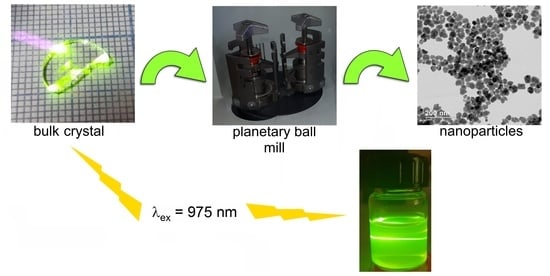
The High-Energy Milling Preparation and Spectroscopic Characterization of Rare-Earth Ions Doped BaY2F8 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystal Growth Process
2.2. Optical Characterization
2.3. Nanodispergation of Bulk Crystals
2.4. Synthesis of Reference β-Na1.5(Y1.17Yb0.3Er0.03)F6 Nanoparticles
2.5. XRD Analysis
2.6. Electron Microscopy Characterization
2.7. Fluorescent Spectroscopy Analysis
3. Results
3.1. Growth and Optical Properties of Ba(Y0.964Yb0.030Er0.006)2F8 Crystal
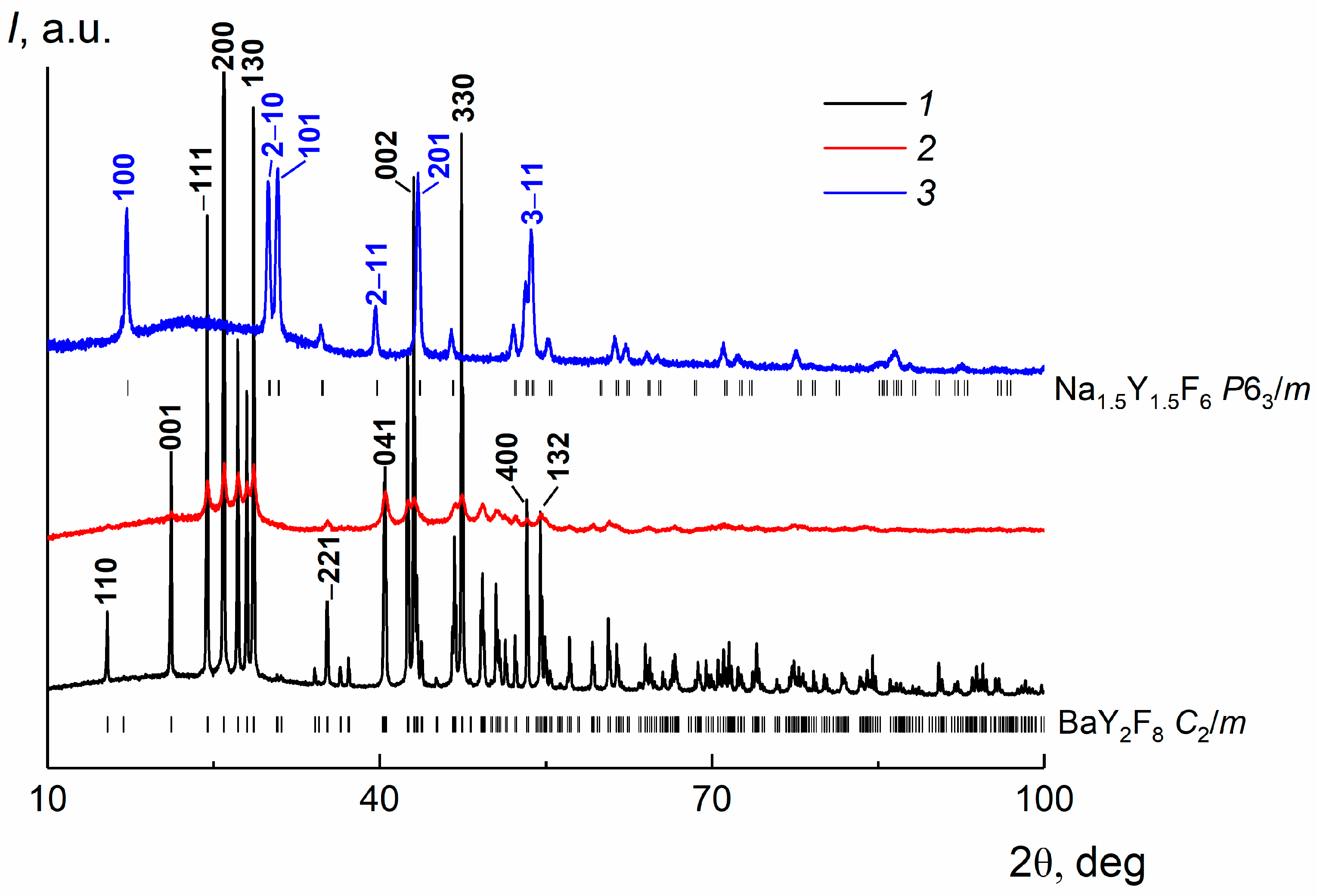
3.2. XRD Analysis of a Bulk Crystal, Ba(Y0.964Yb0.030Er0.006)2F8 and β-Na1.5(Y1.17Yb0.3Er0.03)F6 Nanoparticles
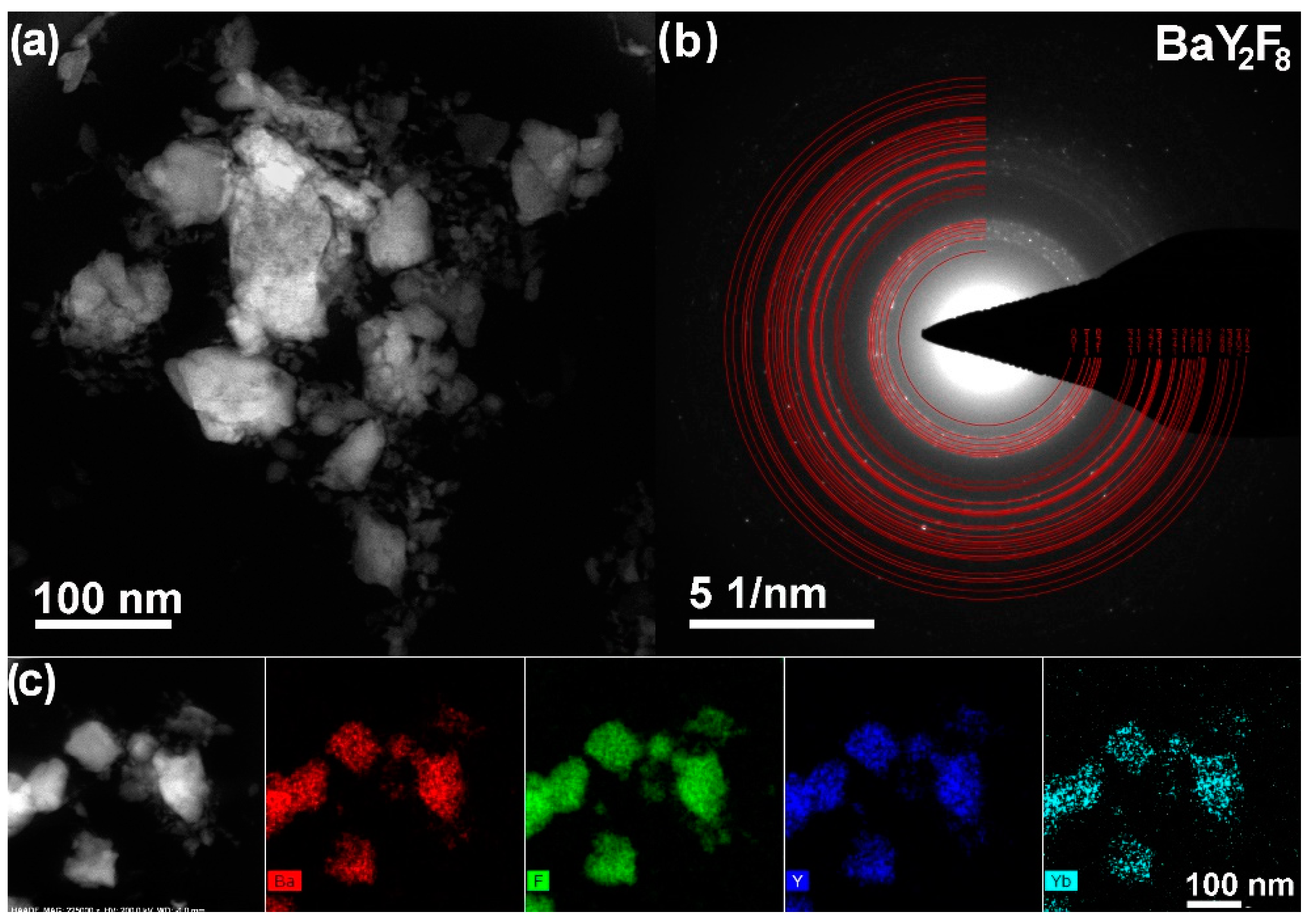
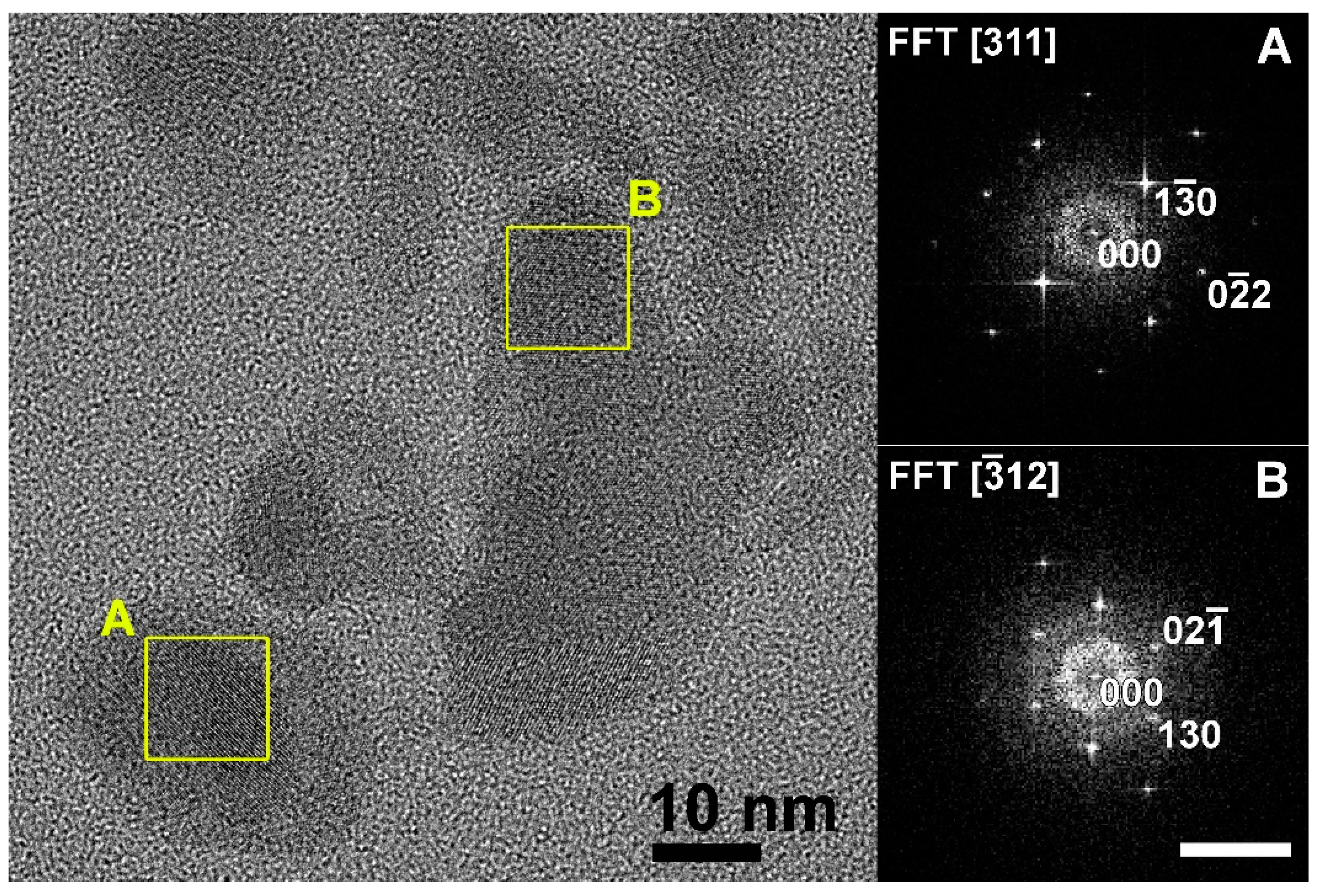
3.3. TEM Analysis of Ba(Y0.964Yb0.030Er0.006)2F8 and β-Na1.5(Y1.17Yb0.3Er0.03)F6 Nanoparticles
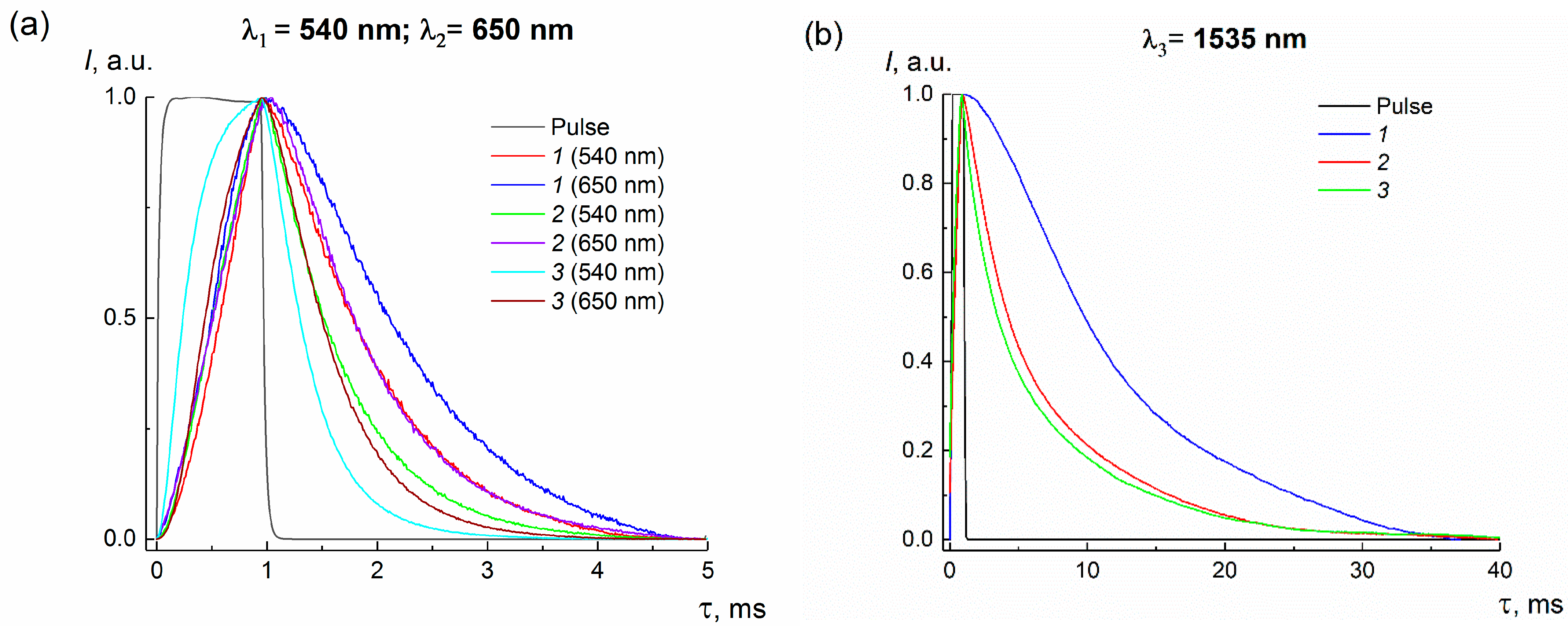
3.4. Photoluminescence Properties of Bulk and Nanocrystals
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meruga, J.M.; Cross, W.M.; May, P.S.; Luu, Q.; Crawford, G.A.; Kellar, J.J. Security printing of covert quick response codes using upconverting nanoparticle inks. Nanotechnology 2012, 23, 395201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.-W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef] [PubMed]
- Generalova, A.; Chichkov, B.; Khaydukov, E. Multicomponent nanocrystals with anti-Stokes luminescence as contrast agents for modern imaging techniques. Adv. Colloid Interface Sci. 2017, 245, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Li, X.; Yi, Z.; Deng, R.; Liang, L.; Xie, X.; Loong, D.T.B.; Song, S.; Fan, D.; et al. Binary temporal upconversion codes of Mn2+-activated nanoparticles for multilevel anti-counterfeiting. Nat. Commun. 2017, 8, 899. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.P.; Maurya, S.K.; Yadav, R.S.; Kumar, A.; Kumar, V.; Joubert, M.-F.; Swart, H.C. Future prospects of fluoride based upconversion nanoparticles for emerging applications in biomedical and energy harvesting. J. Vac. Sci. Technol. B 2018, 36, 060801. