Abstract
The sol-gel process was used to create a new type of polypyrrole-Stannous(II)tungstate nanocomposite by poly(N-methyl pyrrole (PNMPy) sol in Stannous(II)tungstate gel, produced separately using sodium silicotungstic acid and Tn(II)chloride. Tin(II)tungstate (SnWO3) was made by changing the mixing volume ratios of SnWO3 and with a constant amount of an organic polymer. The composite was characterized by TGA, XRD, FTIR, and SEM measurements. A commercially available glassy carbon electrode (GCE) was modified with PNMPy/nano-Stannous(II)WO3 nanocomposites to create a chemical sensor for selective detection of Hg2+ ions using an effective electrochemical methodology. In the I-V technique, selectively toxic Hg2+ ion was targeted selectively, which shows a rapid reaction toward PNMPy/nano-Stannous(II)WO3/Nafion/GCE sensor. It also demonstrates long-term stability, an ultra-low detection limit, exceptional sensitivity, and excellent reproducibility and repeatability. For 0.1 mM to 1.0 nM aqueous Hg2+ ion solution, a linear calibration plot (r2: 0.9993) was achieved, with a suitable sensitivity value of 2.8241 AM−1 cm−2 and an extraordinarily low detection limit (LOD) of 3.40.1 pM (S/N = 3). As a result, the cationic sensor modified by PNMPy/nano-Stannous(II)WO3/GCE could be a promising electrode.
1. Introduction
Despite the widespread potential and everywhere use of conducting polymers research, commercial applications are limited. Only limited practicable applied sciences have progressed from the laboratory perception stage for conducting polymers.
Because of excessive cost, obdurate, limited durability, and thermal stability, the conducting polymers have failed to make large commercialization. To overcome all these shortcomings, efforts by scientists are made by making composites with different initiators [1,2,3,4,5]. Nevertheless, until the early 19th century, nanocomposites did not gain widespread popularity but suddenly gained giant knock when a composite of mica and nylon was reported by Toyota researchers having the strength and yield by five times [6,7].
The growing interest in polymer-nanoparticle composites has been aided by subsequent advances. The appealing applications of conjugated organic polymers are photovoltaics, flexible electronics, and light-emitting displays. Composite of phosphonic acid terminated organic-inorganic hybrid, thiophene quantum dot, and polypyrrole hybrids are great to obtain improved performance with customized ligands and are used in various flexible films supercapacitors and many electronics [8,9,10,11,12]. Mixing of any matrix or nanorods dispersions in the polythiophene cause the increase in device performance. Nanocrystalline materials provide three-times higher power conversion efficiencies as compared to standard blends used in volumes. The improved sensing and catalytic properties offered by the conducting polymer noble metal nanocomposites are as compared to pure conducting polymers [13,14,15].
The most hazardous metal in the environment is mercury (II) [16]. It is the main culprit for the serious nervous system health risk. The trace accumulation of mercury in the kidney, liver, and central nervous system causes serious risk. The immunological system and the human body’s endocrine gland become affected by the pose of mercury in the environment [17,18]. Production of calculators, mercury vapor lamps, batteries, barometers, cameras, medical laboratories chemicals, cathode tubes causes a trace of mercury in industrial waste. Even there is a significant negative impact of monitoring Hg2+ in the environment on human health. For environmental protection and health monitoring, it is necessary to monitor and sens Hg2+. Colorimetrical fluorescent, [19,20,21,22,23] and electrochemical sensors [24,25] for Hg2+ already published.
However, most of these sensors could hardly detect 10 nM, a toxic level of Hg2+ designated by the U.S. Environmental Protection Agency (EPA). To improve the LOD here in we develop PNMPY/NANO-Stannous(II)WO3 composite modified GCE sensor for the Hg2+ ions in an aqueous system.
In this study, we used a co-precipitation approach to make a PNMPy/nano-Stannous(II)WO3 composite for electrochemical detection of the Hg2+ ion at room temperature. Various standard techniques were used to examine the structural and morphological properties of the PNMPy/nano-Stannous(II)WO3 nanocomposite. Following the successful production of the PNMPy/nano-Stannous(II)WO3 composite, we coated the PNMPy/nano-Stannous(II)WO3 composite to the GCE for electrochemical testing of Hg2+ ion detection. Furthermore, we evaluated the electrochemical response of PNMPy/nano-Stannous(II)WO3 composite with bare GCE and PNMPy/nano-Stannous(II)WO3/GCE for the detection of Hg2+ ion and found that the PNMPy/nano-Stannous(II)WO3 composite outperforms the bare GCE.
2. Experimental Section
2.1. Materials and Methods
Gallium nitrate, mercury(II)chloride, manganese (II) sulfate, Nickel(II) chloride, cobalt (II)nitrate, iron (III)chloride, yttrium nitrate, 5% ethanolic Nafion solution, thallium nitrate disodium phosphate, cerium(III)nitrate, antimony(III)chloride, arsenic(III)chloride, were from Sigma-Aldrich and used without further purification.
