The Structure, Magnetic, and Gas Sensing Characteristics of W-Substituted Co-Ferrite Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation Route
2.2. Characterization Techniques
2.3. Gas Sensor Setup
3. Results and Discussion
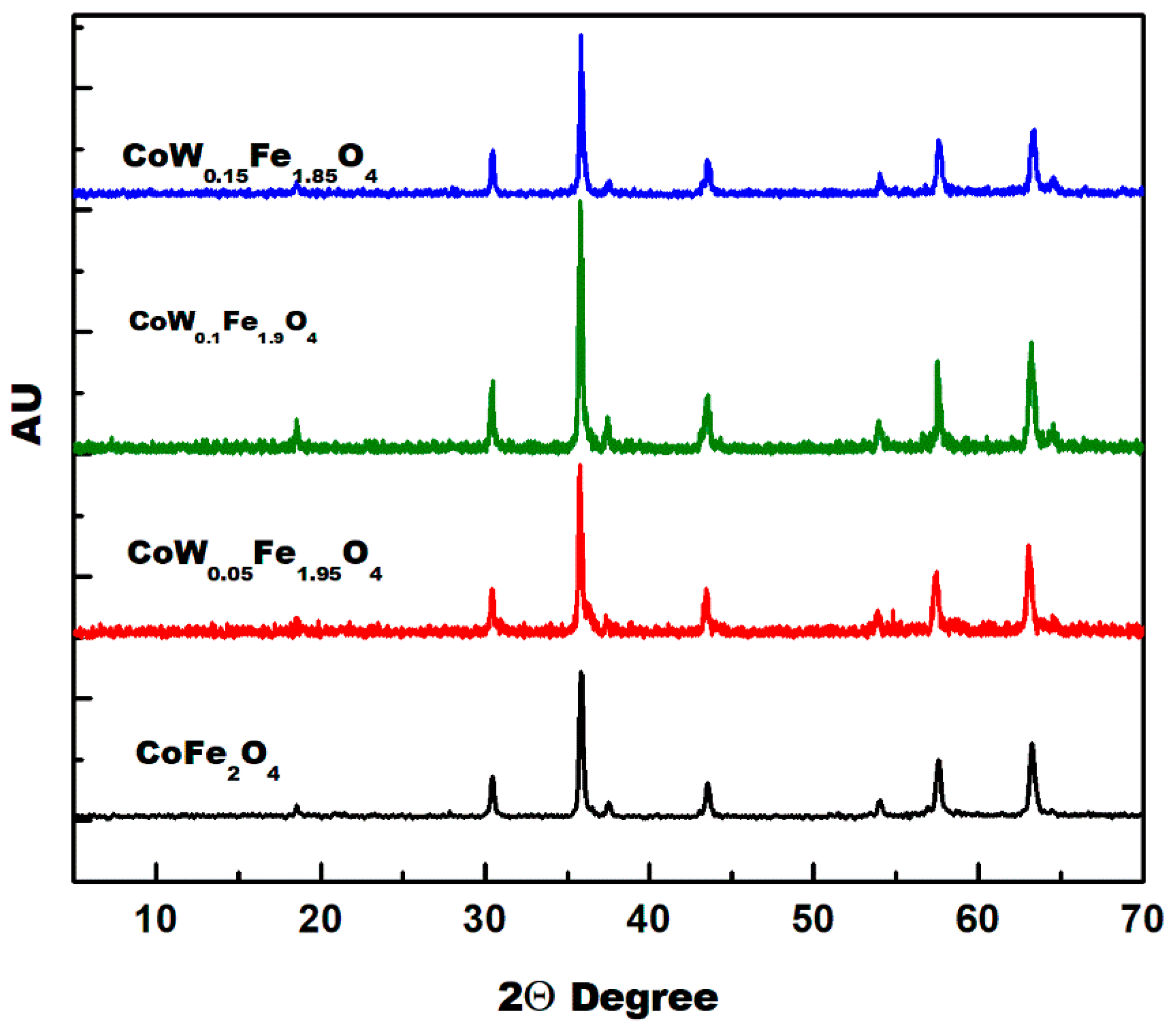
3.1. XRD Analysis
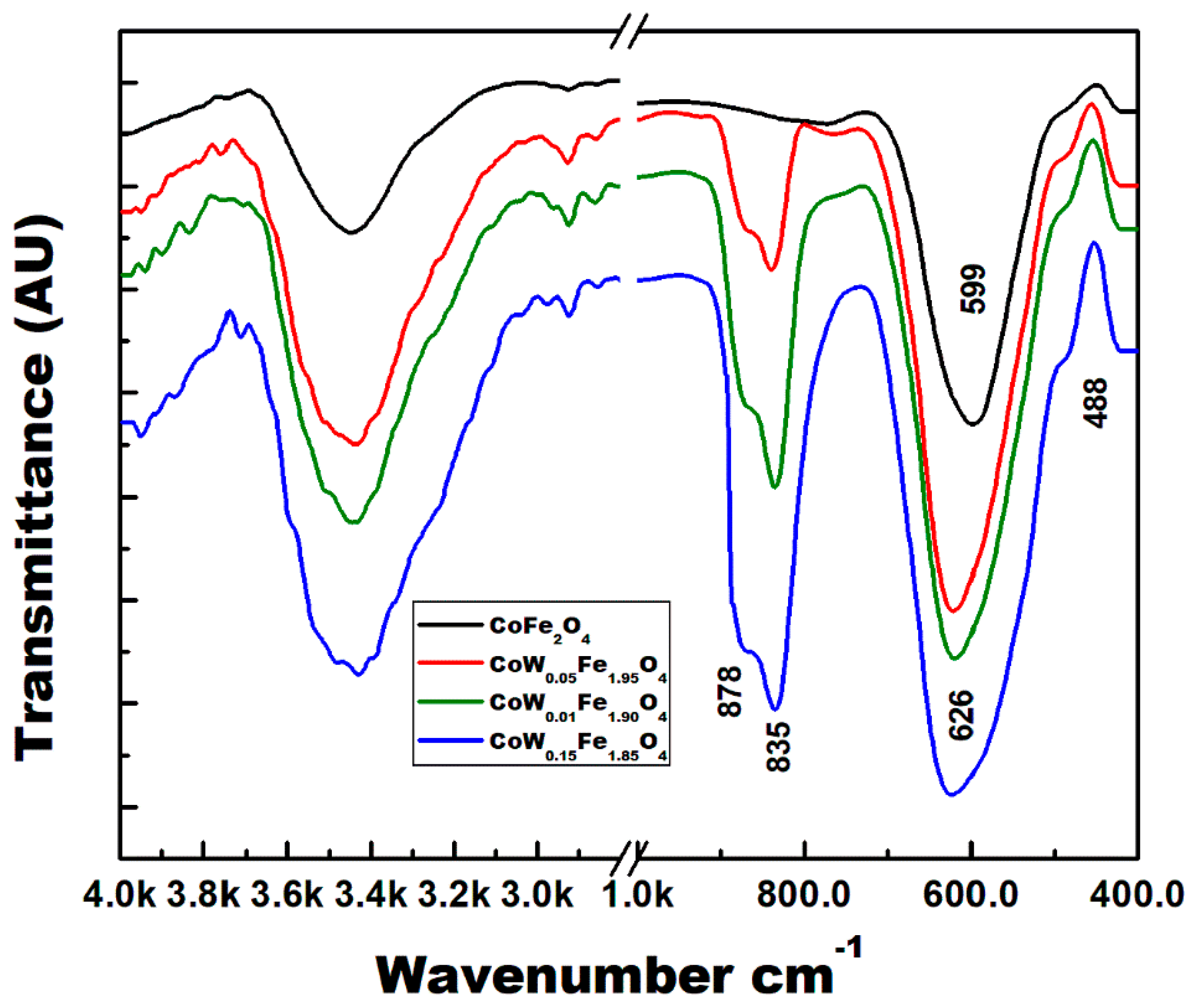
3.2. ATR-FTIR Spectra
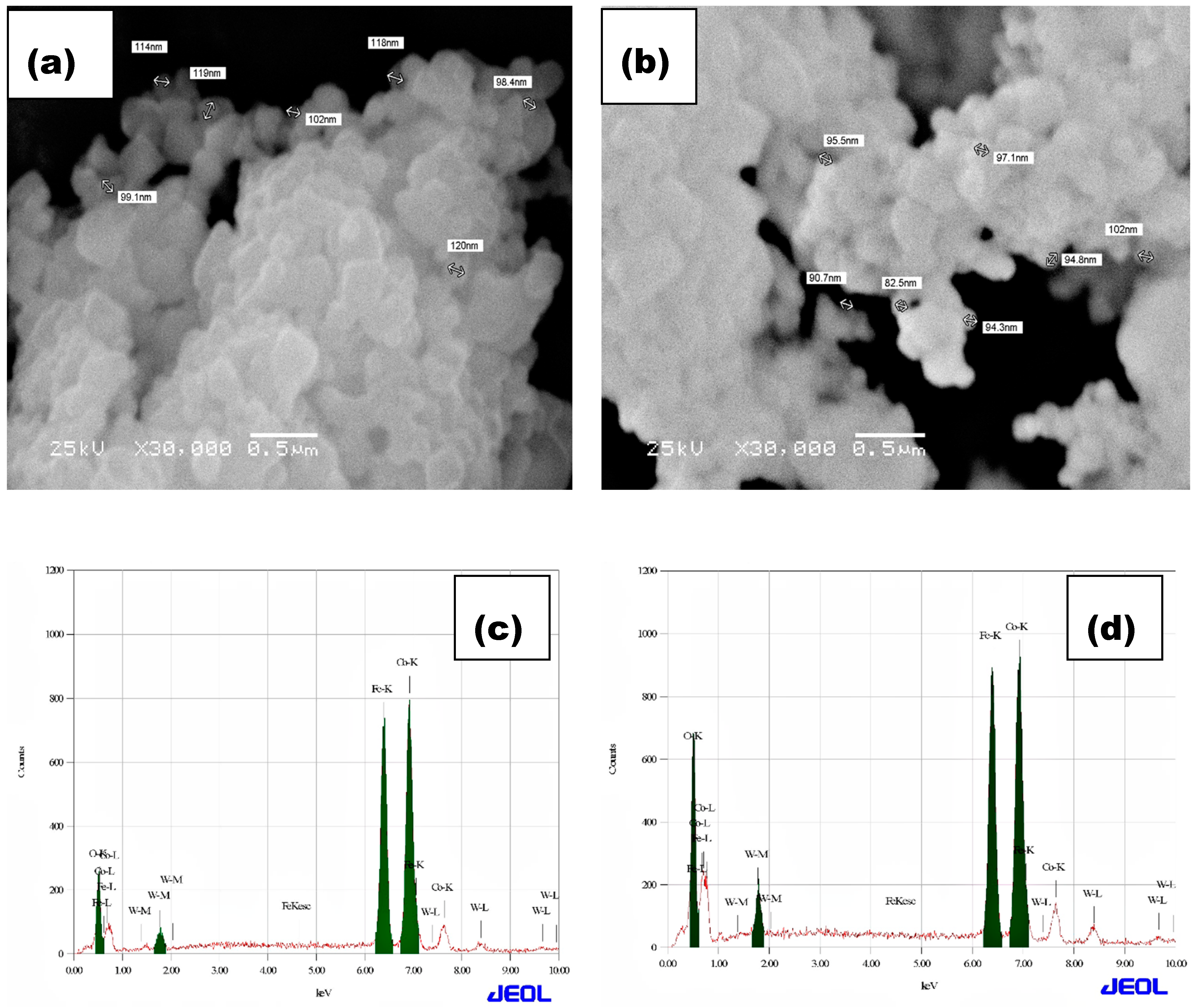
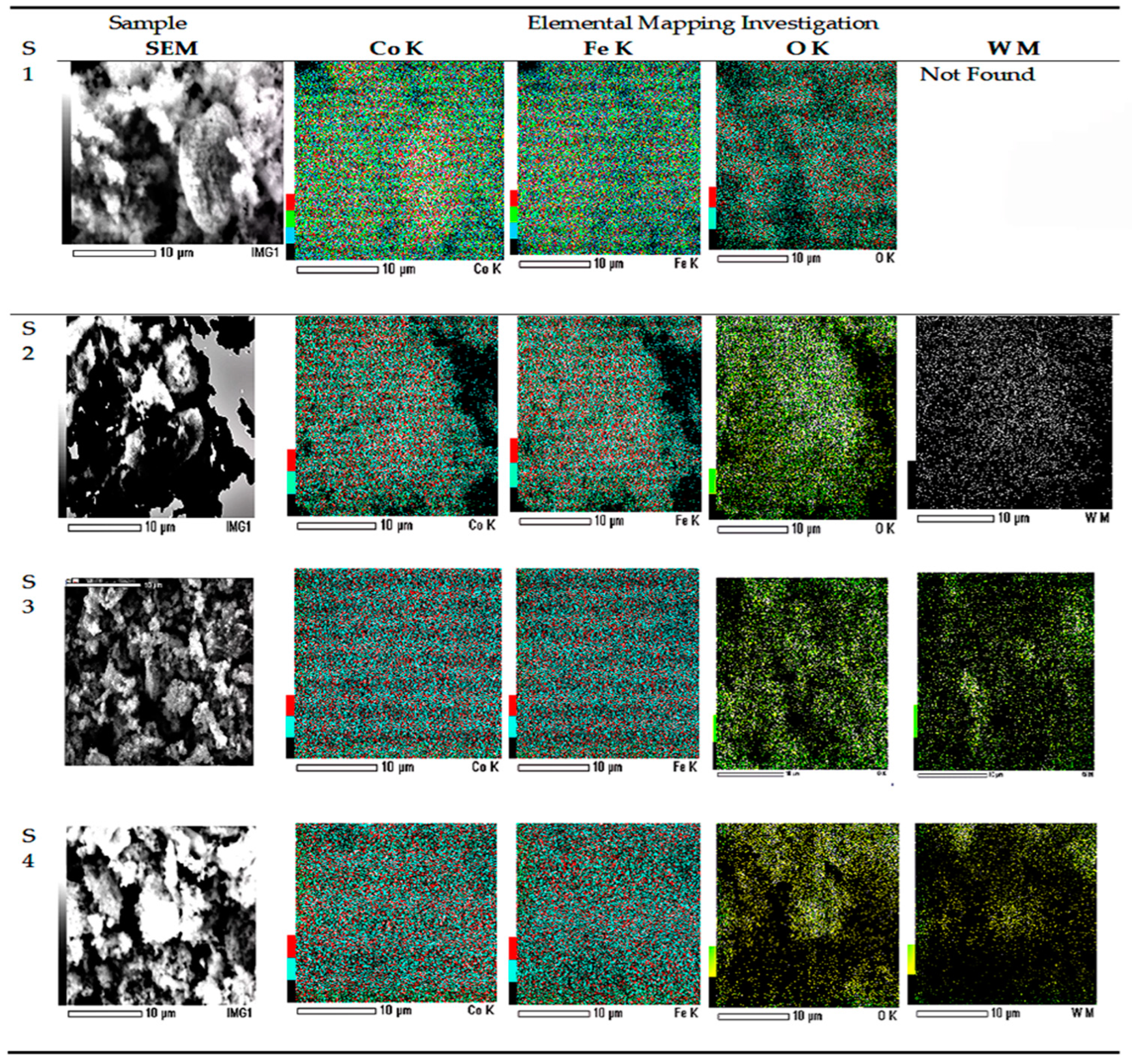
3.3. Microstructure Analysis
3.4. Surface and Pore Size Distribution Analysis
3.5. XPS Analysis
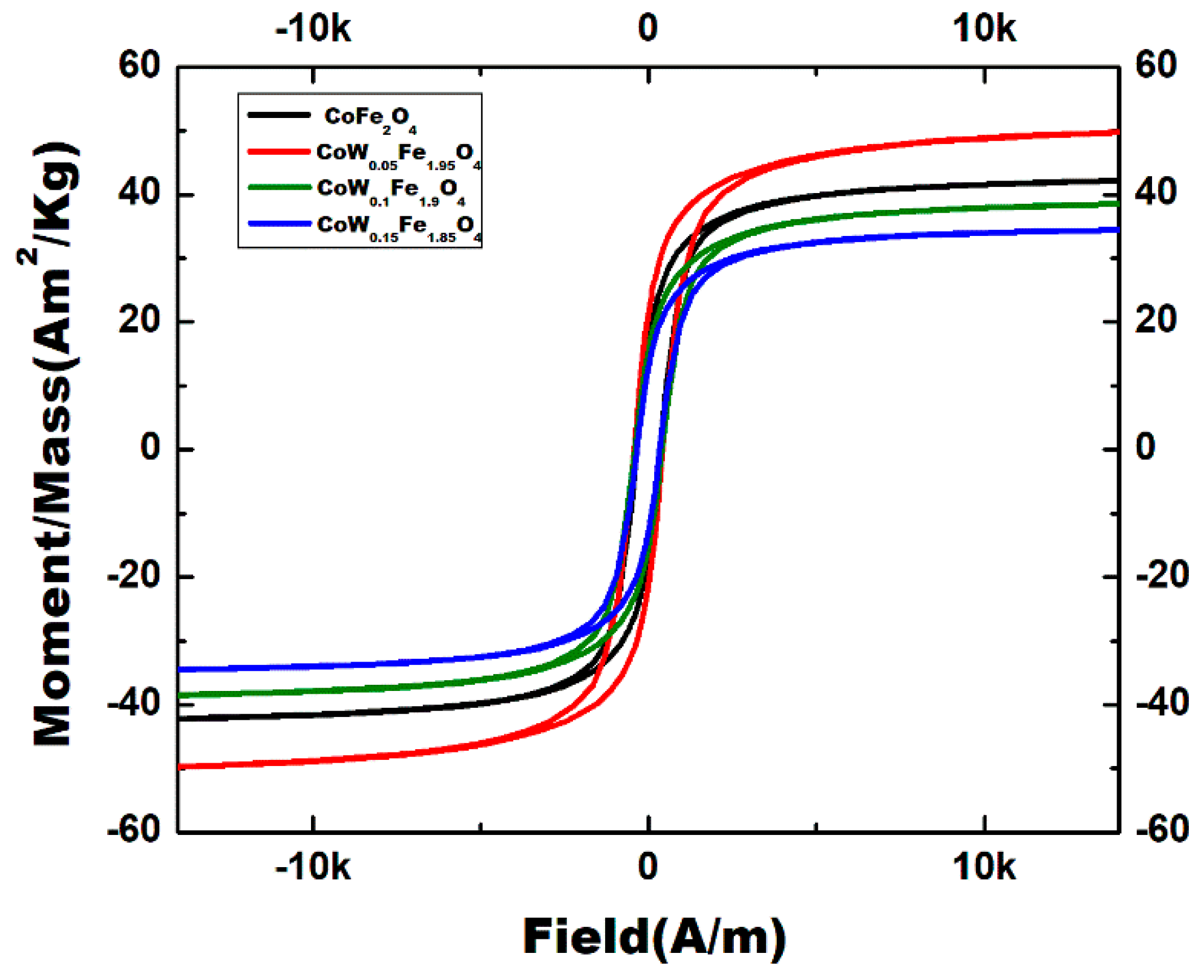
3.6. Magnetic Properties
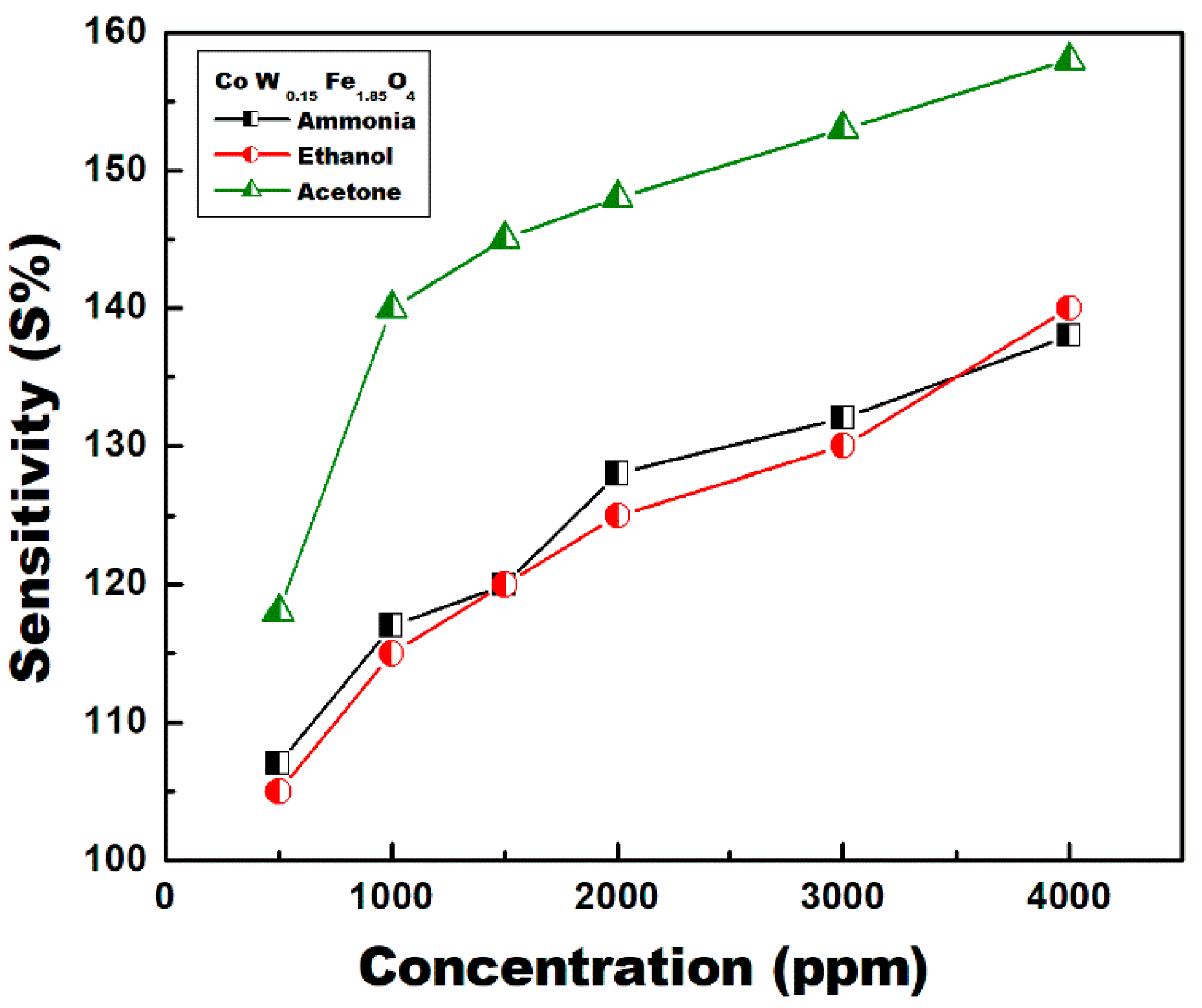
3.7. Gas Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheoran, N.; Kumar, A.; Kumar, K.; Banerjee, A. Structural, Optical, and Multiferroic Properties of Yttrium (Y3+)-Substituted BiFeO3 Nanostructures. J. Supercond. Nov. Magn. 2020, 33, 2017–2029. [Google Scholar] [CrossRef]
- Ranga, R.; Kumar, A.; Kumari, P.; Singh, P.; Madaan, V.; Kumar, K. Ferrite application as an electrochemical sensor: A review. Mater. Charact. 2021, 178, 111269. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. Enhanced Propanol Response Behavior of ZnFe2O4 NP-Based Active Sensing Layer Induced by Film Thickness Optimization. Processes 2021, 9, 1791. [Google Scholar] [CrossRef]
- Xiangfeng, C.; Dongli, J.; Yu, G.; Chenmou, Z. Ethanol gas sensor based on CoFe2O4 nano-crystallines prepared by hydrothermal method. Sens. Actuators B Chem. 2006, 120, 177–181. [Google Scholar] [CrossRef]
