New Co-Crystals/Salts of Gallic Acid and Substituted Pyridines: An Effect of Ortho-Substituents on the Formation of an Acid–Pyridine Heterosynthon
Abstract
:1. Introduction
2. Materials and Methods
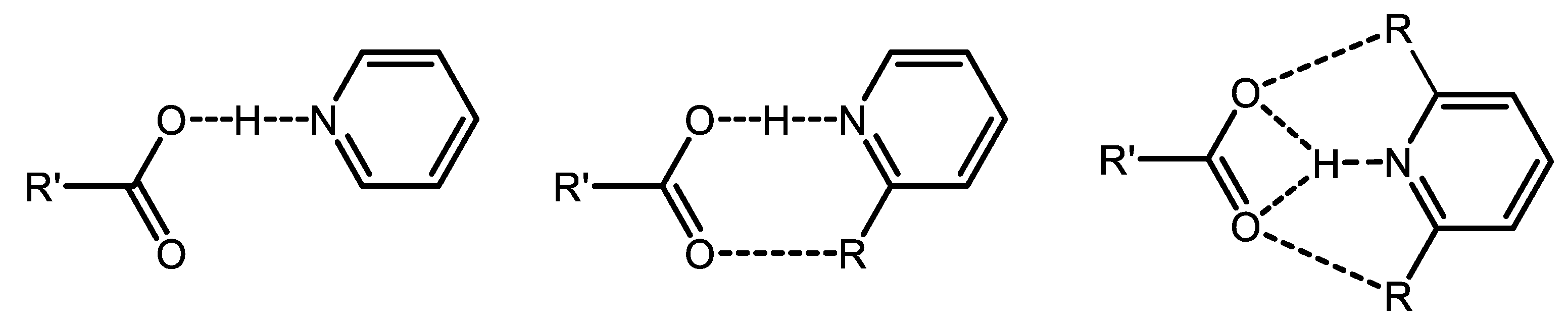
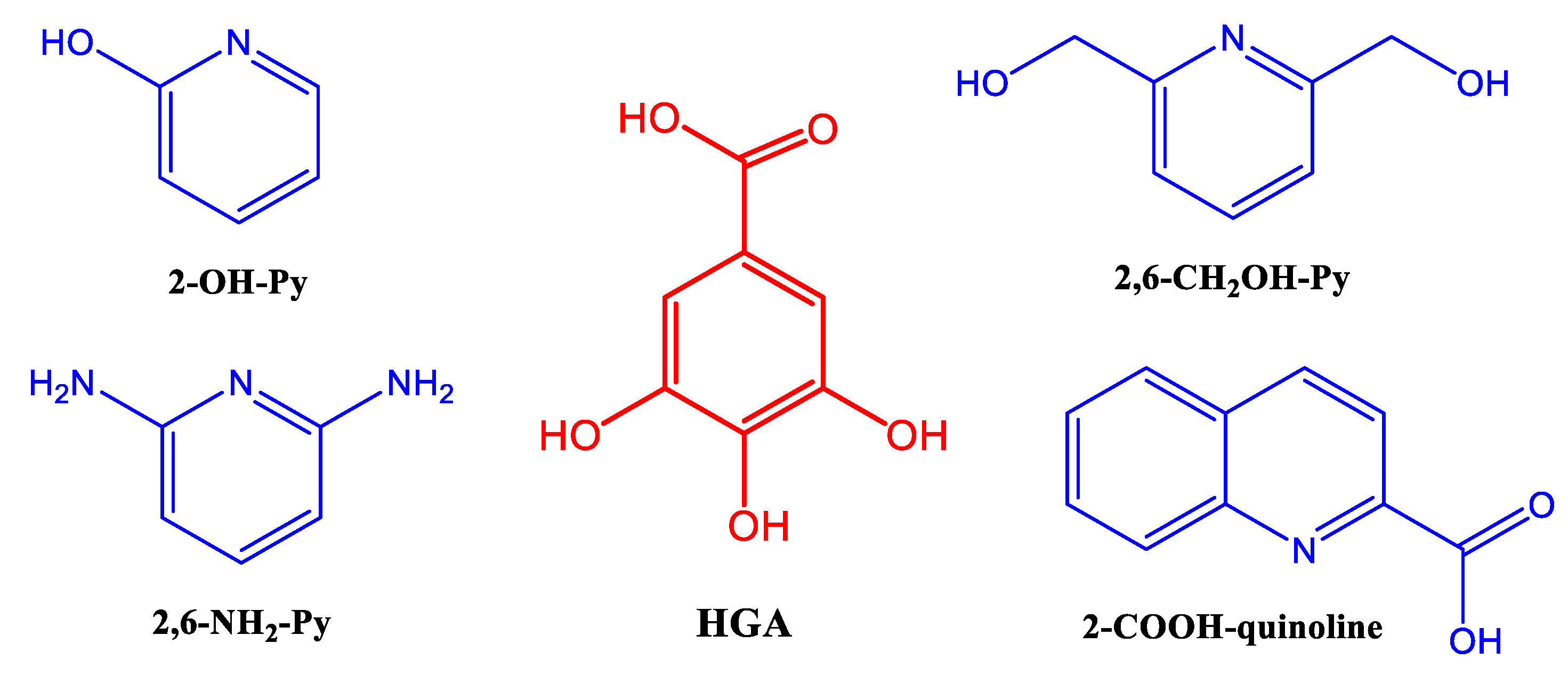
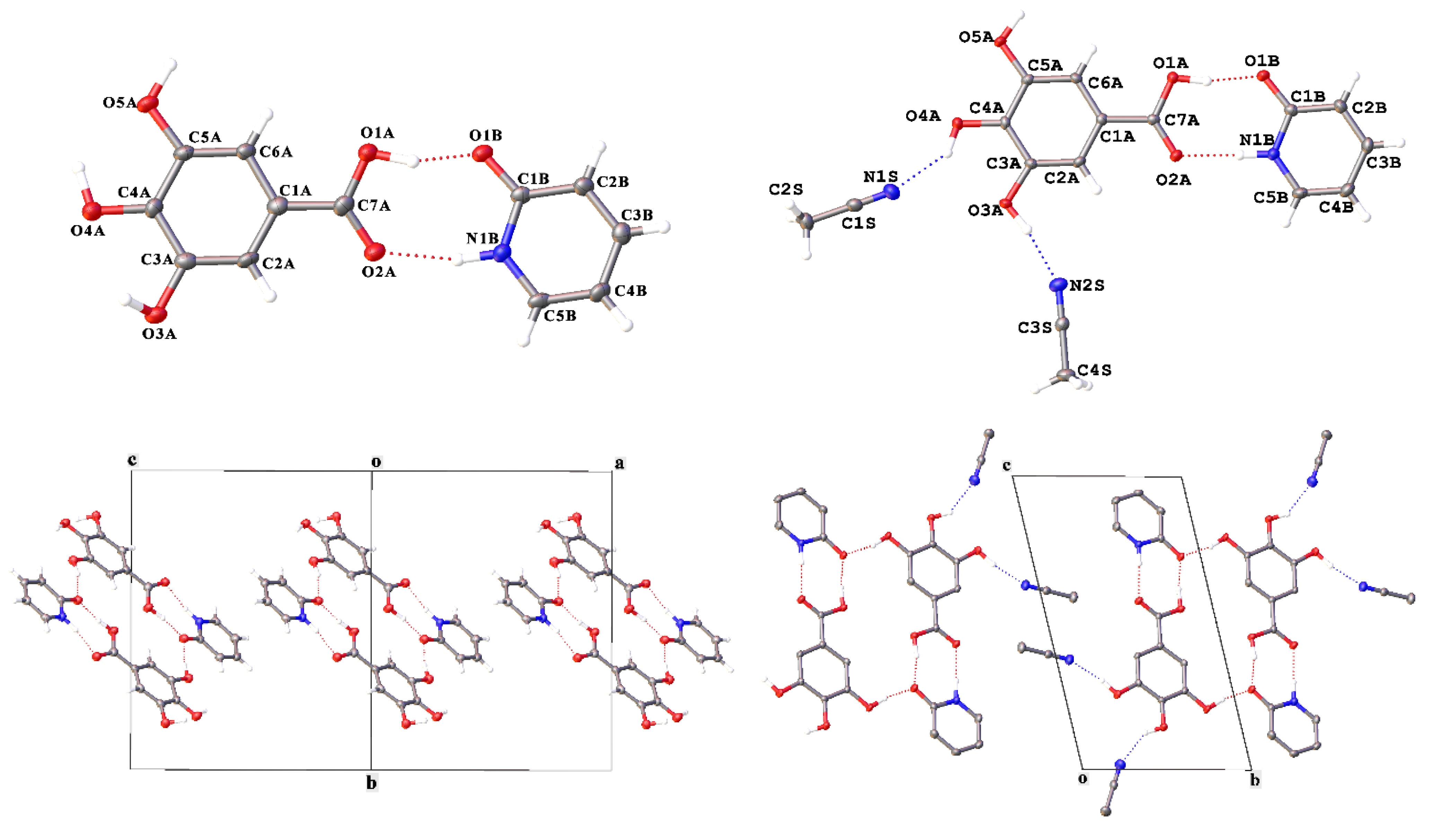
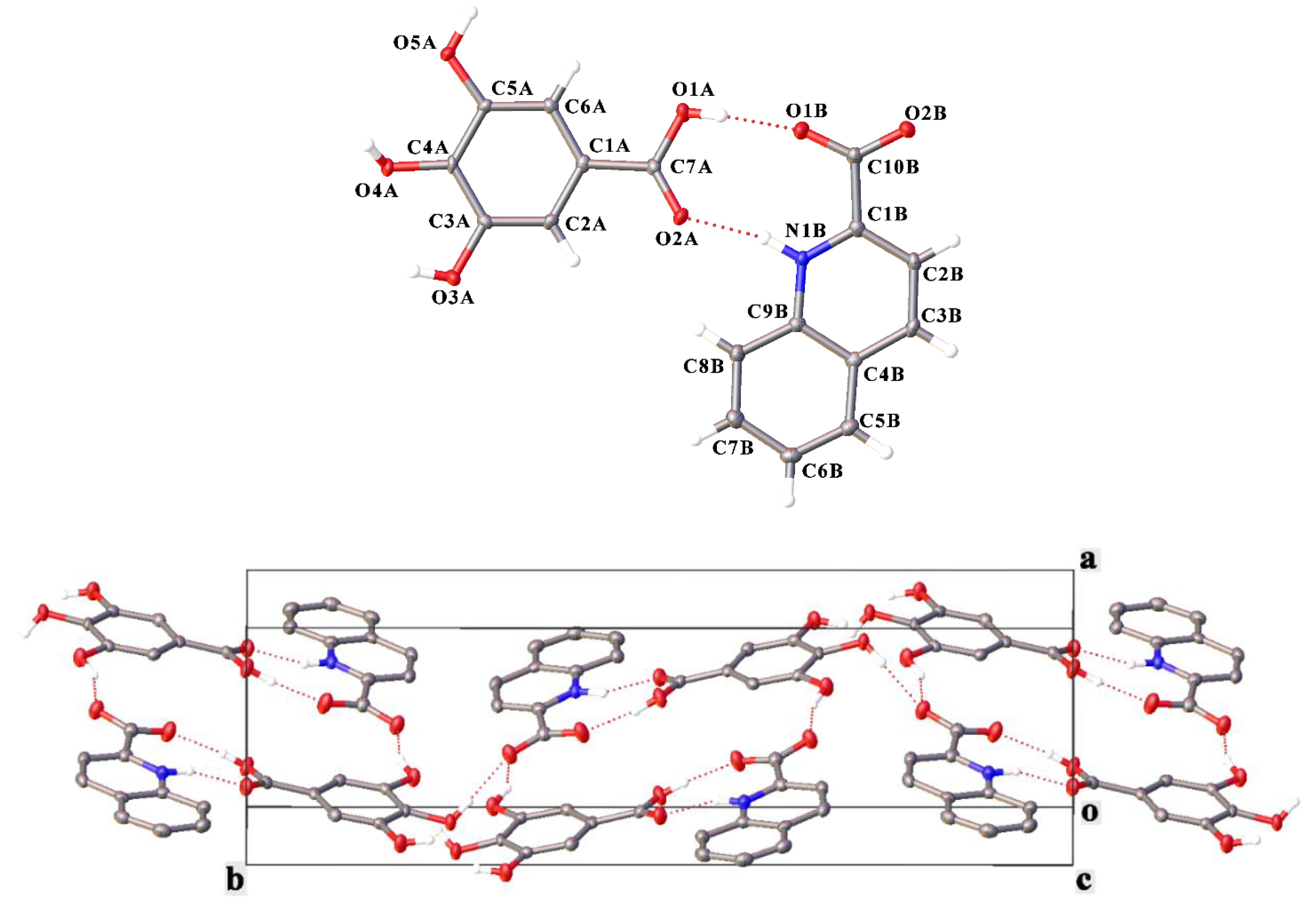
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desiraju, G.R. Crystal Engineering: A Holistic View. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Simanek, E.E.; Mathias, J.P.; Seto, C.T.; Chin, D.; Mammen, M.; Gordon, D.M. Noncovalent Synthesis: Using Physical-Organic Chemistry to Make Aggregates. Acc. Chem. Res. 1995, 28, 37–44. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Nishio, M. CH/π Hydrogen Bonds in Crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The Cation−π Interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; Stoddart, J.F. π–π Interactions in Self-Assembly. J. Phys. Org. Chem. 1997, 10, 254–272. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Krishnamohan, S.; Zaworotko, M.J. X-Ray Crystal Structure of C6H3(CO2H)3-1,3,5·1.5(4,4′-Bipy): A ‘Super Trimesic Acid’ Chicken-Wire Grid. Chem. Commun. 1996, 23, 2655–2656. [Google Scholar] [CrossRef]
- Dale, S.H.; Elsegood, M.R.J.; Hemmings, M.; Wilkinson, A.L. The Co-Crystallisation of Pyridine with Benzenepolycarboxylic Acids: The Interplay of Strong and Weak Hydrogen Bonding Motifs. CrystEngComm 2004, 6, 207–214. [Google Scholar] [CrossRef]