N-methyl pyrrole monomer buys from Merck’s, CDH’s stannous chloride, and Loba Chemie’s sodiummetatungstate were used as essential reagents. All of the other chemicals and reagents were of analytical purity (lead nitrate, nitric acid, sulfuric acid, sodium nitrate, hydrochloric acid, etc.). During this research, we used the instruments: Orion 720 pH/ion meter (Thermo Fisher Scientific, Waltham, MA, USA) for emf and pH measurement having connected saturated calomel reference electrode. Elcon (Eletech Lab Instrument, Ambala, India) water bath incubator shaker with automatic temperature control was used. Digital, Sartorius-21OS, Tokyo, Japan. An electronic balance, 18.6 Mcm−1 from Millipore, BR, Burlington, MA, USA, was used to make aqueous solutions of various concentrations. Link-Oxford-sis (EDX) with a Jeol JSM 6300 (SEM, Tokyo, Japan) was employed. For functional group analysis, model 2000 (Waltham, MA, USA), a Perkin Elmer FTIR spectrophotometer was employed. At ambient conditions, I-V characteristics of produced PNMPY/NANO-Stannous(II)WO3/Nafion/GCE composites were measured to detect the Hg2+ ion using a Keithley electrometer (6517A, Beaverton, OR, USA).
2.2. Preparation of Poly(N-methyl pyrrole)/Nano-Stannous(II)WO3 Composite
In the presence of the oxidizing agent (ammonium persulphate), NMPy was polymerized into PNMPy, then mixing it for further sonicating the mixture for 24 h at 40 °C constant temperature. Mixing an inorganic Stannous(II)tungstate precipitate (produced by combining 1 M HCl stannous chloride in an aqueous sodium meta tungstate in various mixing ratios) and agitating it for a further 2 h was performed using sol-gel methods. The reaction mixture was stirred for another 24 h under the same conditions. Using a Buchner funnel, the powder was filtered out before being washed with methanol, 0.1 M hydrochloric acid, and distilled water. In a vacuum dryer, the resulting greenish-black powder was dried for 24 h at room temperature.
By soaking the samples in a 1 M HNO3 solution for 24 h, shaking periodically, and replenishing the supernatant liquid regularly, the samples were transformed from containing metal ions to H+ form. The excess acid was removed by washing with DMW several times (dematerialized water). The samples were finally dried at 40 °C. As a result, several nanocomposite samples were created and selected for further study based on their Na+ ion exchange capacity (IEC) (1.2 meq/g) and physical appearance.
2.3. Modification of GCE with PNMPy/Nano-Stannous(II)WO3 Composite
The PNMPY/NANO-Stannous(II)WO3 composite was used to fabricate the GCE with the help of 5% ethanolic Nafion solution (95:5, ethanol: Nafion) as well as a 0.4 mm surface (approximately) thick sheet of PNMPY/NANO-Sn(II)WO3. The formatted film on the GCE was then dried in an oven at 45.0 °C for 2 h. The working electrode (WE) in the electrochemical cell was made of PNMPY/NANO-Sn(II)WO3 composite manufactured GCE, the counter electrode (CE) was made of platinum wire, and the supporting electrolyte was made of containing 0.1 M phosphate buffer solution (PBS; pH 7.0). The target analyte was aqueous Hg2+ ion concentrations ranging from 0.1 M to 0.1 nM. In 5.0 mL of PBS (pH = 7.0), all current-voltage (I-V) measurements were performed for electrode calibration. The sensitivity of the proposed Hg2+ ion sensor was calculated using the slope of the calibration plot and the active surface area of the GCE. I-V technique was used to aqueous Hg2+ ion using an electrometer (Keithley, 6517A Electrometer, Beaverton, OR, USA) with PNMPY/NANO-Stannous(II)WO3/GCE as WE.
3. Results and Discussion
3.1. Characterization
To achieve suitable chemical stability, excellent electrical conductivity, a higher surface to volume ratio, well repeatability behavior, and better capacity, a fibrous matrices gel of Sn (II) tungstate and PNMPy sol was mixed by sol-gel method to obtain PNMPY/NANO-Stannous(II)WO3 nanocomposite.
Figure 1A–D show the SEM pictures of PNMPY/NANO-Stannous(II)WO3 composites at various magnifications. Based on the morphological evolution of the SEM images, it is observed that the interaction between the inorganic matrices with the conducting polymer and the morphology of composite is entirely changed. The white luster in the SEM images is because of the presence of metal inorganic, and the other thicker part in SEM photographs shows the conducting organic constituents that make the composite have great characteristics.
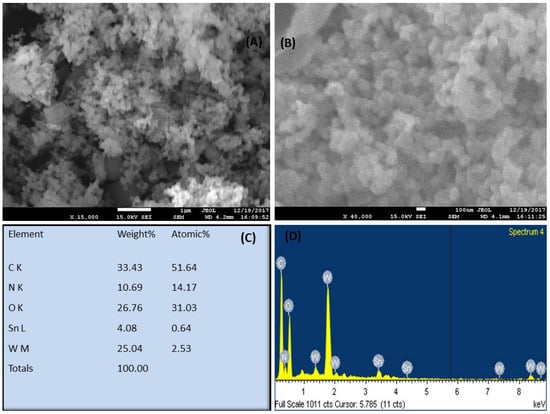
Figure 1.