- Madake, S.B.; Hattali, M.R.; Thorat, J.B.; Pedanekar, R.S.; Rajpure, K.Y. Chemiresistive gas sensing properties of copper substituted zinc ferrite thin films deposited by spray pyrolysis. J. Electron. Mater. 2021, 50, 2460–2465. [Google Scholar] [CrossRef]
- Hernandez, P.T.; Kuznetsov, M.V.; Morozov, Y.G. High-Temperature Synthesis of Nickel-Based Nanoparticles for Use as Materials in Sensors of Potentially Hazardous Gases. Int. J. Self-Propag. High-Temp. Synth. 2019, 28, 159–172. [Google Scholar] [CrossRef]
- Matatagui, D.; Bahos, F.A.; Gràcia, I.; Horrillo, M.D.C. Portable Low-Cost Electronic Nose Based on Surface Acoustic Wave Sensors for the Detection of BTX Vapors in Air. Sensors 2019, 19, 5406. [Google Scholar] [CrossRef] [Green Version]
- Kumar, E.R.; Srinivas, C.; Seehra, M.S.; Deepty, M.; Pradeep, I.; Kamzin, A.S. Particle size dependence of the magnetic, dielectric and gas sensing properties of Co substituted NiFe2O4 nanoparticles. Sens. Actuator A Phys. 2018, 279, 10–16. [Google Scholar] [CrossRef]
- El-Shobaky, G.A.; Turky, A.M.; Mostafa, N.Y.; Mohamed, S.K. Effect of preparation conditions on physicochemical, surface and catalytic properties of cobalt ferrite prepared by coprecipitation. J. Alloys Compd. 2010, 493, 415–422. [Google Scholar] [CrossRef]
