Na0.76V6O15@Boron Carbonitride Nanotube Composites as Cathodes for High-Performance Lithium-Ion Capacitors
Abstract
:1. Introduction
2. Materials and Methods
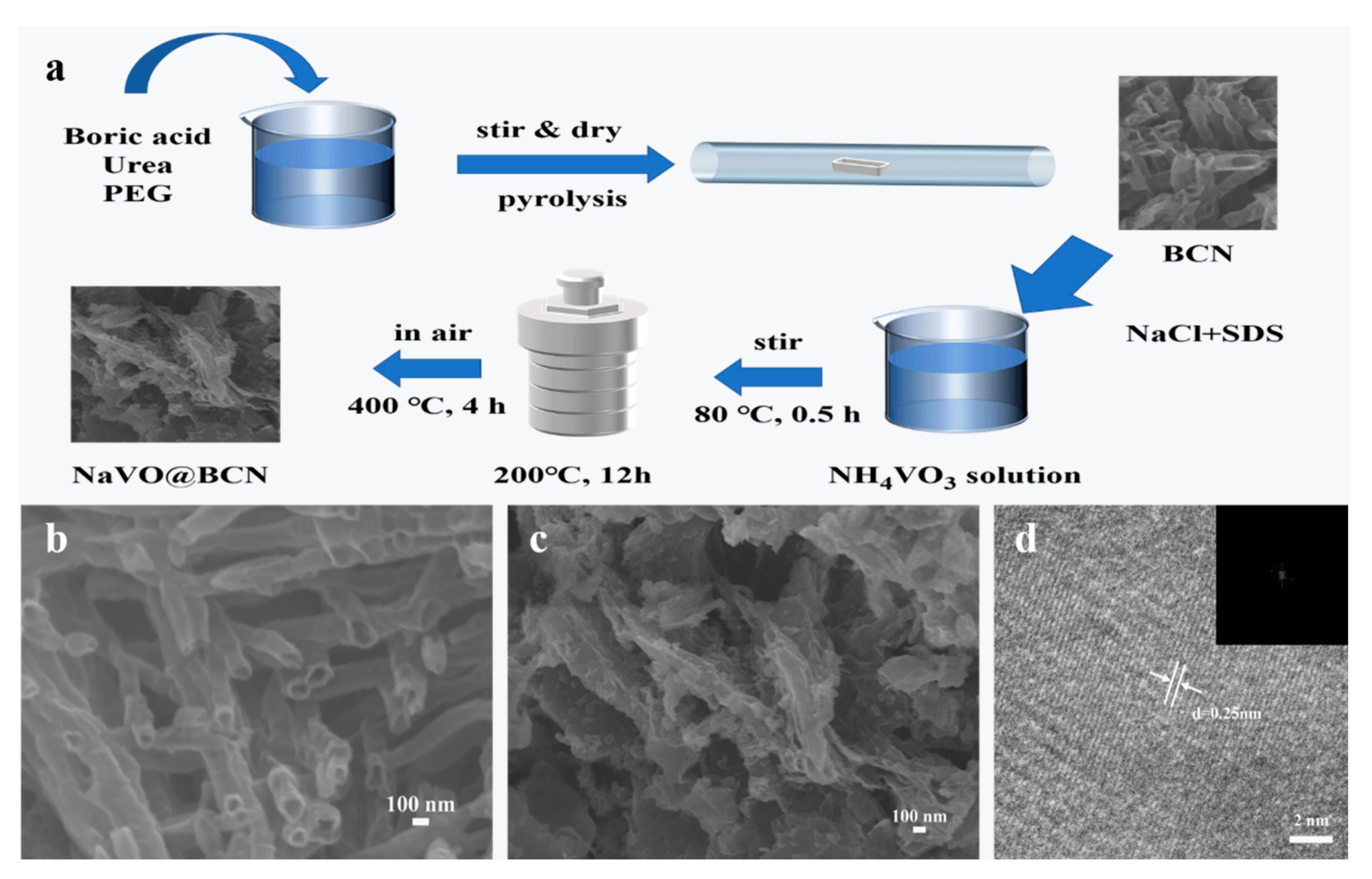
2.1. Material Synthesis
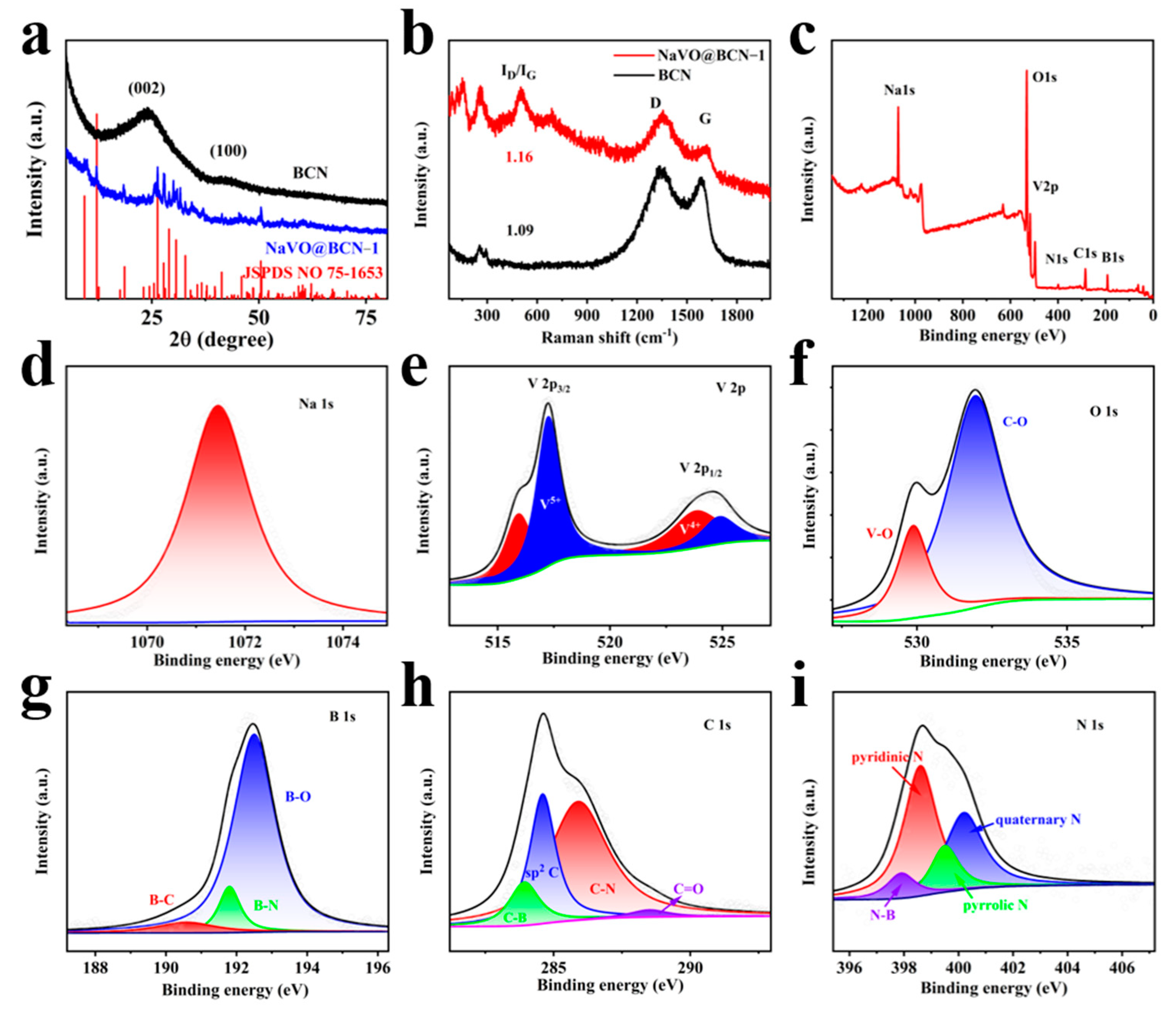
2.2. Structure Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Morphology and Structure
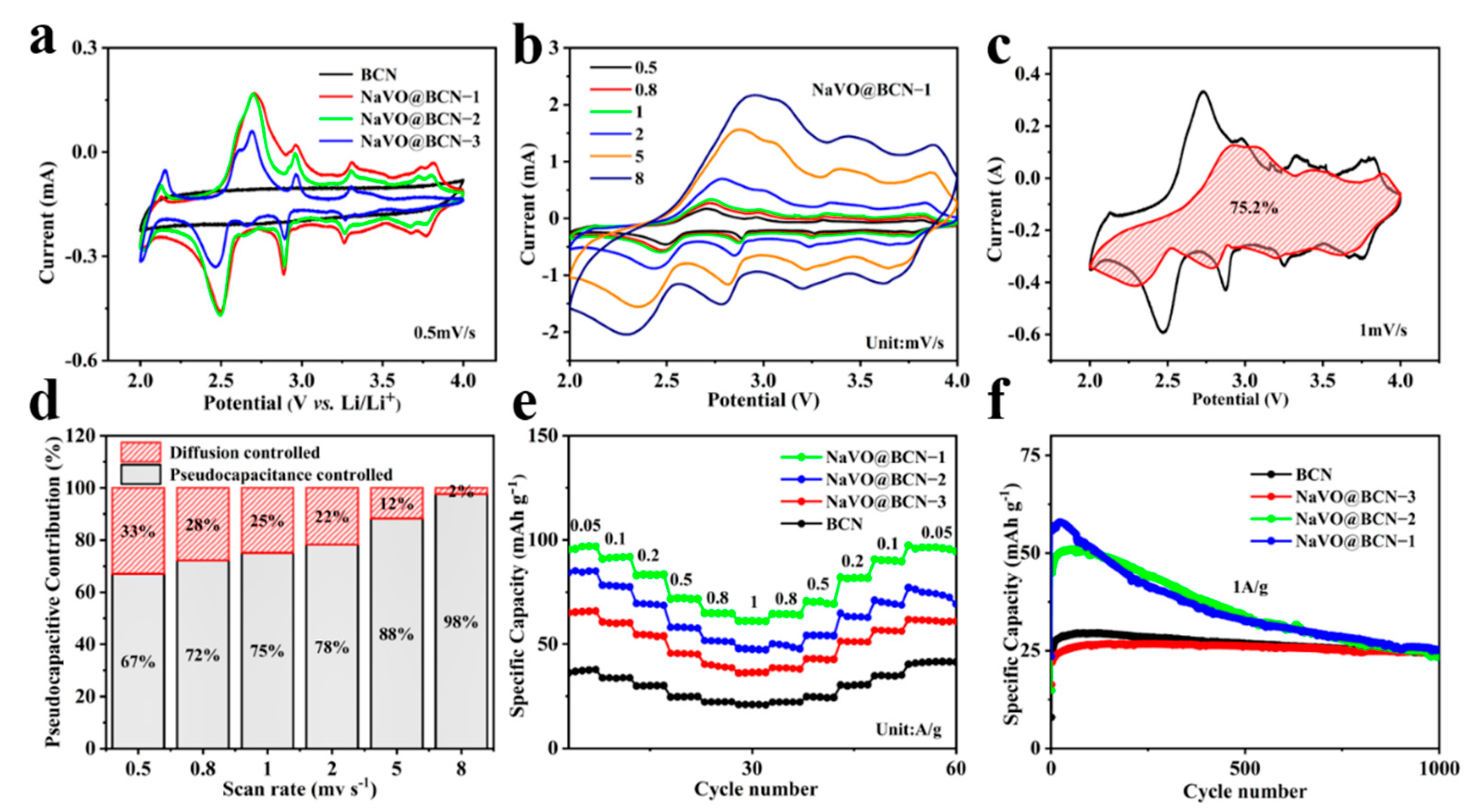
3.2. Electrochemical Performance as the Cathode in the Half-Cell
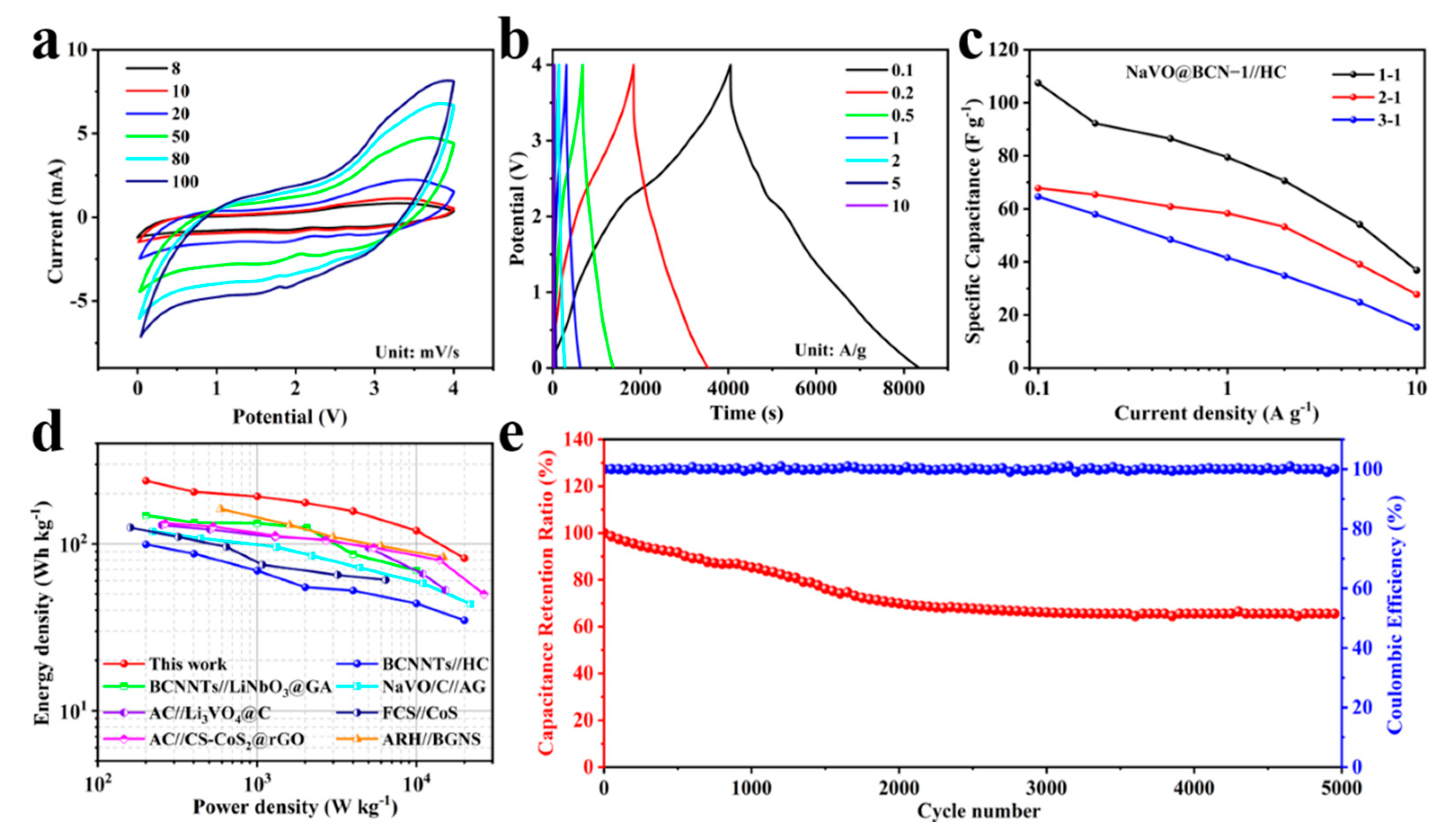
3.3. Electrochemical Performances of LICs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Goodenough, J.B. Energy storage materials: A perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Liu, S.; Shi, D.; Tu, H.; Yao, X.; Deng, L.; Xu, D.; Shao, Y.; Wu, Y.; Hao, X. Self-Supported Fluorine-Doped Boron Carbonitride Porous Aerogels for High-Performance Supercapacitors. Energy Technol. 2021, 9, 2100824. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.-G.; Zheng, J.P. Progress and perspectives on pre-lithiation technologies for lithium ion capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Zou, K.; Cai, P.; Cao, X.; Zou, G.; Hou, H.; Ji, X. Carbon materials for high-performance lithium-ion capacitor. Curr. Opin. Electrochem. 2020, 21, 31–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Chao, D.; Yan, Q.; Fan, H.J. Nonaqueous Hybrid Lithium-Ion and Sodium-Ion Capacitors. Adv. Mater. 2017, 29, 1702093. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode Materials, Electrolytes, and Challenges in Nonaqueous Lithium-Ion Capacitors. Adv. Mater. 2018, 30, e1705670. [Google Scholar] [CrossRef]
- Wang, Y.K.; Liu, M.C.; Cao, J.; Zhang, H.J.; Kong, L.B.; Trudgeon, D.P.; Li, X.; Walsh, F.C. 3D Hierarchically Structured CoS Nanosheets: Li (+) Storage Mechanism and Application of the High-Performance Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2020, 12, 3709–3718. [Google Scholar] [CrossRef]