SEM image of the composite PNMPy/nano-Stannous(II)WO3 (A,B) at different magnifications while from (C,D) showing the elemental composition (C,D) with the help of SEM-EDX.
As shown if Figure 2, the 15% weight loss up to 100 °C is because of the loss of external moisture water molecules present on the surface of the composite [26]. Weight loss of about 20% is maybe because of organic part degradation between 100 and 400 °C. The beginning of pyrophosphate and phosphate of the inorganic part present in the composite takes parts after 400 °C. The peak in the DTA at 700 °C is an indication of exothermic reaction during the material phase change that indicates the exothermic or endothermic behavior of the combustion reaction of PNMPy/nano-Stannous(II)WO3 during heating.
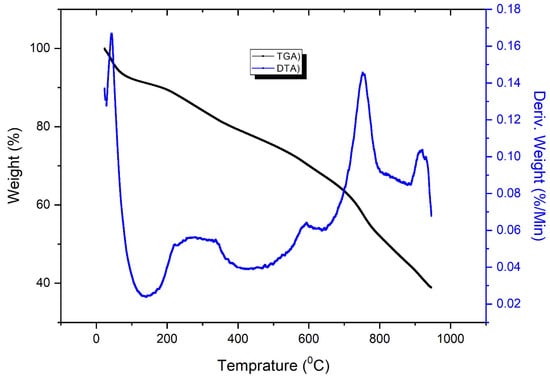
Figure 2.
Thermogravimetric analysis curve (TGA—black in color) for the PNMPy/nano-Stannous(II)WO3 composite in a nitrogen atmosphere while differential thermal analysis (DTA—blue) is shown for reaction endothermic or exothermic.
The presence of C=C and aromatic C-H stretching vibrations in the composite spectra are responsible for high-intensity peaks in the 3100–3200 cm−1 range [27,28]. C-H in-plane deformation mode and ring stretching mode as well as further out of plane deformation mode is the characteristic by the peak presence of at around 786–850 cm−1 in the composite [29,30]. The IR bands positions, as well as the numbers in Figure 3, are the same for the Stannous(II)tungstate that is in the region of around 600–3200 cm−1 range. The peaks at 2800–3200 cm−1 and around 1730 cm−1 are for the C-H stretching vibrations and C=C characteristic peaks, respectively. Ring deformation modes are shifted due to the bulky group of the interaction of the Stannous(II)tungstate and poly(n-methyl pyrrole) backbone. The cause of the shift is bulky groups in the composite that creates the polaron-polaron interaction.
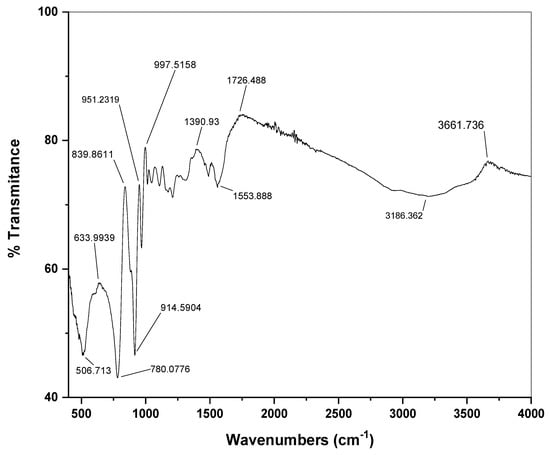
Figure 3.
FTIR spectra of PNMPy/nano-Stannous(II)WO3 composite in KBr showing different bonding stretching.
In the current study, X-ray photoelectron spectroscopy (XPS) is used here to detect the elemental composition and electronic stages of the metals present in the composite. It is a quantitative elemental approach for a substance. The materials are irradiated at the top, typically between 1.0 and 10.0 nm, are low case using their kinetic energy. By XPS measurement, here in PNMPY/NANO-Stannous(II)WO3, C, N, Si, Sn, and O element and their chemical state was analyzed in the composite. The full XPS spectrum of PNMPY/NANO-Stannous(II)WO3 nanocomposite is presented in Figure 4a. A 35.9 eV binding energy is for the W4f7/2 spin-orbit peak in Figure 4b, which is consistent with the tungsten reference data [31]. The C1s spectrum, shown in Figure 4, has peaked at 285.2eV. (c). The presence of carbon in nanocomposite was shown by the C1s peaks. [32]. N1s is executed at 399.3 eV in the PNMPY/NANO-Stannous(II)WO3, as clear in Figure 4d, [33]. A 532.6 eV is the indication of the presence of oxygen (i.e., O2−) in Figure 4e in the PNMPY/NANO-Stannous(II)WO3 nanocomposite [34]. The presence of Si in the form of Si2p in the composite was shifted at roughly 104 eV in Figure 4f. As a result, the PNMPY/NANO-Stannous(II)WO3 nanocomposite is found to include six distinct elements.