- Aljuraide, N.I.; Mousa, M.A.A.; Mostafa, N.Y.; El-Shobaky, G.A.; Hamdeh, H.H. Microstructure analysis of zinc ferrite nanoparticles by means of X-ray powder diffraction and Mössbauer spectroscopy. Int. J. Nanopart. 2012, 5, 56–63. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Hessien, M.M.; Shaltout, A.A. Hydrothermal synthesis and characterizations of Ti substituted Mn-ferrites. J. Alloys Compd. 2012, 529, 29–33. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Mostafa, N.Y.; Abd Elkader, O.H.; Ahmed, M.A. Solubility limits in Mn–Mg ferrites system under hydrothermal conditions. J. Magn. Magn. Mater. 2014, 364, 39–46. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Mostafa, N.Y.; Abd Elkader, O.H.; Hemeda, A.; Tawfik, A. Electrical and morphological properties of magnetocaloric nano ZnNi ferrite. J. Magn. Magn. Mater. 2015, 394, 96–104. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Zaki, Z.; Hessien, M.M.; Shaltout, A.A.; Alsawat, M. Enhancing saturation magnetization of Mg ferrite nanoparticles for better magnetic recoverable photocatalyst. Appl. Phys. A 2018, 124, 12. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Hassan, S.K.; Karydas, A.G.; Zaki, Z.I.; Mostafa, N.Y.; Kregsamer, P.; Wobrauschek, P.; Streli, C. Comparative elemental analysis of fine particulate matter (PM2.5) from industrial and residential areas in Greater Cairo-Egypt by means of a multi-secondary target energy. Spectrochim. Acta Part B At. Spectrosc. 2018, 145, 29–35. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mostafa, N.Y.; Abd-Elkader, O.H. Structural and magnetic properties correlated with cation distribution of Mo-substituted cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 2014, 368, 246–251. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Zaki, Z.I.; Heiba, Z.K. Structural and magnetic properties of cadmium substituted manganese ferrites prepared by hydrothermal route. J. Magn. Magn. Mater. 2013, 329, 71–76. [Google Scholar] [CrossRef]
- Srinivasamurthy, K.M.; Manjunatha, K.; El-Denglawey, A.; Rajaramakrishna, R.; Kubrin, S.P.; Pasha, A.; Angadi, V.J. Evaluation of structural, dielectric and LPG gas sensing behavior of porous Ce3+-Sm3+ doped Cobalt nickel ferrite. Mater. Chem. Phys. 2022, 275, 125222. [Google Scholar] [CrossRef]
- Bartůněk, V.; Sedmidubský, D.; Huber, Š.; Švecová, M.; Ulbrich, P.; Jankovský, O. Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel. Materials 2018, 11, 1241. [Google Scholar] [CrossRef] [Green Version]
- Heiba, Z.K.; Mohamed, M.B.; Mostafa, N.Y.; El-Naggar, A.M. Structural and optical properties of Cd1−xMnxFe2O4/PMMA nanocomposites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1898–1906. [Google Scholar] [CrossRef]
- Dessai, G.P.P.; Singh, A.K.; Verenkar, V.M.S. Mn doped Ni-Zn ferrite thick film as a highly selective and sensitive gas sensor for Cl2 gas with quick response and recovery time. Mater. Res. Bull. 2022, 149, 111699. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. ChemInform Abstract: Biological and Chemical Sensors Based on Graphene Material. Chem. Soc. 2011, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Z.G.; Miyauchi, M. Efficient Visible Light Active CaFe2O4/WO3 Based Composite Photocatalysts: Effect of Interfacial Modification. J. Phys. Chem. C 2009, 113, 17132–17137. [Google Scholar]