- Han, P.; Xu, G.; Han, X.; Zhao, J.; Zhou, X.; Cui, G. Lithium Ion Capacitors in Organic Electrolyte System: Scientific Problems, Material Development, and Key Technologies. Adv. Energy Mater. 2018, 8, 1801243. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, J.; Shellikeri, A.; Naderi, R.; Qin, N.; Lu, Y.; Fan, R.; Wu, Q.; Zheng, J.; Zhang, C.; et al. An Overview on Design Parameters of Practical Lithium-Ion Capacitors. Batter. Supercaps 2021, 4, 749–757. [Google Scholar] [CrossRef]
- Sui, D.; Wu, M.; Liu, Y.; Yang, Y.; Zhang, H.; Ma, Y.; Zhang, L.; Chen, Y. High performance Li-ion capacitor fabricated with dual graphene-based materials. Nanotechnology 2021, 32, 015403. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Chang, M.; Peng, Z.; Li, C.; He, X.; Yang, Y.; Liu, Y.; Lu, Y. Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials 2021, 11, 2771. [Google Scholar] [CrossRef]
- Liang, Z.; Tu, H.; Shi, D.; Chen, F.; Jiang, H.; Shao, Y.; Wu, Y.; Hao, X. In Situ Growing BCN Nanotubes on Carbon Fibers for Novel High-Temperature Supercapacitor with Excellent Cycling Performance. Small 2021, 17, e2102899. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, H.; Wang, M.; Yang, M.; Guo, Q.; Wan, L.; Xia, H.; Yu, Y. High Energy and High Power Lithium-Ion Capacitors Based on Boron and Nitrogen Dual-Doped 3D Carbon Nanofibers as Both Cathode and Anode. Adv. Energy Mater. 2017, 7, 1701336. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, D.; Sun, X.; Wang, S.; Li, Y.; Chang, B.; Zhang, B.; Shao, Y.; Wu, Y.; Hao, X. Boron Carbonitride Lithium-Ion Capacitors with an Electrostatically Expanded Operating Voltage Window. ACS Appl. Mater. Interfaces 2020, 12, 47425–47434. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, C.; Lv, R.; Bai, Y.; Lin, Y.; Huang, Z.; Shen, W.; Qiu, X.; Kang, F. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 15, 43–53. [Google Scholar] [CrossRef]