- Vishweshwar, P.; Nangia, A.; Lynch, V.M. Recurrence of Carboxylic Acid−Pyridine Supramolecular Synthon in the Crystal Structures of Some Pyrazinecarboxylic Acids. J. Org. Chem. 2002, 67, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Beatty, A.M.; Helfrich, B.A. “Total Synthesis” Supramolecular Style: Design and Hydrogen-Bond-Directed Assembly of Ternary Supermolecules. Angew. Chem. Int. Ed. 2001, 40, 3240–3242. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Maini, L.; Polito, M.; Scaccianoce, L.; Cojazzi, G.; Grepioni, F. Design of Organometallic Molecular and Ionic Materials. Coord. Chem. Rev. 2001, 216–217, 225–248. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Wirsig, T.B.; Barbour, L.J.; Atwood, J.L. Crystal Engineering of Nonporous Organic Solids for Methane Sorption. Chem. Commun. 2005, 35, 4420–4422. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, S.; Lin, H.H.; Lee, C.J.; Wei, H.H. A Two-Dimensional CuIIGdIII Compound Self-Assembled by H-Bonding and Intermolecular Weak Coordinate Bonding between the Dinuclear Cores: Structure and Magnetic Properties. Inorg. Chem. Commun. 2002, 5, 585–588. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical Cocrystals, Salts and Multicomponent Systems; Intermolecular Interactions and Property Based Design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almarsson, Ö.; Zaworotko, M.J. Crystal Engineering of the Composition of Pharmaceutical Phases. Do Pharmaceutical Co-Crystals Represent a New Path to Improved Medicines? Chem. Commun. 2004, 17, 1889–1896. [Google Scholar] [CrossRef]
- Etter, M.C. Hydrogen Bonds as Design Elements in Organic Chemistry. Available online: https://pubs.acs.org/doi/pdf/10.1021/j100165a007 (accessed on 11 February 2021).