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gulzar, A.; Yang, P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.; Jin, D. Recent advances in near-infrared emitting lanthanide-doped nanoconstructs: Mechanism, design and application for bioimaging. Coord. Chem. Rev. 2018, 381, 104–134. [Google Scholar] [CrossRef]
- Karimov, D.N.; Demina, P.A.; Koshelev, A.V.; Rocheva, V.V.; Sokovikov, A.V.; Generalova, A.N.; Zubov, V.P.; Khaydukov, E.V.; Koval’Chuk, M.V.; Panchenko, V.Y. Upconversion Nanoparticles: Synthesis, Photoluminescence Properties, and Applications. Nanotechnol. Russ. 2020, 15, 655–678. [Google Scholar] [CrossRef]
- Nadort, A.; Zhao, J.; Goldys, E.M. Lanthanide upconversion luminescence at the nanoscale: Fundamentals and optical properties. Nanoscale 2016, 8, 13099–13130. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.-P.; Sun, X.; Zhang, Y.-W.; Yan, Z.-G.; Sun, L.-D.; Yan, C.-H. Uniform Alkaline Earth Fluoride Nanocrystals with Diverse Shapes Grown from Thermolysis of Metal Trifluoroacetates in Hot Surfactant Solutions. Cryst. Growth Des. 2009, 9, 2013–2019. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Kuznetsov, S.; Osiko, V.V. Nanofluorides. J. Fluor. Chem. 2011, 132, 1012–1039. [Google Scholar] [CrossRef]
- Cheng, T.; Marin, R.; Skripka, A.; Vetrone, F. Small and Bright Lithium-Based Upconverting Nanoparticles. J. Am. Chem. Soc. 2018, 140, 12890–12899. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-X.; Zhang, Y.-W.; Si, R.; Yan, Z.-G.; Sun, L.-D.; You, L.-P.; Yan, C.-H. High-Quality Sodium Rare-Earth Fluoride Nanocrystals: Controlled Synthesis and Optical Properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Tu, D.; Zheng, W.; Shang, X.; Song, X.; Zhou, S.; Liu, Y.; Li, R.; Chen, X. Large-scale synthesis of uniform lanthanide-doped NaREF4 upconversion/downshifting nanoprobes for bioapplications. Nanoscale 2018, 10, 11477–11484. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, T.; Balabhadra, S.; Przybylska, D.; Węcławiak, M. Upconversion luminescence in BaYF5, BaGdF5 and BaLuF5 nanocrystals doped with Yb3+/Ho3+, Yb3+/Er3+ or Yb3+/Tm3+ ions. J. Alloys Compd. 2015, 649, 606–616. [Google Scholar] [CrossRef]