- Pan, A.; Zhang, J.; Cao, G.; Liang, S.; Wang, C.; Nie, Z.; Arey, B.W.; Xu, W.; Liu, D.; Xiao, J.; et al. Nanosheet-structured LiV3O8 with high capacity and excellent stability for high energy lithium batteries. J. Mater. Chem. 2011, 21, 10077. [Google Scholar] [CrossRef]
- Bach, S.; Boudaoud, A.; Emery, N.; Baddour-Hadjean, R.; Pereira-Ramos, J.P. K0.5V2O5: A novel Li intercalation compound as positive electrode material for rechargeable lithium batteries. Electrochim. Acta 2014, 119, 38–42. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, D.; Wang, H.; Huang, X.; Zhang, H.; Liu, S.; Liu, Y. Synthesis and electrochemical properties of NaV3O8 nanoflakes as high-performance cathode for Li-ion battery. RSC Adv. 2014, 4, 8328. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, J.; Liu, J.; Lei, M.; Tang, S.; Lu, P.; Yang, L.; Yang, H.; Yang, Q. Facile Synthesis of Na0.33V2O5 Nanosheet-Graphene Hybrids as Ultrahigh Performance Cathode Materials for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 17433–17440. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.W.; Teh, P.F.; Pramana, S.S.; Wong, C.L.; Su, T.; Li, L.; Madhavi, S. Electrospun Single-Phase Na1.2V3O8 Materials with Tunable Morphologies as Cathodes for Rechargeable Lithium-Ion Batteries. ChemElectroChem 2015, 2, 837–846. [Google Scholar] [CrossRef]
- Yang, K.; Fang, G.; Zhou, J.; Qin, M.; Tang, Y.; Pan, A.; Liang, S. Hydrothermal synthesis of sodium vanadate nanobelts as high-performance cathode materials for lithium batteries. J. Power Sources 2016, 325, 383–390. [Google Scholar] [CrossRef]