- Zou, Y.; Wang, H.; Yang, R.; Lai, X.; Wan, J.; Lin, G.; Liu, D. Controlled synthesis and enhanced toluene-sensing properties of mesoporous NixCo1−xFe2O4 nanostructured microspheres with tunable composite. Sens. Actuators B Chem. 2019, 280, 227–234. [Google Scholar] [CrossRef]
- Patil, J.Y.; Nadargi, D.Y.; Mulla, I.S.; Suryavanshi, S.S. Cerium doped MgFe2O4 nanocomposites: Highly sensitive and fast response-recoverable acetone gas sensor. Heliyon 2019, 14, e01489. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.K.; Singh, R.K.; Singh, P. Fabrication of Lanthanum Ferrite Based Liquefied Petroleum Gas Sensor. Sens. Actuators B Chem. 2016, 229, 25–30. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Qhtani, M.M.; Alotaibi, S.H.; Zaki, Z.I.; Alharthi, S.; Cieslik, M.; Gornicka, K.; Ryl, J.; Boukherroub, R.; Amin, M.A. Cathodic activation of synthesized highly defective monoclinic hydroxyl-functionalized ZrO2 nanoparticles for efficient electrochemical production of hydrogen in alkaline media. Int. J. Energy Res. 2020, 44, 10695–10709. [Google Scholar] [CrossRef]
- Pawar, R.A.; Patange, S.M.; Shitre, A.R.; Gore, S.K.; Jadhavd, S.S.; Shirsath, S.E. Crystal chemistry and single-phase synthesis of Gd3+ substituted Co–Zn ferrite nanoparticles for enhanced magnetic properties. RSC Adv. 2018, 8, 25258. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, N.Y.; Zaki, Z.I.; Mohsen, Q.; Alotaibi, S.H.; El-moemen, A.A.; Amin, M.A. Carboxylate-assisted synthesis of highly-defected monoclinic zirconia nanoparticles. J. Mol. Struct. 2020, 1214, 128232. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Mane, M.I.; Yasukawa, Y.; Liu, X.; Morisako, A. Self-ignited high temperature synthesis and enhanced super-exchange interactions of Ho3+-Mn2+-Fe3+-O2-ferromagnetic nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 2347–2357. [Google Scholar] [CrossRef]
- Abozaid, R.M.; Lazarević, Z.Z.; Radojević, V.; Rabasović, M.S.; Šević, D.; Rabasović, M.D.; Romčević, N.Z. Characterization of Neodymium Doped Calcium Tungstate Single Crystal by Ra-man, IR and Luminescence Spectroscopy. Sci. Sinter. 2018, 50, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.W.; Feng, R. Agglomeration of magnetic nanoparticles. J. Chem. Phys. 2012, 136, 124109. [Google Scholar] [PubMed]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of binding energy positions with C1s for XPS results. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Wang, W.P.; Yang, H.; Xian, T.; Jiang, J.L. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 2012, 53, 1586–1589. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Sun, A.; Zhang, Y.; Yu, L.; Suo, N.; Zuo, Z. Microstructure, XPS and magnetic analysis of Al-doped nickel–manganese–cobalt ferrite. J. Mater. Sci. Mater. Electron. 2021, 32, 20474–20488. [Google Scholar] [CrossRef]