- Li, C.; Xu, Z.; Yang, D.; Cheng, Z.; Hou, Z.; Ma, P.; Lian, H.; Lin, J. Well-dispersed KRE3F10(RE = Sm–Lu, Y) nanocrystals: Solvothermal synthesis and luminescence properties. CrystEngComm 2011, 14, 670–678. [Google Scholar] [CrossRef]
- Razumkova, I.A.; Denisenko, Y.G.; Boyko, A.N.; Ikonnikov, D.A.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V. Synthesis and Upconversion Luminescence in LaF3:Yb3+, Ho3+, GdF3: Yb3+, Tm3+ and YF3:Yb3+, Er3+ obtained from Sulfide Precursors. Z. Anorg. Allg. Chem. 2019, 645, 1393–1401. [Google Scholar] [CrossRef]
- Boyer, J.-C.; Cuccia, A.L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4: Er3+/Yb3+ and Tm3+/Yb3+ Monodisperse Nanocrystals. Nano Lett. 2007, 7, 847–852. [Google Scholar] [CrossRef]
- Gemini, L.; Schmitz, T.; Kling, R.; Barcikowski, S.; Gökce, B. Upconversion Nanoparticles Synthesized by Ultrashort Pulsed Laser Ablation in Liquid: Effect of the Stabilizing Environment. ChemPhysChem 2017, 18, 1210–1216. [Google Scholar] [CrossRef]
- Sajti, L.; Karimov, D.N.; Rocheva, V.V.; Arkharova, N.A.; Khaydukov, K.V.; Lebedev, O.I.; Voloshin, A.E.; Generalova, A.N.; Chichkov, B.N.; Khaydukov, E.V. Pulsed laser reshaping and fragmentation of upconversion nanoparticles—from hexagonal prisms to 1D nanorods through “Medusa”-like structures. Nano Res. 2020, 14, 1141–1148. [Google Scholar] [CrossRef]
- Yuan, D.; Yi, G.S.; Chow, G.M. Effects of size and surface on luminescence properties of submicron upconversion NaYF4: Yb, Er particles. J. Mater. Res. 2009, 24, 2042–2050. [Google Scholar] [CrossRef]
- Patel, D.N.; Sarkisov, S.S.; Darwish, A.M.; Ballato, J. Optical gain in capillary light guides filled with NaYF4: Yb3+, Er3+ nanocolloids. Opt. Express 2016, 24, 21147–21158. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, B.; Tkachenko, N. Phase diagrams of BaF2-(Y, Ln)F3 systems. J. Less Common Met. 1982, 85, 155–170. [Google Scholar] [CrossRef]