- Yang, M.; Shi, D.; Sun, X.; Li, Y.; Liang, Z.; Zhang, L.; Shao, Y.; Wu, Y.; Hao, X. Shuttle confinement of lithium polysulfides in borocarbonitride nanotubes with enhanced performance for lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 296–304. [Google Scholar] [CrossRef]
- Lu, R.; Ren, X.; Wang, C.; Zhan, C.; Nan, D.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.H. Na0.76V6O15/Activated Carbon Hybrid Cathode for High-Performance Lithium-Ion Capacitors. Materials 2021, 14, 122. [Google Scholar] [CrossRef]
- Chang, B.; Li, L.; Shi, D.; Jiang, H.; Ai, Z.; Wang, S.; Shao, Y.; Shen, J.; Wu, Y.; Li, Y.; et al. Metal-free boron carbonitride with tunable boron Lewis acid sites for enhanced nitrogen electroreduction to ammonia. Appl. Catal. B Environ. 2021, 283, 119622. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, J.; Chen, M.; Guo, Y.; Zhou, G.; Li, N.; Zhou, S.; Wong, C.-P. Self-assembled NaV6O15 flower-like microstructures for high-capacity and long-life sodium-ion battery cathode. Nano Energy 2020, 68, 104357. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Bach, S.; Emery, N.; Pereira-Ramos, J.P. The peculiar structural behaviour of β-Na0.33V2O5 upon electrochemical lithium insertion. J. Mater. Chem. 2011, 21, 11296–11305. [Google Scholar] [CrossRef]
- Luo, C.; Xiao, L.; Wu, X. Aqueous zinc ion batteries based on sodium vanadate electrode materials with long lifespan and high energy density. Mater. Adv. 2022, 3, 604–610. [Google Scholar] [CrossRef]
- Xu, X.; Chang, S.; Zeng, T.; Luo, Y.; Fang, D.; Xie, M.; Yi, J. Synthesis of CeVO4-V2O5 nanowires by cation-exchange method for high-performance lithium-ion battery electrode. J. Alloy. Compd. 2021, 887, 161237. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, D.; Wang, Y.; Ni, S. Na3VO4 as a new anode material for lithium-ion batteries. New J. Chem. 2021, 45, 11506–11511. [Google Scholar] [CrossRef]
