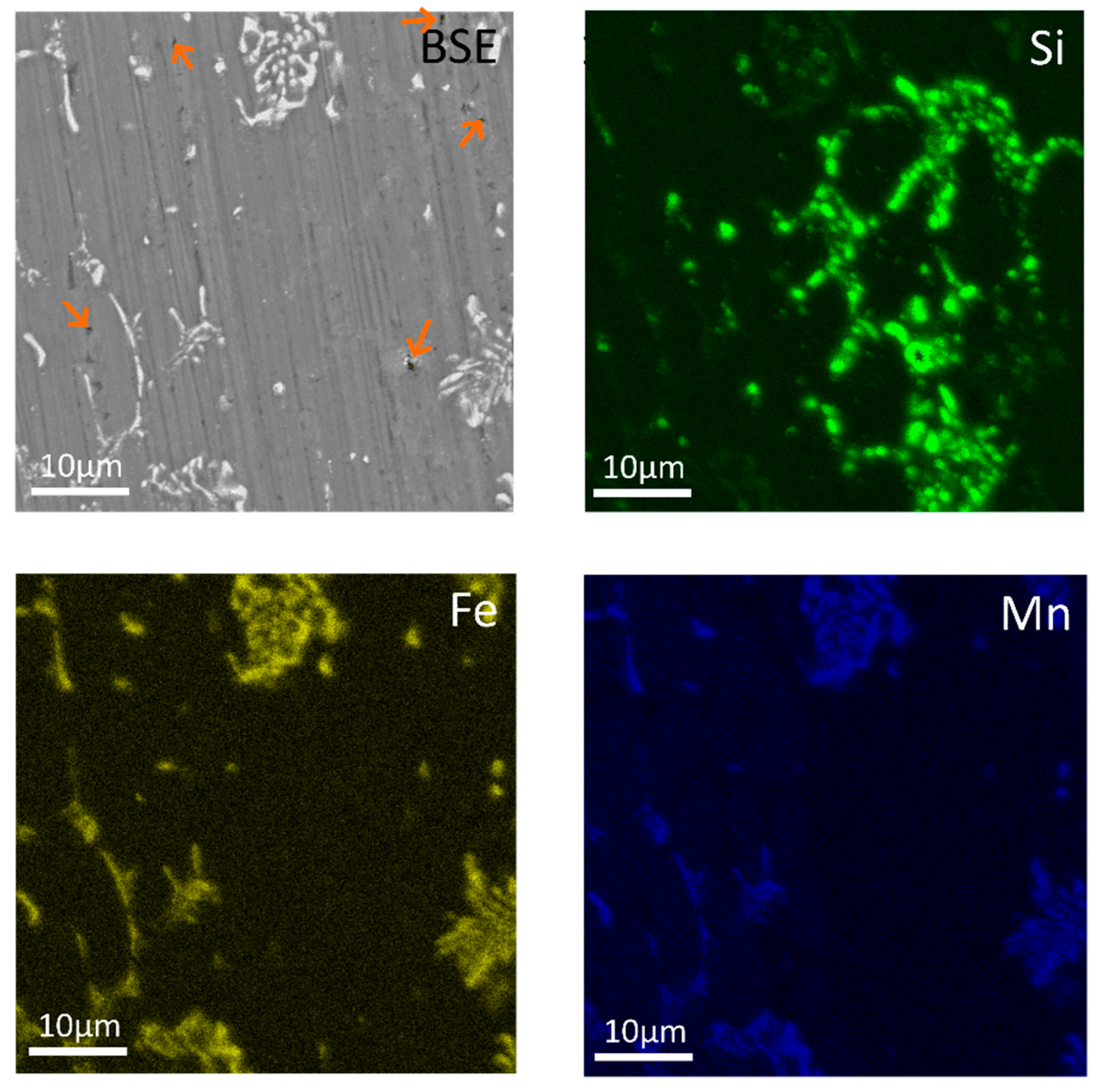
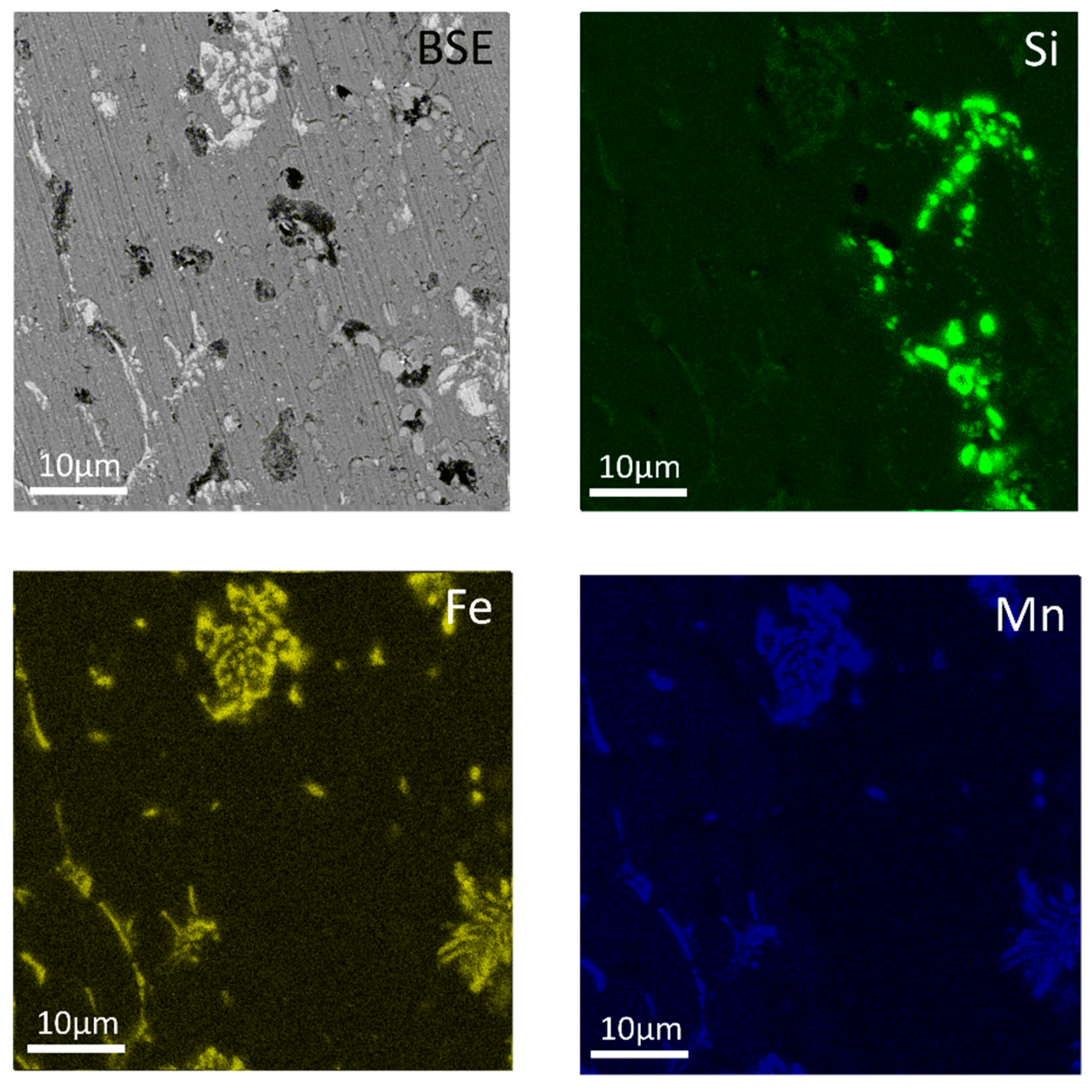
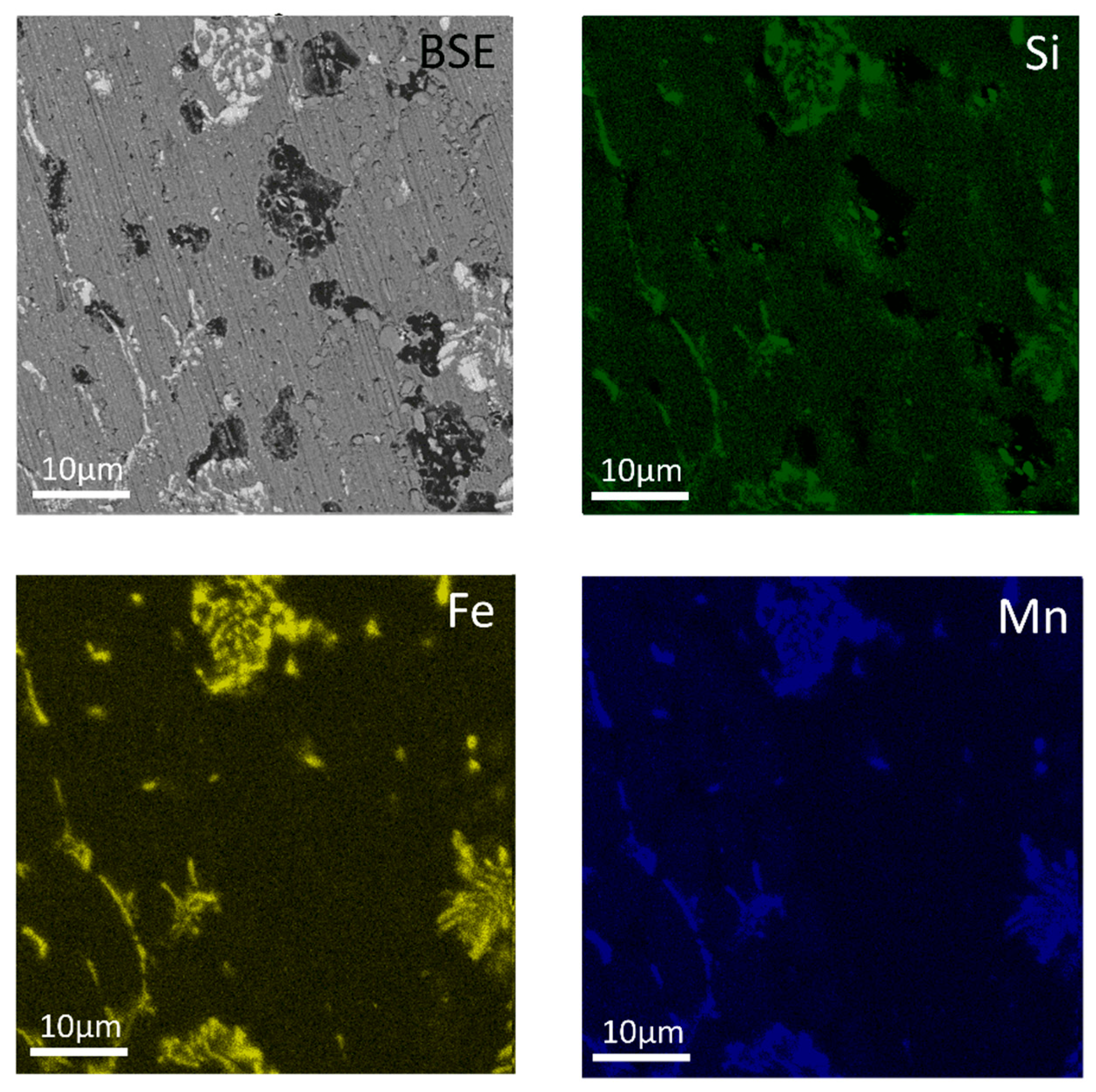

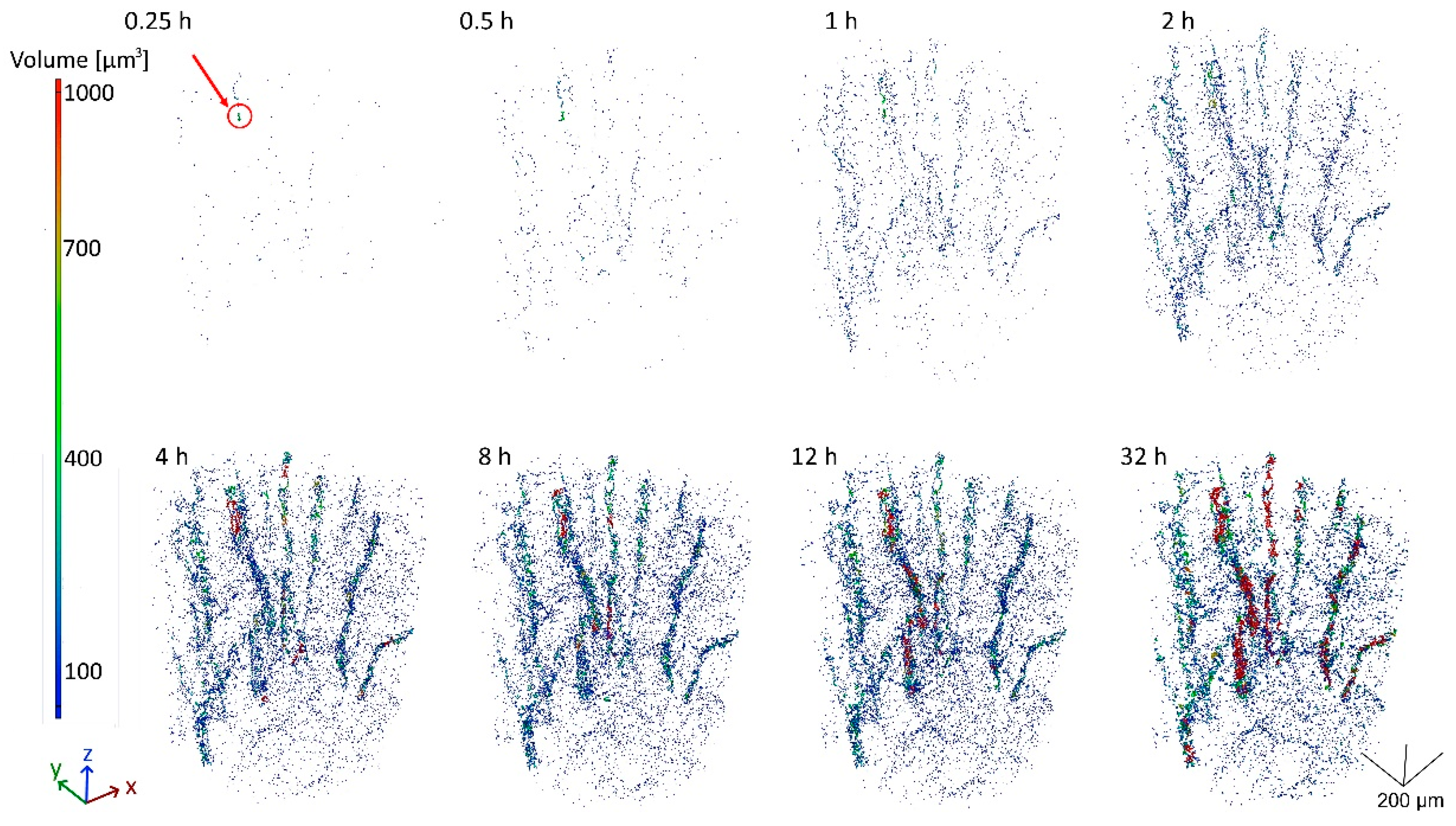

| Al | Mn | Fe | Si | Cu | Zr | Cr |
|---|---|---|---|---|---|---|
| Balance | 1.0 | 0.2 | 0.5 | 0.2 | 0.2 | ∼0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajer, J.; Zaunschirm, S.; Plank, B.; Šlapáková, M.; Bajtošová, L.; Cieslar, M.; Kastner, J. Kirkendall Effect in Twin-Roll Cast AA 3003 Aluminum Alloy. Crystals 2022, 12, 607. https://doi.org/10.3390/cryst12050607
Bajer J, Zaunschirm S, Plank B, Šlapáková M, Bajtošová L, Cieslar M, Kastner J. Kirkendall Effect in Twin-Roll Cast AA 3003 Aluminum Alloy. Crystals. 2022; 12(5):607. https://doi.org/10.3390/cryst12050607
Chicago/Turabian StyleBajer, Jan, Stefan Zaunschirm, Bernhard Plank, Michaela Šlapáková, Lucia Bajtošová, Miroslav Cieslar, and Johann Kastner. 2022. "Kirkendall Effect in Twin-Roll Cast AA 3003 Aluminum Alloy" Crystals 12, no. 5: 607. https://doi.org/10.3390/cryst12050607
APA StyleBajer, J., Zaunschirm, S., Plank, B., Šlapáková, M., Bajtošová, L., Cieslar, M., & Kastner, J. (2022). Kirkendall Effect in Twin-Roll Cast AA 3003 Aluminum Alloy. Crystals, 12(5), 607. https://doi.org/10.3390/cryst12050607