- Shattock, T.R.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Carboxylic Acid·Pyridine Hydrogen Bonds in Cocrystals That also Contain a Hydroxyl Moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Chitra, R.; Choudhury, R.R.; Thiruvenkatam, V.; Hosur, M.V.; Guru Row, T.N. Molecular Interactions in Bis(2-Aminopyridinium) Malonate: A Crystal Isostructural to Bis(2-Aminopyridinium) Maleate Crystal. J. Mol. Struct. 2012, 1010, 46–51. [Google Scholar] [CrossRef]
- Varughese, S.; Pedireddi, V.R. A Competitive Molecular Recognition Study: Syntheses and Analysis of Supramolecular Assemblies of 3,5-Dihydroxybenzoic Acid and Its Bromo Derivative with Some N-Donor Compounds. Chem.-A Eur. J. 2006, 12, 1597–1609. [Google Scholar] [CrossRef]
- Gunawardana, C.A.; Aakeröy, C.B. Co-Crystal Synthesis: Fact, Fancy, and Great Expectations. Chem. Commun. 2018, 54, 14047–14060. [Google Scholar] [CrossRef]
- Ponce, A.; Brostoff, L.B.; Gibbons, S.K.; Zavalij, P.; Viragh, C.; Hooper, J.; Alnemrat, S.; Gaskell, K.J.; Eichhorn, B. Elucidation of the Fe(III) Gallate Structure in Historical Iron Gall Ink. Anal. Chem. 2016, 88, 5152–5158. [Google Scholar] [CrossRef] [Green Version]
- Dewick, P.M.; Haslam, E. Phenol Biosynthesis in Higher Plants. Gallic Acid. Biochem. J. 1969, 113, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ow, Y.Y.; Stupans, I. Gallic Acid and Gallic Acid Derivatives: Effects on Drug Metabolizing Enzymes. Curr. Drug Metab. 2003, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.; Marcolino-Junior, L.H.; Bergamini, M.F. Voltammetric Determination of the Antioxidant Capacity in Wine Samples Using a Carbon Nanotube Modified Electrode. J. Agric. Food Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, Antiviral and Antimicrobial Activities of Alkaloids, Flavonoids, and Phenolic Acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Huang, A.C.; Wu, P.P.; Lin, H.Y.; Chueh, F.S.; Yang, J.S.; Lu, C.C.; Chiang, J.H.; Meng, M.; Chung, J.G. Gallic Acid Suppresses the Migration and Invasion of PC-3 Human Prostate Cancer Cells via Inhibition of Matrix Metalloproteinase-2 and -9 Signaling Pathways. Oncol. Rep. 2011, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, F.; Jiang, H.; Wu, K.; Zheng, X.; Cai, Y.; Katakowski, M.; Chopp, M.; To, S.S.T. Gallic Acid Suppresses Cell Viability, Proliferation, Invasion and Angiogenesis in Human Glioma Cells. Eur. J. Pharmacol. 2010, 641, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priscilla, D.H.; Prince, P.S.M. Cardioprotective Effect of Gallic Acid on Cardiac Troponin-T, Cardiac Marker Enzymes, Lipid Peroxidation Products and Antioxidants in Experimentally Induced Myocardial Infarction in Wistar Rats. Chem.-Biol. Interact. 2009, 179, 118–124. [Google Scholar] [CrossRef]
- Clarke, H.D.; Arora, K.K.; Wojtas, Ł.; Zaworotko, M.J. Polymorphism in Multiple Component Crystals: Forms III and IV of Gallic Acid Monohydrate. Cryst. Growth Des. 2011, 11, 964–966. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Kong, S.; Zheng, X.; Zheng, J.; Shi, R. A Valid Assay for the Pharmacokinetic Study of Gallic Acid from Choerospondiatis Fructus in Rabbit Plasma by LC/MS/MS. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 235–244. [Google Scholar] [CrossRef]
- Jo, C.R.; Jeong, I.Y.; Lee, N.Y.; Kim, K.S.; Byun, M.W. Synthesis of a Novel Compound from Gallic Acid and Linoleic Acid and Its Biological Functions. Food Sci. Biotechnol. 2006, 15, 317–320. [Google Scholar]
- Maldonado, O.S.; Lucas, R.; Comelles, F.; González, M.J.; Parra, J.L.; Medina, I.; Morales, J.C. Synthesis and Characterization of Phenolic Antioxidants with Surfactant Properties: Glucosyl-and Glucuronosyl Alkyl Gallates. Tetrahedron 2011, 67, 7268–7279. [Google Scholar] [CrossRef]