- Maksimov, B.A.; Solans, K.; Dudka, A.P.; Genkina, E.A.; Font-Badria, M.; Buchinskaya, I.I.; Loshmanov, A.A.; Golubev, A.M.; Simonov, V.I.; Font-Altaba, M.; et al. The fluorite-matrix-based Ba4R3F17 (R = Y, Yb) crystal structure. Ordering of cations and specific features of the anionic motif. Crystallogr. Rep. 1996, 41, 56–64. [Google Scholar]
- Greis, O.; Stede, P.; Kieser, M. Darstellung und Eigenschaften der Ternären Verbindungen BaSE2F8 mit SE = Dy—Lu und Y. Z. Anorg. Allg. Chem. 1981, 477, 133–138. [Google Scholar] [CrossRef]
- Guggenheim, H.J.; Johnson, L.F. New fluoride compounds for efficient infrared-to-visible conversion. Appl. Phys. Lett. 1969, 15, 51–52. [Google Scholar] [CrossRef]
- Johnson, L.F.; Guggenheim, H.J. Infrared-Pumped Visible Laser. Appl. Phys. Lett. 1971, 19, 44–47. [Google Scholar] [CrossRef]
- Pollack, S.A.; Chang, D.B.; McFarlane, R.A.; Jenssen, H. Infrared (Er)BaY2F8 laser pumped through di- and tri-ionic upconversion processes. J. Appl. Phys. 1990, 67, 648–653. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Kochubeĭ, S.A.; Naumochkin, K.N.; Pestryakov, E.V.; Trunov, V.I.; Uvarova, T.V. Amplification of ultraviolet radiation due to the 5d–4finterconfigurational transition of the Ce3+ ion in BaY2F8. Sov. J. Quantum Electron. 1989, 19, 340–342. [Google Scholar] [CrossRef]
- Noginov, M.A.; Curley, M.; Venkateswarlu, P.; Williams, A.; Jenssen, H.P. Excitation scheme for the upper energy levels in a Tm:Yb:BaY2F8 laser crystal. J. Opt. Soc. Am. B 1997, 14, 2126–2136. [Google Scholar] [CrossRef]
- Uvarova, T.V.; Pushkar, A.A.; Uvarova, A.G. Multi-bands active media on the basis of BaY2F8 single crystals for up-conversion solid-state lasers with diode pumping. Phys. Status Solidi C 2011, 8, 2911–2914. [Google Scholar] [CrossRef]
- Spijker, J.V.; Dorenbos, P.; van Eijk, C.; Jacobs, J.; Hartog, H.D.; Korolev, N. Luminescence and scintillation properties of BaY2F8:Ce3+, BaLu2F8 and BaLu2F8:Ce3+. J. Lumin. 1999, 85, 11–19. [Google Scholar] [CrossRef]