- Sun, M.; Xu, N.; Cao, Y.W.; Yao, J.N.; Wang, E.G. Nanocrystalline tungsten oxide thin film: Preparation, microstructure, and photochromic behavior. J. Mater. Res. 2000, 15, 927–933. [Google Scholar] [CrossRef]
- Mosivand, S.; Kazeminezhad, I. Synthesis of electrocrystallized cobalt ferrite nanopowders by tuning the cobalt salt concentration. RSC Adv. 2015, 5, 14796–14803. [Google Scholar] [CrossRef]
- Pillai, V.; Shah, D.O. Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions. J. Magn. Magn. Mater. 1996, 163, 243–248. [Google Scholar] [CrossRef]
- Sutka, A.; Doebelin, N. Study of defects by Rietveld technique and gas response of excess-iron zinc ferrite. J. Jpn. Soc. Powder Powder Metall. 2014, 61, S81–S84. [Google Scholar] [CrossRef] [Green Version]


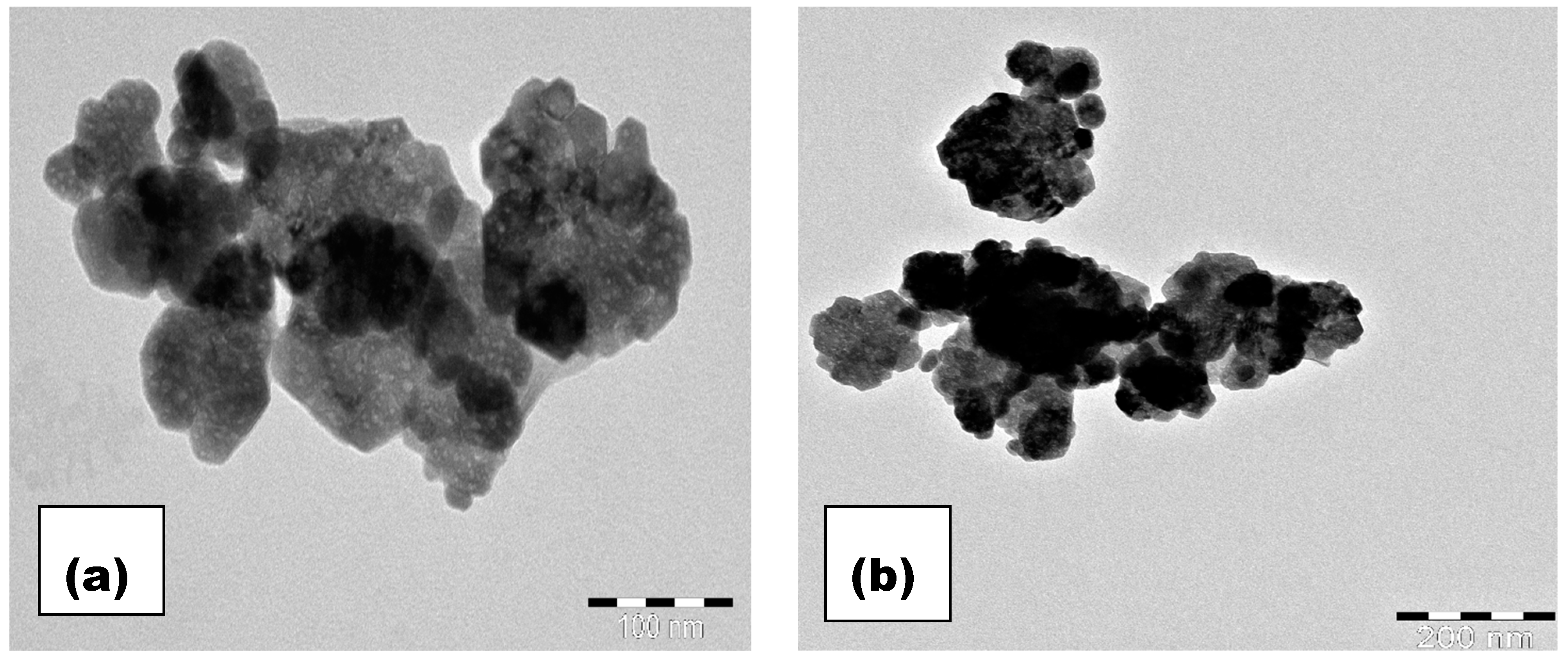
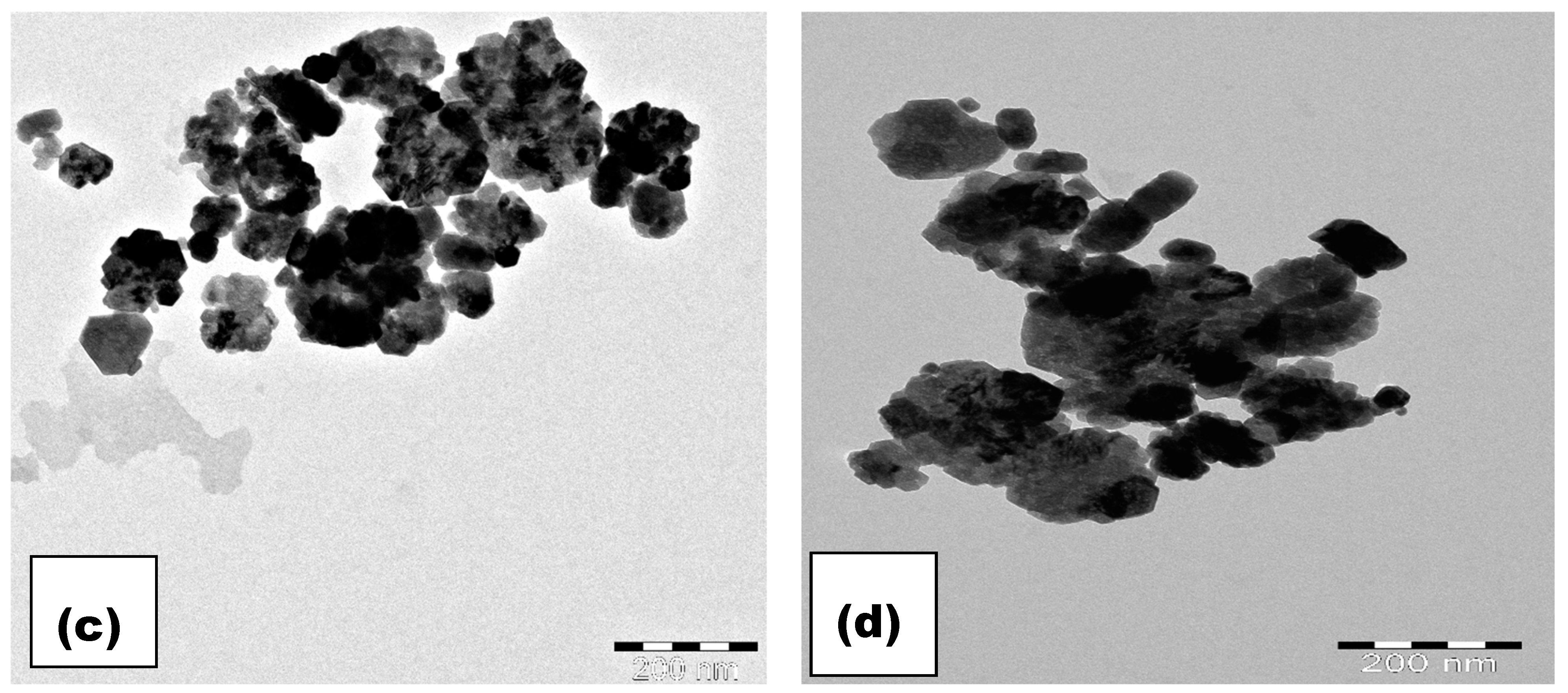





| Parameter | x = 0.0 | x = 0.05 | x = 0.1 | x = 0.15 |
|---|---|---|---|---|
| a (Å) | 8.364 (2) | 8.367 (6) | 8.371 (4) | 8.372 (3) |
| Size (nm) | 71.39 | 72.95 | 78.29 | 80.22 |
| Volume Å3 | 585.04 | 585.77 | 586.59 | 586.69 |
| Strain (ε) × 10−4 | 4.86 | 4.75 | 4.43 | 4.94 |
| Metal Cation | A-Sites (Tetrahedral) | B-Sites (Octahedral) |
|---|---|---|
| Co2+ | 0.72 | 0.79 |
| Fe3+ | 0.49 | 0.55 |
| W6+ | 0.42 | 0.60 |
| Samples | SBET (m2/g) | Vm (cc/g) | Vp (cc/g) | ȓ (nm) |
|---|---|---|---|---|
| Pure | 5.1004 | 0.2318 | 0.023309 | 18.280 |
| 0.05 W | 4.9752 | 0.2138 | 0.020584 | 17.193 |
| 0.10 W | 2.1090 | 0.1968 | 0.00455 | 37.334 |