- Braun, D.E.; Bhardwaj, R.M.; Florence, A.J.; Tocher, D.A.; Price, S.L. Complex Polymorphic System of Gallic Acid—Five Monohydrates, Three Anhydrates, and over 20 Solvates. Cryst. Growth Des. 2013, 13, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Chen, J.M.; Lu, T.B. Lenalidomide–Gallic Acid Cocrystals with Constant High Solubility. Cryst. Growth Des. 2015, 15, 4869–4875. [Google Scholar] [CrossRef]
- Surov, A.O.; Churakov, A.V.; Proshin, A.N.; Dai, X.L.; Lu, T.; Perlovich, G.L. Cocrystals of a 1,2,4-Thiadiazole-Based Potent Neuroprotector with Gallic Acid: Solubility, Thermodynamic Stability Relationships and Formation Pathways. Phys. Chem. Chem. Phys. 2018, 20, 14469–14481. [Google Scholar] [CrossRef] [PubMed]
- Pantwalawalkar, J.; More, H.; Bhange, D.; Patil, U.; Jadhav, N. Novel Curcumin Ascorbic Acid Cocrystal for Improved Solubility. J. Drug Deliv. Sci. Technol. 2021, 61, 102233. [Google Scholar] [CrossRef]
- Gandhi, A.K.; Shi, T.; Li, M.; Jungnelius, U.; Romano, A.; Tabernero, J.; Siena, S.; Schafer, P.H.; Chopra, R. Immunomodulatory Effects in a Phase II Study of Lenalidomide Combined with Cetuximab in Refractory KRAS-Mutant Metastatic Colorectal Cancer Patients. PLoS ONE 2013, 8, e80437. [Google Scholar] [CrossRef]
- Goswami, S.; Ghosh, A.; Borah, K.; Mahanta, A.; Guha, A.K.; Bora, S.J. Structure-Property Correlation in Gallic Acid and 4-Cyanopyridine Cocrystal and Binding Studies with Drug Efflux Pump in Bacteria. J. Mol. Struct. 2021, 1225, 129279. [Google Scholar] [CrossRef]
- Chen, J.M.; Li, S.; Lu, T.B. Pharmaceutical Cocrystals of Ribavirin with Reduced Release Rates. Cryst. Growth Des. 2014, 14, 6399–6408. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, H.; Chen, W. Synthesis, Characterization and Antimicrobial Activity of Three Gallates Containing Imidazole, Benzimidazole and Triclosan Units. Asian J. Chem. 2014, 26, 513–520. [Google Scholar] [CrossRef]
- Steiner, T. Competition of Hydrogen-Bond Acceptors for the Strong Carboxyl Donor. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57, 103–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, J.A.; Bis, J.A.; Vishweshwar, P.; Shattock, T.R.; McLaughlin, O.L.; Zaworotko, M.J. Crystal Engineering of the Composition of Pharmaceutical Phases. 3. Primary Amide Supramolecular Heterosynthons and Their Role in the Design of Pharmaceutical Co-Crystals. Z. Für Krist.-Cryst. Mater. 2005, 220, 340–350. [Google Scholar] [CrossRef]
- Bora, P.; Saikia, B.; Sarma, B. Regulation of Π···π Stacking Interactions in Small Molecule Cocrystals and/or Salts for Physiochemical Property Modulation. Cryst. Growth Des. 2018, 18, 1448–1458. [Google Scholar] [CrossRef]
- Sarma, B.; Nath, N.K.; Bhogala, B.R.; Nangia, A. Synthon Competition and Cooperation in Molecular Salts of Hydroxybenzoic Acids and Aminopyridines. Cryst. Growth Des. 2009, 9, 1546–1557. [Google Scholar] [CrossRef]
- Hemamalini, M.; Loh, W.S.; Quah, C.K.; Fun, H.K. Investigation of Supramolecular Synthons and Structural Characterisation of Aminopyridine-Carboxylic Acid Derivatives. Chem. Cent. J. 2014, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmerer, A.; Govindraju, S.; Johnston, M.; Motloung, X.; Savig, K.L. Co-Crystals and Molecular Salts of Carboxylic Acid/Pyridine Complexes: Can Calculated p K a’s Predict Proton Transfer? A Case Study of Nine Complexes. CrystEngComm 2015, 17, 3591–3595. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.H.; Motherwell, W.D.S.; Raithby, P.R.; Shields, G.P.; Taylor, R. Systematic Analysis of the Probabilities of Formation of Bimolecular Hydrogen-Bonded Ring Motifs in Organic Crystal Structures. New J. Chem. 1999, 23, 25–34. [Google Scholar] [CrossRef]