- de Mello, A.C.; Andrade, A.B.; Nakamura, G.H.; Baldochi, S.L.; Valerio, M.E. Scintillation mechanism of Tb3+ doped BaY2F8. Opt. Mater. 2010, 32, 1337–1340. [Google Scholar] [CrossRef]
- Kurosawa, S.; Yanagida, T.; Pejchal, J.; Fukuda, K.; Kawaguchi, N.; Ishizu, S.; Suyama, T.; Nakagawa, M.; Yokota, Y.; Nikl, M.; et al. Evaluation of Nd:BaY2F8 for VUV scintillator. Radiat. Meas. 2013, 55, 108–111. [Google Scholar] [CrossRef]
- Liu, H.; Lu, W.; Wang, H.; Rao, L.; Yi, Z.; Zeng, S.; Hao, J. Simultaneous synthesis and amine-functionalization of single-phase BaYF5:Yb/Er nanoprobe for dual-modal in vivo upconversion fluorescence and long-lasting X-ray computed tomography imaging. Nanoscale 2013, 5, 6023–6029. [Google Scholar] [CrossRef] [PubMed]
- Nampi, P.P.; Vakurov, A.; Viswambharan, H.; Schneider, J.E.; Drummond-Brydson, R.; Millner, P.A.; Saha, S.; Jose, G. Barium yttrium fluoride based upconversion nanoparticles as dual mode image contrast agents. Mater. Sci. Eng. C 2021, 124, 111937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, P.A.; Li, C.; Li, G.; Huang, S.; Yang, D.; Shang, M.; Kang, X.; Lin, J. Controllable and white upconversion luminescence in BaYF5:Ln3+ (Ln = Yb, Er, Tm) nanocrystals. J. Mater. Chem. 2010, 21, 717–723. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, S.; Jiang, G.; Chen, Y.; Duan, C.; Yin, M. Temperature dependent luminescence of Dy3+ doped BaYF5 nanoparticles for optical thermometry. Curr. Appl. Phys. 2014, 14, 1067–1071. [Google Scholar] [CrossRef]
- Haritha, P.; Martin, I.R.; Viswanath, C.D.; Vijaya, N.; Krishnaiah, K.V.; Jayasankar, C.; Haranath, D.; Lavín, V.; Venkatramu, V. Structure, morphology and optical characterization of Dy3+-doped BaYF5 nanocrystals for warm white light emitting devices. Opt. Mater. 2017, 70, 16–24. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Kuznetsov, S.V.; Voronov, V.V.; Ermakov, R.P.; Samarina, K.S.; Popov, A.I.; Osiko, V.V. Co-precipitation of yttrium and barium fluorides from aqueous solutions. Mater. Res. Bull. 2012, 47, 1794–1799. [Google Scholar] [CrossRef]