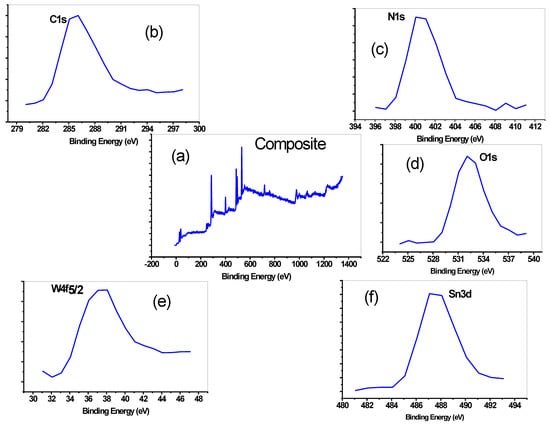
Figure 4.
X-ray photoelectron spectroscopy (XPS) study of the PNMPy/nano-Stannous(II)SiO3 composite showing different peaks for different metals in (a) for composite, (b) carbon, (c) nitrogen, (d) oxygen, (e) tungsten, and (f) for Tin.
3.2. Applications: Chemical Sensor Development
3.2.1. Detection of Hg2+ Ion Using PNMPY/NANO-Stannous(II)WO3/GCE by the I-V Method
As a chemical sensor, the toxic Hg2+ ion in an aqueous medium was detected using a PNMPY/NANO-Stannous(II)WO3 composite modified GCE. The Hg2+ ion strongly responded in the I-V technique when it comes into touch with PNMPY/NANO-Stannous(II)WO3. The potential application of a Hg2+ ion sensor made from the PNMPY/NANO-Stannous(II)WO3/Nafion/GCE assembly to detect and quantify the designated toxin, Hg2+ ion, in a buffer solution was investigated. The development of the Hg2+ ion sensor based on the PNMPY/NANO-Stannous(II)WO3/Nafion/GCE assembly is still in its early stages, and there have been no other reports found. The suggested PNMPY/NANO-Stannous(II)WO3/GCE sensor has several advantages, including increased air stability, better electrochemical property during the determination, ease of operation, assembly and manufacturing, and safe chemo-characteristic [35]. The potential application of a chemical sensor with PNMPY/NANO-Stannous(II)WO3/Nafion/GCE for the detection of targeted analytes Hg2+ ion in a suitable buffer system was evaluated in detail, with PNMPY/NANO-Stannous(II)WO3/Nafion/GCE acting as brilliant electron mediator during the sensing operation. The proposed sensor was built using an ordered PNMPY/NANO-Stannous(II)WO3/GCE assembly as the WE, with the targeted analyte being an Hg2+ ion. The applied current vs. potential (I-V) was investigated on a thin layer of PNMPY/NANO-Stannous(II)WO3 composite on GCE employed as the WE in the sensing operation of Hg2+ ion in the phosphate buffer system. In the electrode, a 1.0 s timer was set as the holding period in the electrometer.
Scheme 1 depicts a possible reduction pathway for the Hg2+ ion. The electrode fabrication in layer-by-layer is given in Scheme 1a, which followed the drop-coating method [31,32]. The phenomena of two-electrode systems in the detection of target mercury ion is presented in Scheme 1b. The reduction phenomena of mercury with the fabricated electrode are given in Scheme 1c. The result of a two-electrode system in the detection of target mercury ion is found and presented in Scheme 1d. A decrease in an electron from the conduction band of A PNMPY/NANO-Stannous(II)WO3 composite was identified during the Hg2+ ion reduction by the manufactured PNMPY/NANO-Stannous(II)WO3/Nafion/GCE assembly, which caused the reducing current response during the I-V measurement at ambient condition. Hg2+ ion was converted to Hg using the hypothesized Hg2+ ion reduction mechanism. The built WE were used to detect Hg2+ ions after GCE was coated with a slurry of PNMPY/NANO-Stannous(II)WO3 composite nanomaterials. Two electrons were removed from the conduction band of the targeted Hg2+ ion during the electrochemical reduction process, lowering the I-V response of PNMPY/NANO-Stannous(II)WO3/Nafion/GCE sensors [36].
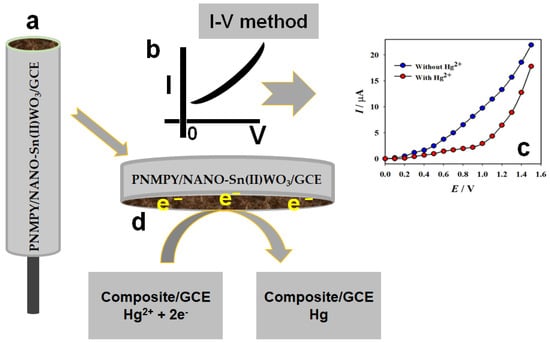
Scheme 1.
Schematic representation of mercury detection with PNMPY/NANO-Stannous(II)WO3/Nafion/GCE sensor probe by electrochemical approach. (a) Fabricated electrode with the PNMPY/NANO-Stannous(II)WO3. (b) Detection method, (c) outcome of the result, and (d) detection phenomena.