- Mohamed, S.; Tocher, D.A.; Vickers, M.; Karamertzanis, P.G.; Price, S.L. Salt or Cocrystal? A New Series of Crystal Structures Formed from Simple Pyridines and Carboxylic Acids. Cryst. Growth Des. 2009, 9, 288–2889. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, G.R. Combinatorial Exploration of the Structural Landscape of Acid–Pyridine Cocrystals. Cryst. Growth Des. 2014, 14, 1375–1385. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Does It Really Matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt−Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutmann, F. Charge Transfer Complexes in Biological Systems; M. Dekker: New York, NY, USA, 1997; ISBN 0-8247-9986-0. [Google Scholar]
- Khan, I.M.; Ahmad, A. Synthesis, Characterization, Structural, Spectrophotometric and Antimicrobial Activity of Charge Transfer Complex of p-Phenylenediamine with 3,5-Dinitrosalicylic Acid. J. Mol. Struct. 2010, 975, 381–388. [Google Scholar] [CrossRef]
- Khan, I.M.; Ahmad, A.; Aatif, M. Synthesis, Single-Crystal Characterization, Antimicrobial Activity and Remarkable In Vitro DNA Interaction of Hydrogen-Bonded Proton-Transfer Complex of 1,10-Phenanthroline with 2,4,6-Trinitrophenol. J. Photochem. Photobiol. B Biol. 2011, 105, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Chow, P.S.; Tan, R.B. Polymorphism in Cocrystals: A Review and Assessment of Its Significance. CrystEngComm 2014, 16, 3451–3465. [Google Scholar] [CrossRef]
- Liu, L.D.; Liu, S.L.; Liu, Z.X.; Hou, G.G. Synthesis, Structure, Antitumor Activity of Novel Pharmaceutical Co-Crystals Based on Bispyridyl-Substituted α, β-Unsaturated Ketones with Gallic Acid. J. Mol. Struct. 2016, 1112, 1–8. [Google Scholar] [CrossRef]
- Kavuru, P.; Aboarayes, D.; Arora, K.K.; Clarke, H.D.; Kennedy, A.; Marshall, L.; Ong, T.T.; Perman, J.; Pujari, T.; Wojtas, Ł.; et al. Hierarchy of Supramolecular Synthons: Persistent Hydrogen Bonds between Carboxylates and Weakly Acidic Hydroxyl Moieties in Cocrystals of Zwitterions. Cryst. Growth Des. 2010, 10, 3568–3584. [Google Scholar] [CrossRef]
- Dong, F.Y.; Wu, J.; Tian, H.Y.; Ye, Q.M.; Jiang, R.W. Gallic Acid Pyridine Monosolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o3096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyothi, K.L.; Kumara, K.; Hema, M.K.; Mahesha; Gautam, R.; Guru Row, T.N.; Lokanath, N.K. Structural Elucidation, Theoretical Insights and Thermal Properties of Three Novel Multicomponent Molecular Forms of Gallic Acid with Hydroxypyridines. J. Mol. Struct. 2020, 1207, 127828. [Google Scholar] [CrossRef]
- Kaur, R.; Perumal, S.S.R.R.; Bhattacharyya, A.J.; Yashonath, S.; Guru Row, T.N. Structural Insights into Proton Conduction in Gallic Acid—Isoniazid Cocrystals. Cryst. Growth Des. 2014, 14, 423–426. [Google Scholar] [CrossRef]
- Shen, F.M.; Lush, S.F. 4,4′-Ethyl enedipyridinium Bis (3,4,5-Trihy droxy benzoate) Sesquihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o2056–o2057. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Q.; Qing, X.; Yu, H.; Wang, G. Crystal structure of 4,4′-bipyridine—3,4,5-trihydroxybenzoic acid—water (1:2:2), C10H8N2·2C7H6O5·2H2O. Z. Für Krist.-New Cryst. Struct. 2010, 225, 701–702. [Google Scholar] [CrossRef]
- De Kowalewski, D.G.; Contreras, R.H.; Díez, E.; Esteban, A. NMR J(C,C) Scalar Coupling Analysis of the Effects of Substituents on the Keto–Enol Tautomeric Equilibrium in 2-OH-n-X-Pyridines. An Experimental and DFT Study. Mol. Phys. 2004, 102, 2607–2615. [Google Scholar] [CrossRef]
- Yang, H.W.; Craven, B.M. Charge Density Study of 2-Pyridone. Acta Crystallogr. Sect. B Struct. Sci. 1998, 54, 912–920. [Google Scholar] [CrossRef]