- Karbowiak, M.; Cichos, J. Does BaYF5 nanocrystals exist?—The BaF2-YF3 solid solution revisited using photoluminescence spectroscopy. J. Alloys Compd. 2016, 673, 258–264. [Google Scholar] [CrossRef]
- Grube, J.; Krieke, G. How activator ion concentration affects spectroscopic properties on Ba4Y3F17: Er3+, Yb3+, a new perspective up-conversion material. J. Lumin. 2018, 203, 376–384. [Google Scholar] [CrossRef]
- Pudovkin, M.; Kuznetsov, S.; Proydakova, V.Y.; Voronov, V.; Semashko, V. Luminescent thermometry based on Ba4Y3F17:Pr3+ and Ba4Y3F17:Pr3+,Yb3+ nanoparticles. Ceram. Int. 2020, 46, 11658–11666. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Q.; Li, Y. Synthesis and upconversion luminescence of BaY2F8:Yb3+/Er3+ nanobelts. Chem. Commun. 2010, 46, 7528–7529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, Y.; Qu, J.; Ding, J.; Lin, H.; Liu, L.; Zhou, Y.; Zeng, F.; Li, C.; Su, Z. Up-conversion photoluminescence properties and energy transfer process of Ho3+,Yb3+ Co-doped BaY2F8 fine fibers. J. Lumin. 2019, 212, 154–159. [Google Scholar] [CrossRef]
- Nair, G.B.; Kumar, A.; Swart, H.; Dhoble, S. Facile precipitation synthesis of green-emitting BaY2F8:Yb3+, Ho3+ upconverting phosphor. Ceram. Int. 2019, 45, 14205–14213. [Google Scholar] [CrossRef]
- Toncelli, A.; Ahmadi, B.; Marchetti, F. Upconversion enhancement in Yb3+,Tm3+:BaY2F8 quasi-nanoparticles. J. Lumin. 2012, 132, 2268–2274. [Google Scholar] [CrossRef]
- Hakim, R.; Damak, K.; Gemmi, M.; Luin, S.; Maalej, R.; Toncelli, A. Pr3+:BaY2F8 Crystal Nanoparticles (24 nm) Produced by High-Energy Ball Milling: Spectroscopic Characterization and Comparison with Bulk Properties. J. Phys. Chem. C 2015, 119, 2844–2851. [Google Scholar] [CrossRef]
- Boyer, J.-C.; Vetrone, F.; Cuccia, L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4 Nanocrystals Doped with Er3+, Yb3+ and Tm3+, Yb3+ via Thermal Decomposition of Lanthanide Trifluoroacetate Precursors. J. Am. Chem. Soc. 2006, 128, 7444–7445. [Google Scholar] [CrossRef]
- JEMS. Available online: http://www.jems-saas.ch (accessed on 15 March 2020).