The current responses for 13 hazardous heavy metal ions are shown in Figure 5a, where the Hg2+ ion solution (red-dotted) in PBS (pH = 7.0) causes a unique I-V response with the PNMPY/NANO-Stannous(II)WO3/Nafion/GCE surface (1.0 M; 25.0 µL). The electrochemical property of the PNMPY/NANO-Stannous(II)WO3 COMPOSITE was investigated in PBS with pH values ranging from 5.7 to 8.0, as shown in Figure 5b. The results reveal that the PNMPY/NANO-Stannous(II)WO3 composite performs different electrochemical responses in various pH levels. The electrocatalytic property of the PNMPY/NANO-Stannous(II)WO3 composite changes with various pH levels, as seen by the variation in the current response. The highest current response is obtained when pH is optimized by using Hg2+ ion (1.0 M; 25.0 µL) in PBS. As a result, throughout the rest of the experiments in this Hg2+ ion determination by the PNMPY/NANO -Stannous(II)WO3/GCE assembly, pH value 7.0 was employed. Current intensities using Hg2+ ion (1.0 µM; 25.0 µL) in PBS (5.0 mL, pH = 7.0) for bare GCE (blue-dotted) and PNMPY/NANO-STANNOUS(II)WO3 COMPOSITE fabricated GCE (red-dotted) are given in Figure 5c. The PNMPY/NANO-Stannous(II)WO3/GCE provides a considerably higher response than bare GCE, which demonstrates the PNMPY/NANO-STANNOUS(II)WO3 composite’s exhibited excellent electrochemical property toward the detection of Hg2+ ion. In 5.0 mL of PBS, Figure 5d illustrates the current response of the PNMPY/NANO-Stannous(II)WO3 composite manufactured GCE in the absence of Hg2+ ion (blue-dotted) and the presence of Hg2+ ion (red-dotted; 1.0 M; 25.0 L). The proposed PNMPY/NANO-Stannous(II)WO3/GCE sensor’s Hg2+ ion sensing capabilities is demonstrated by a significant decrease in current responsiveness when Hg2+ ions are present in the electrochemical system [37].
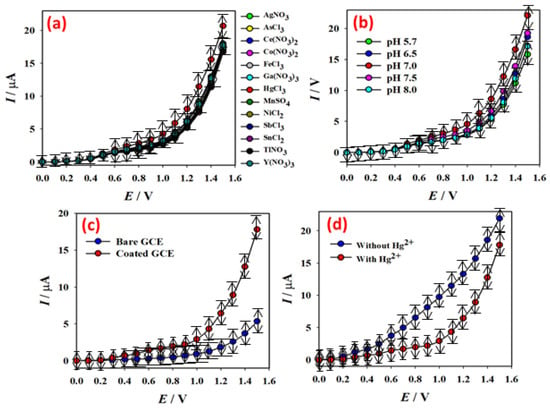
Figure 5.
I-V response of modified electrode in the detection of mercury. (a) Selectivity study using thirteen chemicals, (b) pH optimization, (c) bare and PNMPy/nano-Stannous(II)WO3 modified GCE, and (d) without and with the presence of Hg2+ ion (1.0 µM; 25.0 µL).
The surface current response variation was examined after each injection of Hg2+ ion solutions ranging from low (0.1 nM) to high (0.1 M) concentrations in 5.0 mL PBS. Using an aqueous Hg2+ ion solution of various concentrations (0.1 nM to 0.1 M), I-V responses from the PNMPY/NANO-STANNOUS(II)WO3 composite manufactured GCE surface were determined and presented in Figure 6a. The amplification peak current at 0.4 V is shown in Figure 6b. At ambient temperature, it indicates that as the potential increases, the I-V responses for the PNMPY/NANO-Stannous(II)WO3 composite manufactured GCE sensor rise as well (25.0 °C). The I-V responses likewise decreased regularly as the concentration of Hg2+ ion solution increased from dilute (0.1 nM) to concentrated (0.1 M). The LOD of the devised sensor was determined using aqueous Hg2+ ions (0.1 nM to 0.1 M). Figure 6 shows the calibration plot (at +0.4 V) for the entire concentration range (c). The calibration plot revealed a higher sensitivity value of 2.8241 AM-1 cm−2. From the calibration plot, the linear dynamic range of the developed PNMPY/NANO-Stannous(II)WO3/GCE sensor was attained as 0.1 nM to 1.0 mM (r2 = 0.9993), and the LOD was estimated as 3.4 ± 0.1 pM (3 × noise (N)/slope(S)). I-t responses for Hg2+ ion (1.0 µM; 25.0 µL) in 5.0 mL of PBS solutions using the PNMPY/NANO-Stannous(II)WO3 composite nanostructures fabricated GCE and presented in Figure 6d. Approximately in 10 s, a constant current response was achieved with Hg2+ ion (1.0 µM; 25.0 µL) in 5.0 mL of PBS.