- Zhoujin, Y.; Yang, X.; Zhang, M.; Guo, J.; Parkin, S.; Li, T.; Yu, F.; Long, S. Synthon Polymorphism and π–π Stacking in N-Phenyl-2-Hydroxynicotinanilides. Cryst. Growth Des. 2021, 21, 6155–6165. [Google Scholar] [CrossRef]
- Li, P.; Zhao, C.; Smith, M.D.; Shimizu, K.D. Comprehensive Experimental Study of N-Heterocyclic π-Stacking Interactions of Neutral and Cationic Pyridines. J. Org. Chem. 2013, 78, 5303–5313. [Google Scholar] [CrossRef]
- Campos-Gaxiola, J.J.; García-Grajeda, B.A.; Hernández-Ahuactzi, I.F.; Guerrero-Álvarez, J.A.; Höpfl, H.; Cruz-Enríquez, A. Supramolecular Networks in Molecular Complexes of Pyridine Boronic Acids and Polycarboxylic Acids: Synthesis, Structural Characterization and Fluorescence Properties. CrystEngComm 2017, 19, 3760–3775. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. Acta 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Hou, B.; Wang, J.K.; Lin, G. Effect of Solvent on Crystallization Behavior of Xylitol. J. Cryst. Growth 2006, 290, 192–196. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Trivedi, D.R. Pharmaceutical Salts of Ethionamide with GRAS Counter Ion Donors to Enhance the Solubility. Eur. J. Pharm. Sci. 2017, 96, 578–589. [Google Scholar] [CrossRef]
- Khan, I.M.; Ahmad, A.; Kumar, S. Synthesis, Spectroscopic Characterization and Structural Investigations of a New Charge Transfer Complex of 2,6-Diaminopyridine with 3,5-Dinitrobenzoic Acid: DNA Binding and Antimicrobial Studies. J. Mol. Struct. 2013, 1035, 38–45. [Google Scholar] [CrossRef]
- Draguta, S.; Fonari, M.S.; Bejagam, S.N.; Storms, K.; Lindline, J.; Timofeeva, T.V. Structural Similarities and Diversity in a Series of Crystalline Solids Composed of 2-Aminopyridines and Glutaric Acid. Struct. Chem. 2016, 27, 1303–1315. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Wen, X.; Liu, B.; Jin, S.; Wang, D. Structure of Seven Organic Salts Assembled from 2,6-Diaminopyridine with Monocarboxylic Acids, Dicarboxylic Acids, and Tetracarboxylic Acids. J. Mol. Struct. 2015, 1093, 82–95. [Google Scholar] [CrossRef]
- Blackholly, L.R.; Shepherd, H.J.; Hiscock, J.R. ‘Frustrated’ Hydrogen Bond Mediated Amphiphile Self-Assembly—A Solid State Study. CrystEngComm 2016, 18, 7021–7028. [Google Scholar] [CrossRef] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Long, S.; Li, T. Enforcing Molecule’s π-Conjugation and Consequent Formation of the Acid−Acid Homosynthon over the Acid−Pyridine Heterosynthon in 2-Anilinonicotinic Acids. Cryst. Growth Des. 2010, 10, 2465–2469. [Google Scholar] [CrossRef]
- Rusu, E.; Shova, S.; Rusu, G. 1,2-Bis(Pyridin-4-Yl)Diazene–3,4,5-Trihy droxy benzoic Acid–Methanol (3/2/2). Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seaton, C.C.; Munshi, T.; Williams, S.E.; Scowen, I.J. Multi-Component Crystals of 4-Phenylpyridine: Challenging the Boundaries between Co-Crystal and Organic Salt Formation with Insight into Solid-State Proton Transfer. CrystEngComm 2013, 15, 5250–5260. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting Hydrogen-Bond Strengths from Acid-Base Molecular Properties. The PK(a) Slide Rule: Toward the Solution of a Long-Lasting Problem. Acc. Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, S.E.; Clymer, T.M.; Pakkala, V.S.; Hebert, S.P.; Reeping, K.; Firestine, S.M.; Evanseck, J.D. Intramolecular Charge-Assisted Hydrogen Bond Strength in Pseudochair Carboxyphosphate. J. Phys. Chem. B 2015, 119, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Sedghiniya, S.; Soleimannejad, J.; Janczak, J. The Salt–Cocrystal Spectrum in Salicylic Acid–Adenine: The Influence of Crystal Structure on Proton-Transfer Balance. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 412–421. [Google Scholar] [CrossRef]