- Sani, E.; Toncelli, A.; Tonelli, M. Effect of Cerium codoping on Er:BaY2F8 crystals. Opt. Express 2005, 13, 8980–8992. [Google Scholar] [CrossRef]
- Izotova, O.E.; Aleksandrov, V.B. The Crystal Structure of BaTm2F8. Sov. Phys. Dokl. 1970, 15, 525–527. [Google Scholar]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Upconversion Phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]

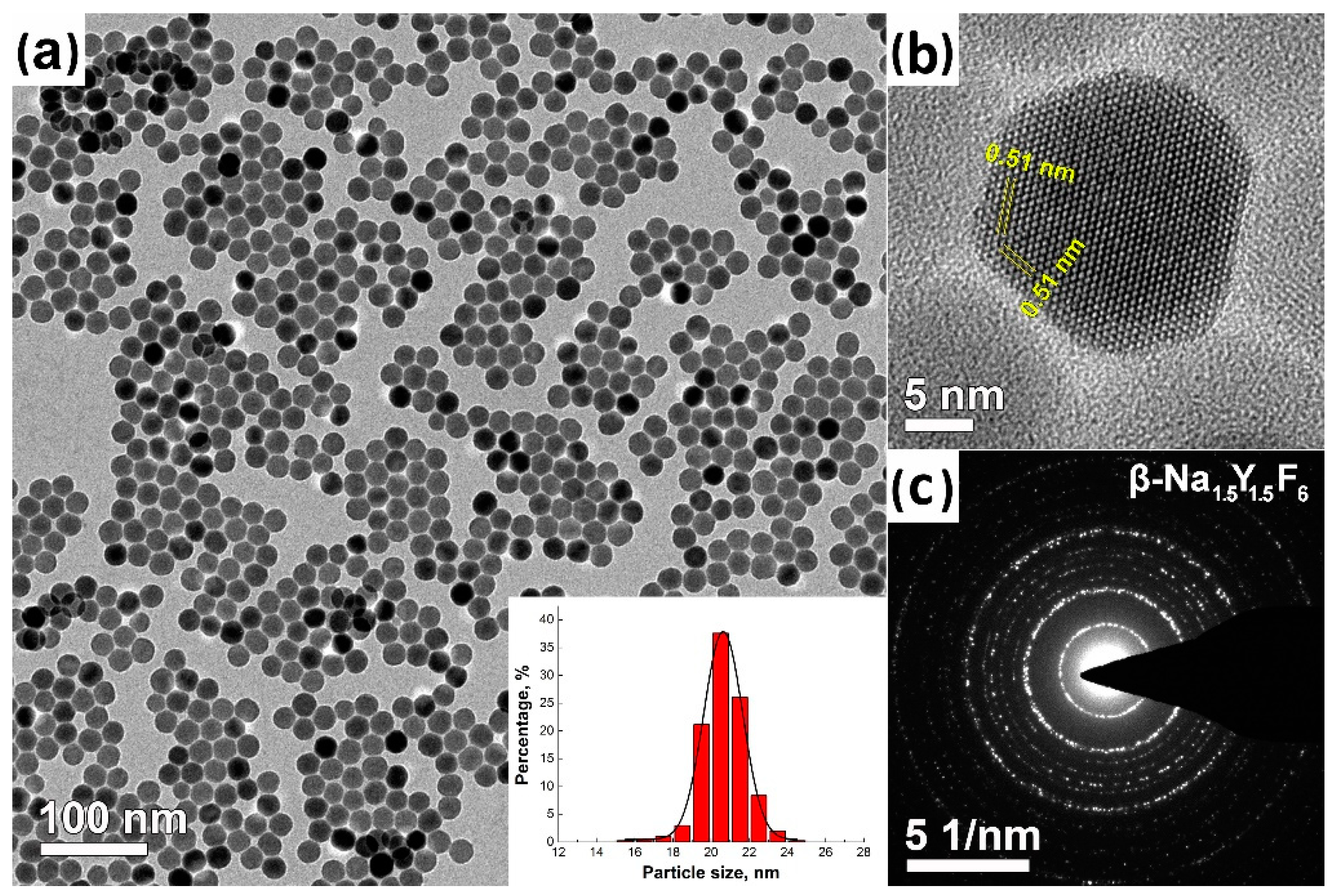
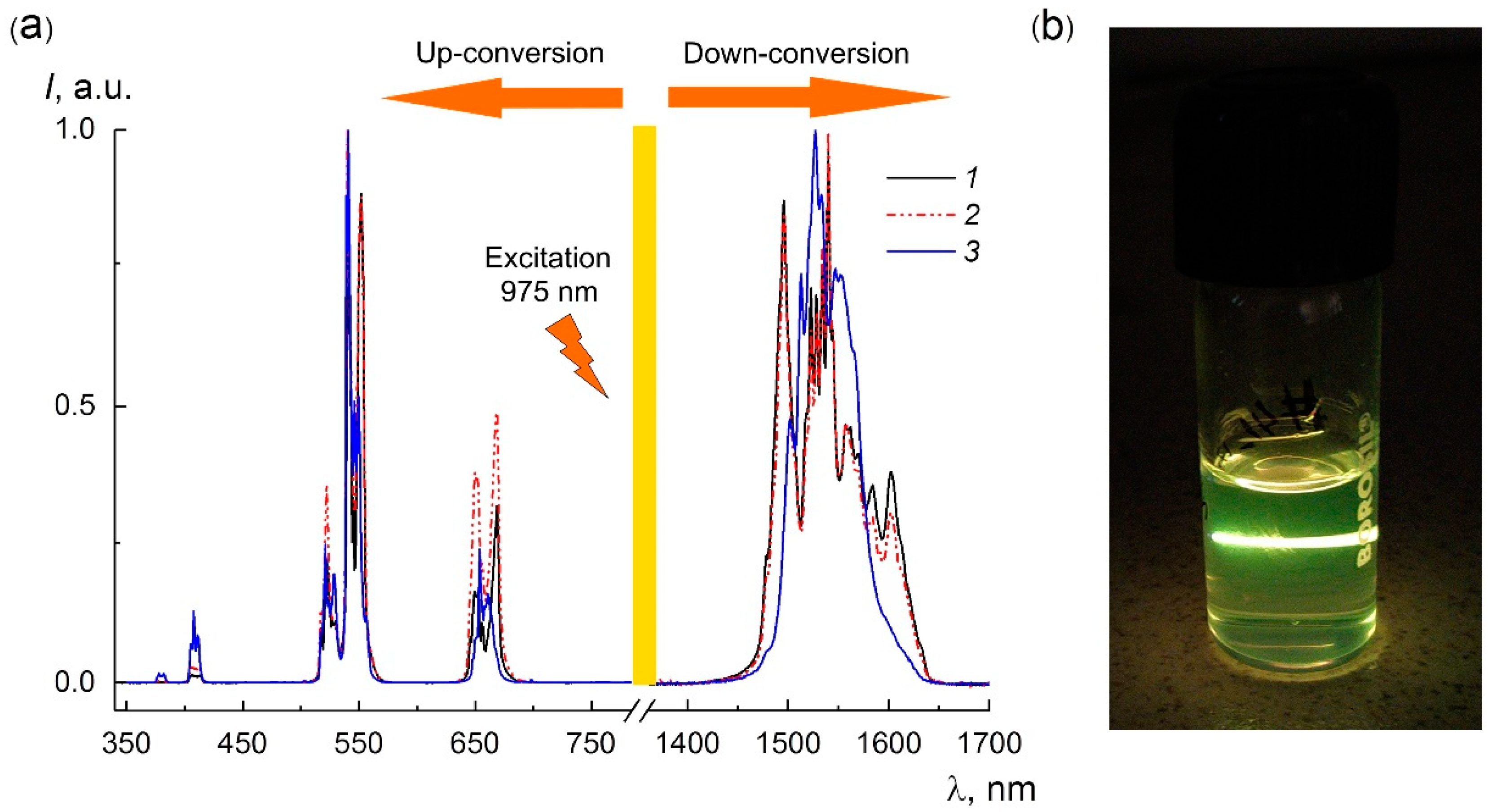

| Space Group | Unit Cell Parameters, Å | |
|---|---|---|
| bulk Ba(Y0.964Yb0.030Er0.006)2F8 | C2/m | a = 6.971(1), b = 10.506(1), c = 4.257(1), β = 99.676° |
| milled Ba(Y0.964Yb0.030Er0.006)2F8 | C2/m | a = 6.969(3), b = 10.502(1), c = 4.254(1), β = 99.676° |
| β-Na1.5(Y1.17Yb0.3Er0.03)F6 | P63/m | a = 5.972(1), c = 3.505(1) |
| τ, ms | |||
|---|---|---|---|
| λ1 = 540 nm | λ2 = 650 nm | λ3 = 1535 nm | |
| bulk Ba(Y0.964Yb0.030Er0.006)2F8 | 0.873 | 1.200 | 11.420 |
| milling Ba(Y0.964Yb0.030Er0.006)2F8 | 0.679 | 0.823 | 7.530 |