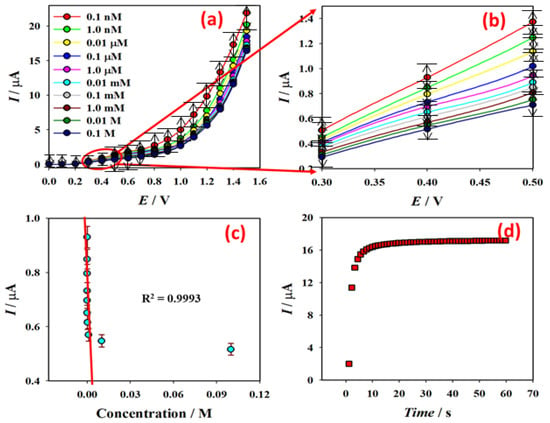
Figure 6.
(a) Current variations for different concentration (0.1 nM to 0.1 M) of aqueous Hg2+ ion in 0.0 to +1.5 V, (b) magnification of I-V responses at 0.4 V, (c) calibration plot of PNMPy/nano-Stannous(II)WO3/CNTs composite fabricated GCE at +0.4 V, and (d) current variation with time for the PNMPy/nano-Stannous(II)WO3/Nafion/GCE in 1.0 µM; 25.0 µL Hg2+ ion.
Current response repeatability, as shown in Figure 7a, depicts the PNMPY/NANO-Stannous(II)WO3 composite manufactured by GCE employing 25.0 L of 0.1 M Hg2+ ion and seven different WE in runs R1–R6 under identical conditions. The superb repeatability (RSD = 3.28%, n = 6) is confirmed because of the similar current response in all experiments. When the same WE were used, as shown by Figure 7b, in run r1~r6, that is the proof of reproducibility. Outstanding reproducibility confirms for the sensor as we obtain almost similar I-V responses in seven repeated experiments (RSD = 3.34%, n = 6). I-V response drops somewhat when the same WE are used in multiple solutions under the same conditions. This could be because the number of active sites in the PNMPY/NANO-Stannous(II)WO3 Composite decreases with each run. Furthermore, the long-stability of the fabricated PNMPY/NANO-Stannous(II)WO3/GCE sensor probe has been performed and presented in Figure 7c. It is measured in every one-hour interval with a similar electrode in the identical solution system (0.1 µM, 25.0 µL Hg2+ ion; 5.0 mL, 0.1 M PBS at pH 7.0). It is found that the sensor current responses exhibited the almost remained similar response in every measurement, which concluded that PNMPY/NANO-Stannous(II)WO3/GCE sensor probe is very stable. From the stability data after 8 h, the current intensity is retained at 98% of its original value, i.e., recorded just after modification of PNMPY/NANO-Stannous(II)WO3/GCE. The prepared sensor is stable enough to detect the target analyte in the identical conditions of the electrochemical system by the reliable I-V method [38] in a short response time. The possible interfering effect of the common inorganic cations during this mercury detection by using PNMPY/NANO-Stannous(II)WO3/GCE electrode was examined. The outcome is indicated of 100-fold of Na+, Ca2+, K+, Cu2+, Cd2+, Mn2+, Pb2+, Mg2+, and Fe3+. There has no interference effect in this Hg2+ determination (interference < ±5%) in I-V response by PNMPY/NANO-Stannous(II)WO3/GCE electrode in identical conditions.
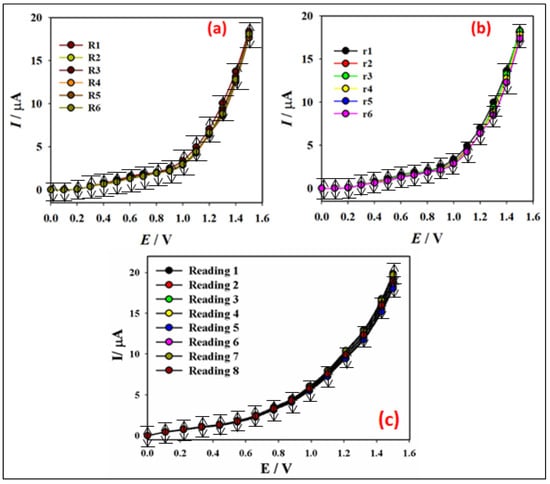
Figure 7.
(a) Repeatability using different WE (0.1 µM, 25.0 µL Hg2+ ion; 5.0 mL, 0.1 M PBS at pH 7.0), (b) reproducibility using the same WE (0.1 µM, 25.0 µL Hg2+ ion; 5.0 mL, 0.1 M PBS at pH 7.0), and (c) stability test (Every reading is taken after 1 h (0.1 µM, 25.0 µL Hg2+ ion; 5.0 mL, 0.1 M PBS at pH 7.0).
3.2.2. Real Sample Analysis by PNMPY/NANO-Stannous(II)WO3/GCE Sensor
In industrial wastewater as well as from plastic bottled water (inside the care irradiation) after seven days of sunshine, the PNMPY/NANO-Stannous(II)WO3/GCE sensor (S2) was used to determine Hg2+ ion. The accuracy of the aqueous Hg2+ ion determination is checked using the standard addition method. A total of 25.0 mL of aqueous Hg2+ ion of various concentrations and an equivalent quantity of genuine sample were mixed and examined by PNMPY/NANO-Stannous(II)WO3/GCE as a WE in PBS (5.0 mL, pH 7.0). Table 1 represents the obtained outcomes, which demonstrated that the PNMPY/NANO-Stannous(II)WO3/GCE modified sensor exhibited a quantitative (~100%) recovery of Hg2+ ion. So, we can conclude that the I-V method is proper and reliable in analyzing real samples by the PNMPY/NANO-Stannous(II)WO3/Nafion/GCE assembly.