- Garg, U.; Azim, Y.; Kar, A.; Pradeep, C.P. Cocrystals/Salt of 1-Naphthaleneacetic Acid and Utilizing Hirshfeld Surface Calculations for Acid–Aminopyrimidine Synthons. CrystEngComm 2020, 22, 2978–2989. [Google Scholar] [CrossRef]
- Huang, K.S.; Britton, D.; Byrn, S.R. A Novel Class of Phenol–Pyridine Co-Crystals for Second Harmonic Generation. J. Mater. Chem. 1997, 7, 713–720. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–Base Crystalline Complexes and the pKa Rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Bekö, S.L.; Schmidt, M.U.; Bond, A.D. An Experimental Screen for Quinoline /Fumaric Acid Salts and Co-Crystals. CrystEngComm 2012, 14, 1967–1971. [Google Scholar] [CrossRef]
- Stilinović, V.; Kaitner, B. Salts and Co-Crystals of Gentisic Acid with Pyridine Derivatives: The Effect of Proton Transfer on the Crystal Packing (and Vice Versa). Cryst. Growth Des. 2012, 12, 5763–5772. [Google Scholar] [CrossRef]
- Wróbel, R.; Chmurzyński, L. Potentiometric PKa Determination of Standard Substances in Binary Solvent Systems. Anal. Chim. Acta 2000, 405, 303–308. [Google Scholar] [CrossRef]
- Delori, A.; Galek, P.T.; Pidcock, E.; Patni, M.; Jones, W. Knowledge-Based Hydrogen Bond Prediction and the Synthesis of Salts and Cocrystals of the Anti-Malarial Drug Pyrimethamine with Various Drug and GRAS Molecules. CrystEngComm 2013, 15, 2916–2928. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, H.; Li, Y.; Chen, R.; Yang, Y.; Wang, L. Supramolecular Assemblies of Tetrafluoroterephthalic Acid and N-Heterocycles via Various Strong Hydrogen Bonds and Weak CH⋯F Interactions: Synthons Cooperation, Robust Motifs and Structural Diversity. J. Mol. Struct. 2016, 1122, 256–267. [Google Scholar] [CrossRef]
- Pang, Y.; Xing, P.; Geng, X.; Zhu, Y.; Liu, F.; Wang, L. Supramolecular Assemblies of 2-Hydroxy-3-Naphthoic Acid and N-Heterocycles via Various Strong Hydrogen Bonds and Weak X⋯π (X = C–H, π) Interactions. RSC Adv. 2015, 5, 40912–40923. [Google Scholar] [CrossRef]
- Basu, T.; Sparkes, H.A.; Mondal, R. Construction of Extended Molecular Networks with Heterosynthons in Cocrystals of Pyrazole and Acids. Cryst. Growth Des. 2009, 9, 5164–5175. [Google Scholar] [CrossRef]
- Jetti, R.K.R.; Boese, R.; Sarma, J.A.R.P.; Reddy, L.S.; Vishweshwar, P.; Desiraju, G.R. Auf Der Suche Nach Einem Polymorph: Eine Zweite Kristallform von 6-Amino-2-Phenylsulfonylimino-1,2-Dihydropyridin. Angew. Chem. 2003, 115, 2008–2012. [Google Scholar] [CrossRef]
- Sreekanth, B.R.; Vishweshwar, P.; Vyas, K. Supramolecular Synthon Polymorphism in 2:1 Co-Crystal of 4-Hydroxybenzoic Acid and 2,3,5,6-Tetramethylpyrazine. Chem. Commun. 2007, 23, 2375–2377. [Google Scholar] [CrossRef]
- Kumar, S.S.; Nangia, A. A Solubility Comparison of Neutral and Zwitterionic Polymorphs. Cryst. Growth Des. 2014, 14, 1865–1881. [Google Scholar] [CrossRef]
- Cao, H.L.; Zhou, J.R.; Cai, F.Y.; Lü, J.; Cao, R. Two-Component Pharmaceutical Cocrystals Regulated by Supramolecular Synthons Comprising Primary N···H···O Interactions. Cryst. Growth Des. 2019, 19, 3–16. [Google Scholar] [CrossRef]
- Babu, N.J.; Cherukuvada, S.; Thakuria, R.; Nangia, A. Conformational and Synthon Polymorphism in Furosemide (Lasix). Cryst. Growth Des. 2010, 10, 1979–1989. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.; Chow, P.S.; Tan, R.B. Cocrystallization with Flufenamic Acid: Comparison of Physicochemical Properties of Two Pharmaceutical Cocrystals. CrystEngComm 2014, 16, 5793–5801. [Google Scholar] [CrossRef]
- Wang, L.; Tan, B.; Zhang, H.; Deng, Z. Pharmaceutical Cocrystals of Diflunisal with Nicotinamide or Isonicotinamide. Org. Process Res. Dev. 2013, 17, 1413–1418. [Google Scholar] [CrossRef]