- Shi, D.; Chang, B.; Ai, Z.; Jiang, H.; Chen, F.; Shao, Y.; Shen, J.; Wu, Y.; Hao, X. Boron carbonitride with tunable B/N Lewis acid/base sites for enhanced electrocatalytic overall water splitting. Nanoscale 2021, 13, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Zou, R.; Mahmood, A.; Liang, Z.; Guo, S. A catalyst-free synthesis of B, N co-doped graphene nanostructures with tunable dimensions as highly efficient metal free dual electrocatalysts. J. Mater. Chem. A 2016, 4, 16469–16475. [Google Scholar] [CrossRef]
- Florent, M.; Bandosz, T.J. Irreversible water mediated transformation of BCN from a 3D highly porous form to its nonporous hydrolyzed counterpart. J. Mater. Chem. A 2018, 6, 3510–3521. [Google Scholar] [CrossRef]
- Shi, D.; Yang, M.; Zhang, B.; Ai, Z.; Hu, H.; Shao, Y.; Shen, J.; Wu, Y.; Hao, X. BCN-Assisted Built-In Electric Field in Heterostructure: An Innovative Path for Broadening the Voltage Window of Aqueous Supercapacitor. Adv. Funct. Mater. 2021, 32, 2108843. [Google Scholar] [CrossRef]
- Yan, M.; Zhao, L.; Zhao, K.; Wei, Q.; An, Q.; Zhang, G.; Wei, X.; Ren, W.; Mai, L. The Capturing of Ionized Oxygen in Sodium Vanadium Oxide Nanorods Cathodes under Operando Conditions. Adv. Funct. Mater. 2016, 26, 6555–6562. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Zhai, W.; Li, D.; Lin, Y.; Guo, S.; Feng, J.; Zhang, L.; Song, L.; Si, P.; et al. Tunable synthesis of LixMnO2 nanowires for aqueous Li-ion hybrid supercapacitor with high rate capability and ultra-long cycle life. J. Power Sources 2019, 413, 302–309. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, W.; Lyu, Y.; Zhang, Y.; Wang, H.; Liu, Y.; Li, D. All-carbon lithium capacitor based on salt crystal-templated, N-doped porous carbon electrodes with superior energy storage. J. Mater. Chem. A 2018, 6, 18276–18285. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Chernova, N.A.; Omenya, F.; Tong, L.; Rastogi, A.C.; Stanley Whittingham, M. Effect of electrode charge balance on the energy storage performance of hybrid supercapacitor cells based on LiFePO4 as Li-ion battery electrode and activated carbon. J. Solid State Electrochem. 2017, 22, 1063–1078. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, S.; Zhang, B.; Shao, Y.; Wu, Y.; Zhao, H.; Lei, Y.; Hao, X. High performance lithium-ion capacitors based on LiNbO3-arched 3D graphene aerogel anode and BCNNT cathode with enhanced kinetics match. Chem. Eng. J. 2020, 396, 125207. [Google Scholar] [CrossRef]
- Ren, X.; Ai, D.; Zhan, C.; Lv, R.; Kang, F.; Huang, Z.-H. 3D porous Li3VO4@C composite anodes with ultra-high rate capacity for lithium-ion capacitors. Electrochim. Acta 2020, 355, 136819. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Bhowmik, S.; Ghosh, S.; Vangapally, N.; Martha, S.K. Boron-doped graphene anode coupled with microporous activated carbon cathode for lithium-ion ultracapacitors. Chem. Eng. J. 2022, 430, 132835. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, X.; Chen, Z.; Xiong, K.; Zhou, Y.; Cai, S.; Zheng, C. Carbon-doped surface unsaturated sulfur enriched CoS2@rGO aerogel pseudocapacitive anode and biomass-derived porous carbon cathode for advanced lithium-ion capacitors. Front. Chem. Sci. Eng. 2021, 15, 1500–1513. [Google Scholar] [CrossRef]
- Ren, J.J.; Su, L.W.; Qin, X.; Yang, M.; Wei, J.P.; Zhou, Z.; Shen, P.W. Pre-lithiated graphene nanosheets as negative electrode materials for Li-ion capacitors with high power and energy density. J. Power Sources 2014, 264, 108–113. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Guo, X.; Wang, S.; Wu, W.; Mondal, A.K.; Wang, C.; Wang, G. A novel lithium-ion hybrid capacitor based on an aerogel-like MXene wrapped Fe2O3 nanosphere anode and a 3D nitrogen sulphur dual-doped porous carbon cathode. Mat. Chem. Front. 2018, 2, 1811–1821. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Peng, R.; Huang, Y.; Guo, L.; Qi, Y. LiMn2O4/graphene composites as cathodes with enhanced electrochemical performance for lithium-ion capacitors. RSC Adv. 2016, 6, 54866–54873. [Google Scholar] [CrossRef]
- Zhuang, B.; Wu, Z.; Chu, W.; Gao, Y.; Cao, Z.; Bold, T.; Yang, N. High-Performance Lithium-ion Supercapatteries Constructed Using Li3V2(PO4)3/C Mesoporous Nanosheets. ChemistrySelect 2019, 4, 9822–9828. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Jiang, H.; Liang, Z.; Kong, Z.; Liu, S.; Deng, L.; Shao, Y.; Wu, Y.; Hao, X. Na0.76V6O15@Boron Carbonitride Nanotube Composites as Cathodes for High-Performance Lithium-Ion Capacitors. Crystals 2022, 12, 597. https://doi.org/10.3390/cryst12050597
Xu D, Jiang H, Liang Z, Kong Z, Liu S, Deng L, Shao Y, Wu Y, Hao X. Na0.76V6O15@Boron Carbonitride Nanotube Composites as Cathodes for High-Performance Lithium-Ion Capacitors. Crystals. 2022; 12(5):597. https://doi.org/10.3390/cryst12050597
Chicago/Turabian StyleXu, Deqin, Hehe Jiang, Zhenyan Liang, Zhen Kong, Shengfu Liu, Lequan Deng, Yongliang Shao, Yongzhong Wu, and Xiaopeng Hao. 2022. "Na0.76V6O15@Boron Carbonitride Nanotube Composites as Cathodes for High-Performance Lithium-Ion Capacitors" Crystals 12, no. 5: 597. https://doi.org/10.3390/cryst12050597
APA StyleXu, D., Jiang, H., Liang, Z., Kong, Z., Liu, S., Deng, L., Shao, Y., Wu, Y., & Hao, X. (2022). Na0.76V6O15@Boron Carbonitride Nanotube Composites as Cathodes for High-Performance Lithium-Ion Capacitors. Crystals, 12(5), 597. https://doi.org/10.3390/cryst12050597