| 0.15 W | 2.6587 | 0.1614 | 0.0014 | 24.292 |
| Samples | Ms (Am2/kg) | Mr (Am2/kg) | Mr/Ms (Am2/kg) | Hci (KA/m) |
|---|---|---|---|---|
| CoF2O4 | 42.481 | 16.575 | 0.3901 | 27.683 |
| 0.05 W | 50.151 | 21.456 | 0.4278 | 34.025 |
| 0.10 W | 38.726 | 15.308 | 0.3952 | 32.186 |
| 0.15 W | 34.676 | 12.99 | 0.3746 | 26.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elkader, O.; Al-Enizi, A.M.; Shaikh, S.F.; Ubaidullah, M.; Abdelkader, M.O.; Mostafa, N.Y. The Structure, Magnetic, and Gas Sensing Characteristics of W-Substituted Co-Ferrite Nanoparticles. Crystals 2022, 12, 393. https://doi.org/10.3390/cryst12030393
Abd-Elkader O, Al-Enizi AM, Shaikh SF, Ubaidullah M, Abdelkader MO, Mostafa NY. The Structure, Magnetic, and Gas Sensing Characteristics of W-Substituted Co-Ferrite Nanoparticles. Crystals. 2022; 12(3):393. https://doi.org/10.3390/cryst12030393
Chicago/Turabian StyleAbd-Elkader, Omar, Abdullah M. Al-Enizi, Shoyebmohamad F. Shaikh, Mohd Ubaidullah, Mohamed O. Abdelkader, and Nasser Y. Mostafa. 2022. "The Structure, Magnetic, and Gas Sensing Characteristics of W-Substituted Co-Ferrite Nanoparticles" Crystals 12, no. 3: 393. https://doi.org/10.3390/cryst12030393
APA StyleAbd-Elkader, O., Al-Enizi, A. M., Shaikh, S. F., Ubaidullah, M., Abdelkader, M. O., & Mostafa, N. Y. (2022). The Structure, Magnetic, and Gas Sensing Characteristics of W-Substituted Co-Ferrite Nanoparticles. Crystals, 12(3), 393. https://doi.org/10.3390/cryst12030393