Table 1.
Analysis of real environmental samples with PNMPy/nano-Stannous(II)WO3/Nafion/GCE by electrochemical approach.
Nano-porosity, size, as well as the shape of PNMPY/NANO-Stannous(II)WO3 all influence the I-V response in the Hg2+ ion detection. A surface-mediated reduction reaction occurs when the PNMPY/NANO-Stannous(II)WO3 composite surface comes into contact with the target Hg2+ ion. Reduction in Hg2+ ion removes electrons from the PNMPY/NANO-Stannous(II)WO3 composite surface, which ultimately decreases the conductance of the PNMPY/NANO-Stannous(II)WO3/GCE assembly; hence, the current response decreases. Superior consistency as well as stability also displayed by PNMPY/NANO-Stannous(II)WO3/GCE sensor [39,40]. Despite these developments in this proposed sensor, there are still a few challenges that need to be addressed before it can be commercialized. A comparison of the electrode parameters of the published study [41,42,43,44,45,46] with the current electrode is given in Table 2.

Table 2.
Comparison of Hg(II)-selective PNMPy/nano-Stannous(II)WO3/GCE modified GCE with other Hg (II)-selective electrodes.
4. Conclusions
PNMPy/Stannous(II)WO3 ternary nanocomposite was synthesis by chemical oxidative polymerization sol-gel process by initiators of N-methyl pyrrole monomer, tin(II)tungstate. Based on morphological investigations, N-methyl pyrrole molecules were in situ polymerized on separately prepared layered Stannous(II)tungstate. The core in the whole process is the Stannous(II)tungstate. In comparison to the homopolymer, the PNMPy/Stannous(II)WO3 has better temperature stability. Due to the electronic characteristics of the PNMPy, the composite demonstrated improved durability. The PNMPY/NANO-Stannous(II)WO3/Nafion/GCE electrode was effectively used as a chemical sensor for different metal ions but found highly selective for aqueous Hg2+ ion concentration. The constructed Hg2+ ion chemical sensor based on PNMPY/NANO-Stannous(II)WO3 composite coated on GCE performed exceptionally well as an electron mediator in the reduction in Hg2+ ion in the PBS system. The suggested PNMPY/NANO-Stannous(II)WO3/Nafion/GCE sensor for the Hg2+ ion has better sensitivity and ultra-low limit of detection over a broad range of concentrations in a fast reaction time, as well as an excellent linear response. Effective chemical sensors could be produced by employing PNMPY/NANO-Stannous(II)WO3 for sustainability.
Author Contributions
Conceptualization, A.K. and M.M.R.; methodology, A.K.; validation, A.A.P.K., A.M.A. and I.K.; formal analysis, A.K.; investigation, A.K.; resources, A.K.; funding by A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Under Grant Number (G-31-130-1442). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This project was funded by the deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Under Grant Number (G-31-130-1442). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M.; Inamuddin; Alamry, K.A.; Hameed, S.A. Preparation and characterization of PANI@G/CWO nanocomposite for enhanced 2-nitrophenol sensing. Appl. Surf. Sci. 2018, 433, 696–704. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Khan, A.A.P.; Rub, M.A.; Azum, N.; Rahman, M.M.; Al-Youbi, A.O.; Qusti, A.H. Dual nature, self oxidized poly(o-anisidine) functionalized multiwall carbon nanotubes composite: Preparation, thermal and electrical studies. Compos. Part B Eng. 2014, 58, 451–456. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Asiri, A.M.; Rahman, M.M.; Alhogbi, B.G. Preparation and properties of novel sol-gel-derived quaternized poly(n-methyl pyrrole)/Sn(II)SiO3/CNT composites. J. Solid State Electrochem. 2015, 19, 1479–1489. [Google Scholar] [CrossRef]
- Khan, A. Electrical conductivity and cation exchange kinetic studies on poly-o-toluidine Th(IV) phosphate nano-composite cation exchange material. Talanta 2007, 73, 850–856. [Google Scholar] [CrossRef]
- Khan, A.A.; Habiba, U.; Khan, A. Synthesis and Characterization of Organic-Inorganic Nanocomposite Poly-o-anisidine Sn(IV) Arsenophosphate: Its Analytical Applications as Pb(II) Ion-Selective Membrane Electrode. Int. J. Anal. Chem. 2009, 2009, 659215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Liu, J.; Tanaka, T.; Sivula, K.; Alivisatos, A.P.; Fréchet, J.M.J. Employing End-Functional Polythiophene to Control the Morphology of Nanocrystal−Polymer Composites in Hybrid Solar Cells. J. Am. Chem. Soc. 2004, 126, 6550–6551. [Google Scholar] [CrossRef]