| β-Na1.5(Y1.17Yb0.3Er0.03)F6 | 0.380 | 0.527 | 6.950 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshelev, A.V.; Arkharova, N.A.; Khaydukov, K.V.; Seyed Dorraji, M.S.; Karimov, D.N.; Klechkovskaya, V.V. The High-Energy Milling Preparation and Spectroscopic Characterization of Rare-Earth Ions Doped BaY2F8 Nanoparticles. Crystals 2022, 12, 599. https://doi.org/10.3390/cryst12050599
Koshelev AV, Arkharova NA, Khaydukov KV, Seyed Dorraji MS, Karimov DN, Klechkovskaya VV. The High-Energy Milling Preparation and Spectroscopic Characterization of Rare-Earth Ions Doped BaY2F8 Nanoparticles. Crystals. 2022; 12(5):599. https://doi.org/10.3390/cryst12050599
Chicago/Turabian StyleKoshelev, Aleksander V., Natalia A. Arkharova, Kirill V. Khaydukov, Mir Saeed Seyed Dorraji, Denis N. Karimov, and Vera V. Klechkovskaya. 2022. "The High-Energy Milling Preparation and Spectroscopic Characterization of Rare-Earth Ions Doped BaY2F8 Nanoparticles" Crystals 12, no. 5: 599. https://doi.org/10.3390/cryst12050599
APA StyleKoshelev, A. V., Arkharova, N. A., Khaydukov, K. V., Seyed Dorraji, M. S., Karimov, D. N., & Klechkovskaya, V. V. (2022). The High-Energy Milling Preparation and Spectroscopic Characterization of Rare-Earth Ions Doped BaY2F8 Nanoparticles. Crystals, 12(5), 599. https://doi.org/10.3390/cryst12050599