- Bavishi, D.D.; Borkhataria, C.H. Spring and Parachute: How Cocrystals Enhance Solubility. Prog. Cryst. Growth Charact. Mater. 2016, 62, 1–8. [Google Scholar] [CrossRef]
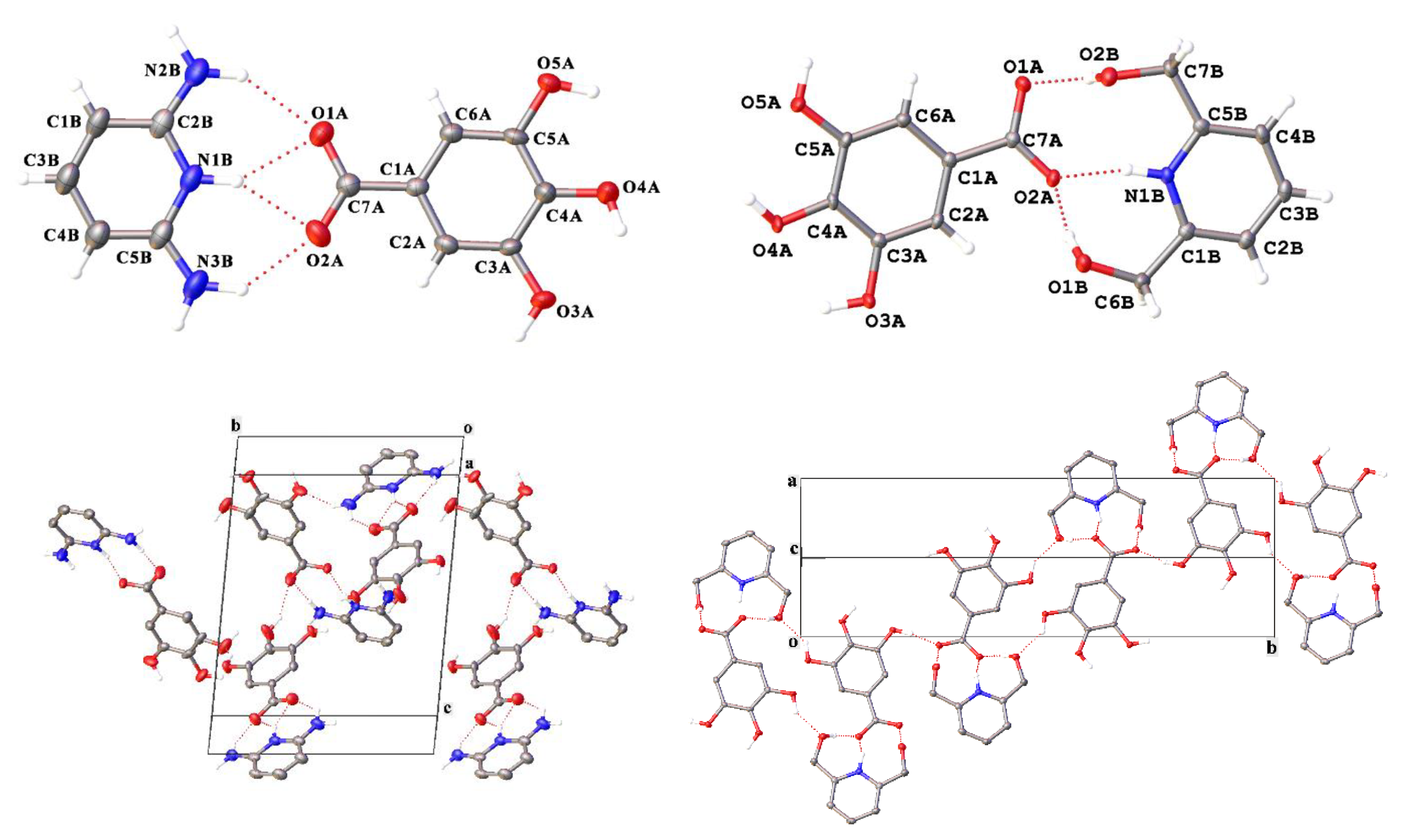
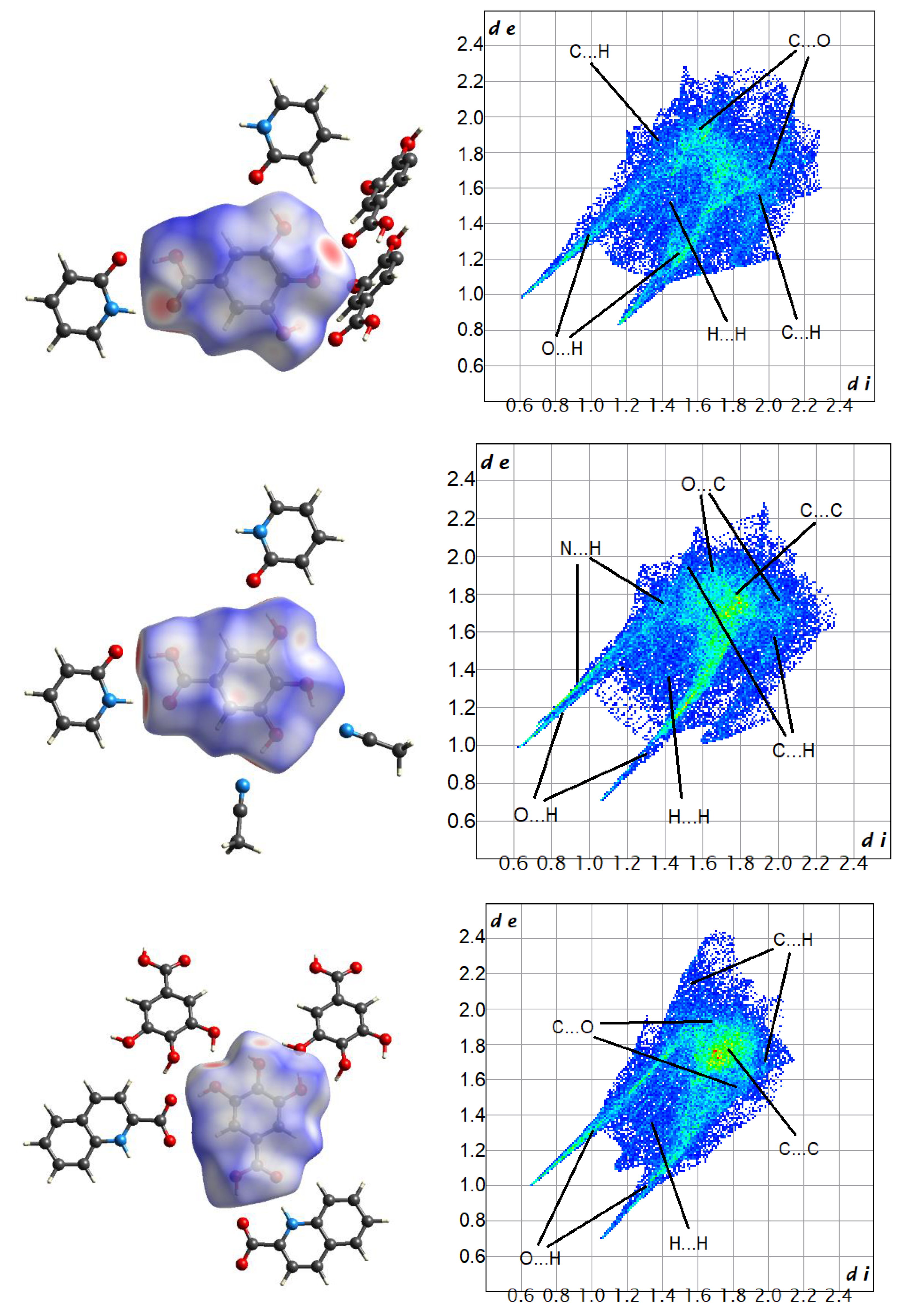
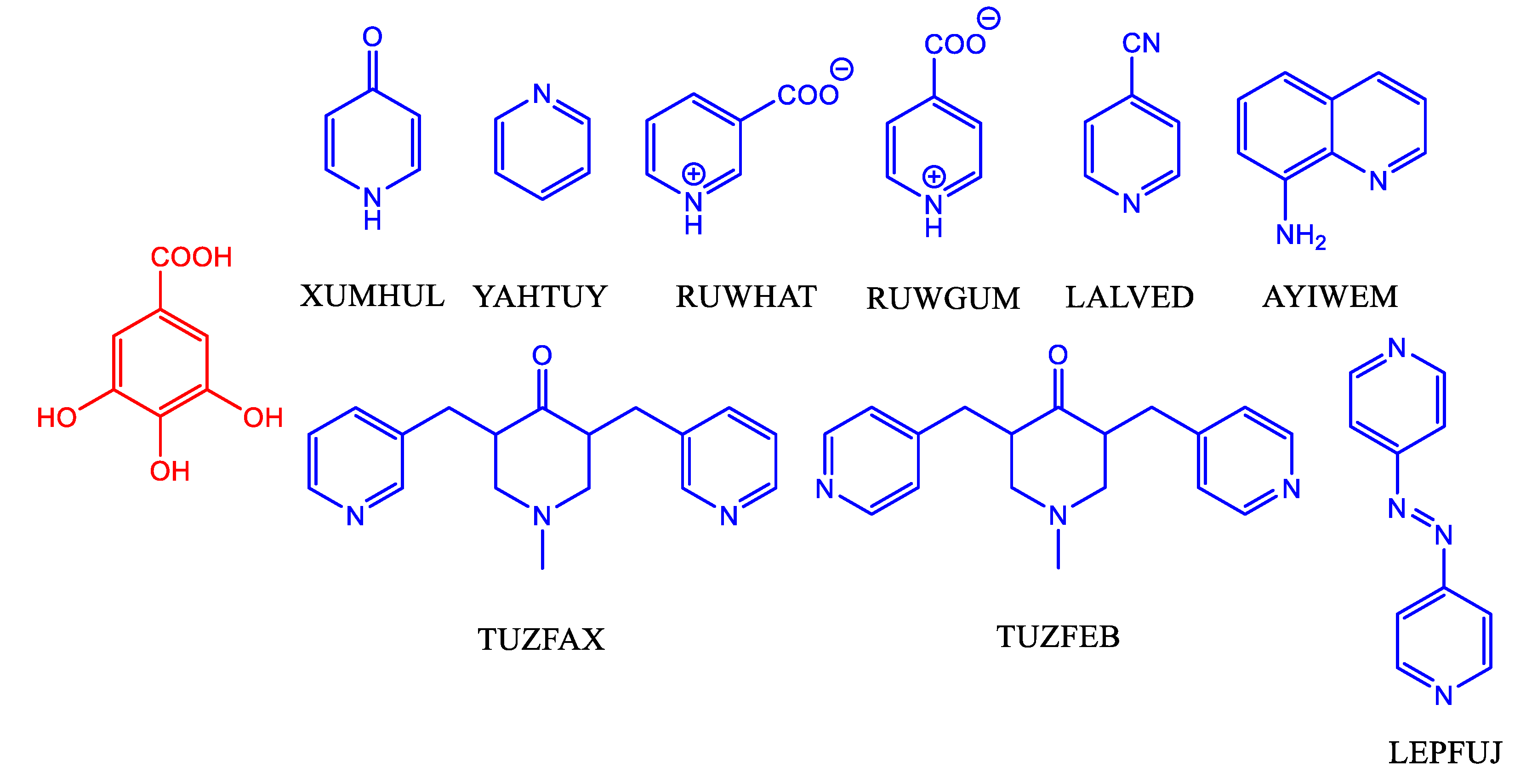
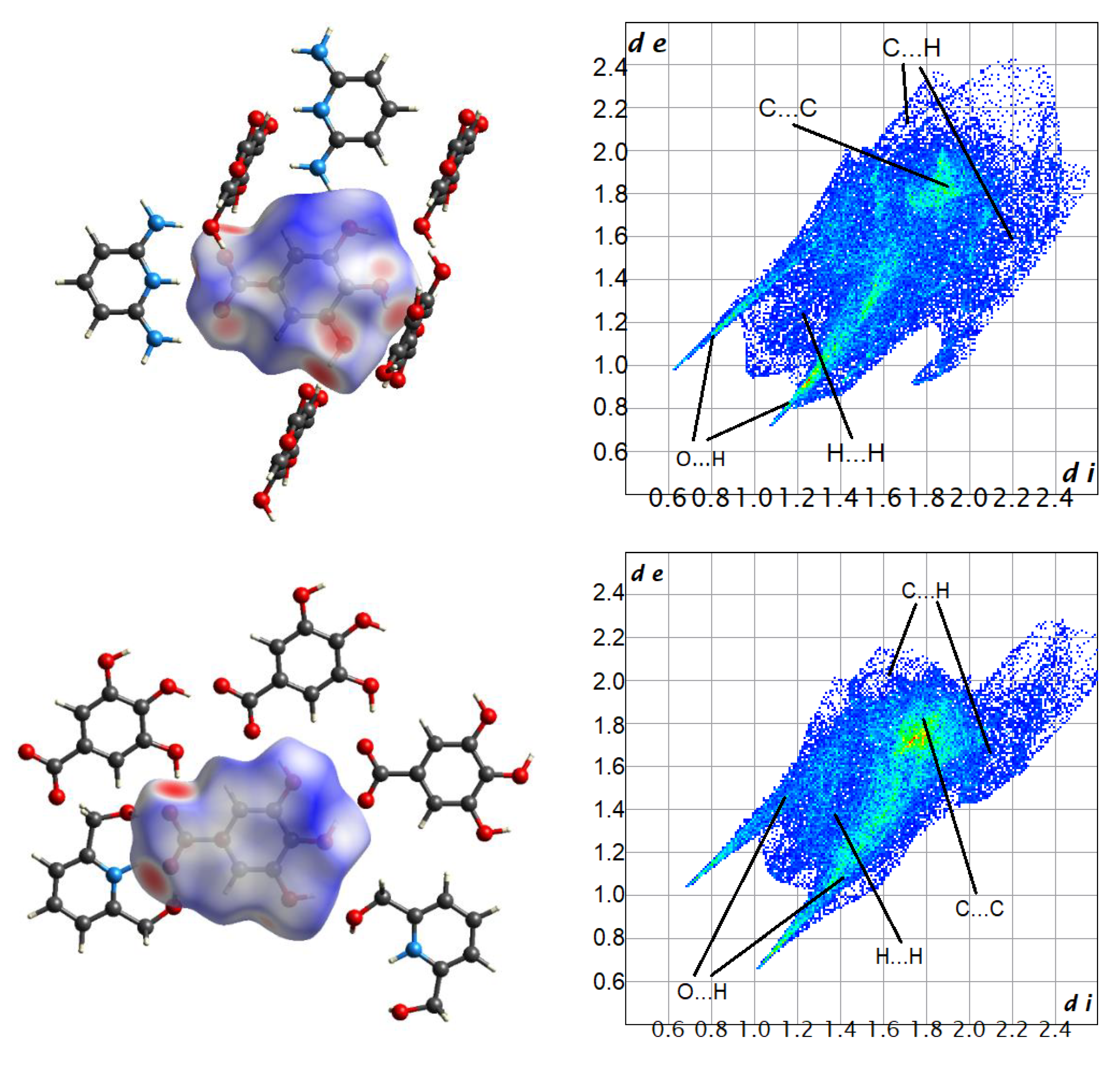

| Parameter | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula unit | C12H11NO6 | C16H17N3O6 | C17H13NO7 | C12.83H14.25N3.42O5 | C14H15NO7 |
| Formula weight | 265.22 | 347.32 | 343.28 | 296.36 | 309.27 |
| Temperature, K | 120 | 120 | 100 | 120 | 120 |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Triclinic | Monoclinic |
| Space group | P21/c | P-1 | P21/c | P-1 | P21/n |
| Z | 4 | 2 | 4 | 4 | 4 |
| a, Å | 13.003(4) | 7.4243(9) | 8.6047(3) | 8.7264(17) | 7.2058(12) |
| b, Å | 15.819(5) | 7.9649(10) | 24.5784(7) | 11.645(2) | 24.941(4) |
| c, Å | 13.037(5) | 14.5075(18) | 9.6157(4) | 14.032(3) | 7.7817(13) |
| α, ° | 90 | 103.247(3) | 90 | 84.710(4) | 90 |
| β, ° | 155.854(6) | 102.030(3) | 135.550(2) | 76.573(4) | 111.937(3) |
| γ, ° | 90 | 90.662(3) | 90 | 74.909(4) | 90 |
| V, Å3 | 1096.8(6) | 815.13(18) | 1424.11(9) | 1338.3(4) | 1297.3(4) |
| Dcalc (g cm−3) | 1.606 | 1.415 | 1.601 | 1.471 | 1.583 |
| Linear absorption, μ (cm−1) | 1.31 | 1.10 | 1.27 | 1.15 | 1.29 |
| F(000) | 552 | 364 | 712 | 621 | 648 |
| 2Θmax, ° | 52 | 54 | 54 | 52 | 54 |
| Reflections measured | 10,262 | 8783 | 11,930 | 13,215 | 12,477 |
| Independent reflections | 2153 | 3551 | 3071 | 5262 | 2750 |
| Observed reflections [I > 2σ(I)] | 1187 | 2546 | 2328 | 2597 | 1877 |
| Parameters | 174 | 228 | 226 | 398 | 199 |
| R1 | 0.0565 | 0.0488 | 0.0463 | 0.0683 | 0.0465 |
| wR2 | 0.1490 | 0.1207 | 0.1127 | 0.2101 | 0.1069 |
| GOF | 0.970 | 1.048 | 1.022 | 1.038 | 0.905 |
| Δρmax/Δρmin (e Å−3) | 0.263/−0.411 | 0.296/−0.343 | 0.324/−0.288 | 0.627/−0.531 | 0.235/−0.291 |
| Contacts | 1 | 2 | 3 | Average |
|---|---|---|---|---|
| O…H | 44.7 | 39.8 | 44.6 | 43.0 |
| N…H | 0.6 | 8.7 | 0.7 | 3.3 |
| C…H | 15.9 | 11.7 | 8.5 | 12.0 |
| H…H | 25.2 | 24.5 | 24.3 | 24.7 |
| C…C | 2.1 | 4.9 | 12.4 | 6.5 |
| O…O | 3.1 | 0.6 | 0.6 | 1.4 |
| C…N | 0.4 | 2.6 | 1.6 | 1.5 |
| C…O | 7.6 | 6.4 | 7.3 | 7.1 |
| N…O | 0.6 | 0.7 | 0.0 | 0.4 |
| Contacts | 4 * | 5 | Average |
|---|---|---|---|
| O…H | 46.7 (44.6) | 49.6 | 47.0 |
| N…H | 0.7 (0.2) | 0.5 | 0.5 |
| C…H | 14.9 (18.2) | 10.0 | 17.2 |
| H…H | 28.6 (30.1) | 24.6 | 27.4 |
| C…C | 4.9 (3.3) | 8.4 | 5.4 |
| O…O | 1.5 (1.3) | 2.6 | 2.0 |
| C…N | 1.5 (0.8) | 1.6 | 1.3 |
| C…O | 0.7 (1.0) | 2.5 | 1.4 |
| N…O | 0.6 (0.3) | 0.2 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denisov, G.L.; Nelyubina, Y.V. New Co-Crystals/Salts of Gallic Acid and Substituted Pyridines: An Effect of Ortho-Substituents on the Formation of an Acid–Pyridine Heterosynthon. Crystals 2022, 12, 497. https://doi.org/10.3390/cryst12040497
Denisov GL, Nelyubina YV. New Co-Crystals/Salts of Gallic Acid and Substituted Pyridines: An Effect of Ortho-Substituents on the Formation of an Acid–Pyridine Heterosynthon. Crystals. 2022; 12(4):497. https://doi.org/10.3390/cryst12040497
Chicago/Turabian StyleDenisov, Gleb L., and Yulia V. Nelyubina. 2022. "New Co-Crystals/Salts of Gallic Acid and Substituted Pyridines: An Effect of Ortho-Substituents on the Formation of an Acid–Pyridine Heterosynthon" Crystals 12, no. 4: 497. https://doi.org/10.3390/cryst12040497
APA StyleDenisov, G. L., & Nelyubina, Y. V. (2022). New Co-Crystals/Salts of Gallic Acid and Substituted Pyridines: An Effect of Ortho-Substituents on the Formation of an Acid–Pyridine Heterosynthon. Crystals, 12(4), 497. https://doi.org/10.3390/cryst12040497