- Zhang, M.; Nautiyal, A.; Du, H.; Li, J.; Liu, Z.; Zhang, X.; Wang, R. Polypyrrole film based flexible supercapacitor: Mechanistic insight into influence of acid dopants on electrochemical performance. Electrochim. Acta 2020, 357, 136877. [Google Scholar] [CrossRef]
- Shimoga, G.; Palem, R.; Choi, D.-S.; Shin, E.-J.; Ganesh, P.-S.; Saratale, G.; Saratale, R.; Lee, S.-H.; Kim, S.-Y. Polypyrrole-Based Metal Nanocomposite Electrode Materials for High-Performance Supercapacitors. Metals 2021, 11, 905. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Hasik, M.; Kloc, M. Pd/polyaniline as the catalysts for 2-ethylanthraquinone hydrogenation. The effect of palladium dispersion. Catal. Lett. 2000, 64, 41–47. [Google Scholar] [CrossRef]
- Radford, P.; Creager, S. Dual-stream flow injection method for amplified electrochemical detection of ferrocene derivatives. Anal. Chim. Acta 2001, 449, 199–209. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Khan, A.A.P.; Rub, M.A.; Azum, N.; Rahman, M.M.; Khan, S.B.; Alamry, K.A.; Ab Ghani, S. Sol–gel synthesis and characterization of conducting polythiophene/tin phosphate nano tetrapod composite cation-exchanger and its application as Hg(II) selective membrane electrode. J. Sol-Gel Sci. Technol. 2013, 65, 160–169. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Xu, Y.; Liu, Y.; Li, D.; Fan, C. A highly sensitive chemiluminescence sensor for detecting mercury (II) ions: A combination of Exonuclease III-aided signal amplification and graphene oxide-assisted background reduction. Sci. China Ser. B Chem. 2015, 58, 514–518. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Hou, Y.; Chen, J. Ultrasensitive Quantum Dot Fluorescence quenching Assay for Selective Detection of Mercury Ions in Drinking Water. Sci. Rep. 2015, 4, 5624. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; She, S.; Zhang, J.; Bayaguud, A.; Wei, Y. Label-free colorimetric detection of mercury via Hg2+ ions-accelerated structural transformation of nanoscale metal-oxo clusters. Sci. Rep. 2015, 5, 16316. [Google Scholar] [CrossRef] [Green Version]
- Moreton, J.A.; Delves, H.T. Simple direct method for the determination of total mercury levels in blood and urine and nitric acid digests of fish by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1998, 13, 659–665. [Google Scholar] [CrossRef]
- Xiao, X.; Ulstrup, J.; Li, H.; Wang, M.; Zhang, J.; Si, P. Nanoporous gold assembly of glucose oxidase for electrochemical biosensing. Electrochim. Acta 2014, 130, 559–567. [Google Scholar] [CrossRef]
- Lang, X.-Y.; Fu, H.-Y.; Hou, C.; Han, G.-F.; Yang, P.; Liu, Y.-B.; Jiang, Q. Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nat. Commun. 2013, 4, 2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Kim, Y. Gold nanoparticle-based fluorescent “turn-on” sensing system for the selective detection of mercury ions in aqueous solution. RSC Adv. 2015, 5, 95268–95272. [Google Scholar] [CrossRef]
- Wegner, S.V.; Okesli, A.; Chen, A.P.; He, C. Design of an Emission Ratiometric Biosensor from MerR Family Proteins: A Sensitive and Selective Sensor for Hg2+. J. Am. Chem. Soc. 2007, 129, 3474–3475. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Togashi, H. Highly Selective Oligonucleotide-Based Sensor for Mercury(II) in Aqueous Solutions. Angew. Chem. 2004, 116, 4400–4402. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ting, R.; Perrin, D.M. High affinity DNAzyme-based ligands for transition metal cations? A prototype sensor for Hg2+. Org. Biomol. Chem. 2004, 2, 307–312. [Google Scholar] [CrossRef]
- Duval, C. Inorganic Thermogravimetric Analysis; Elsevier: Amsterdam, The Netherlands, 1963. [Google Scholar]
- Wang, Y.; Rubner, M. Stability studies of the electrical conductivity of various poly(3-alkylthiophenes). Synth. Met. 1990, 39, 153–175. [Google Scholar] [CrossRef]
- Wang, J.; Keene, F. Mechanism of mediation of the electrochemical oxidation of K4Fe(CN)6 at poly-[tris(3-{ω-[4-(2,2′-bipyridyl)] alkyl}-thiophene)iron(II)]-film modified electrodes in aqueous solutions. Electrochim. Acta 1996, 41, 2563–2569. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly(thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Toshima, N.; Hara, S. Direct synthesis of conducting polymers from simple monomers. Prog. Polym. Sci. 1995, 20, 155–183. [Google Scholar] [CrossRef]
- Solonin, Y.M.; Khyzhun, O.Y.; Graivoronskaya, E.A. Nonstoichiometric Tungsten Oxide Based on Hexagonal WO3. Cryst. Growth Des. 2001, 1, 473–477. [Google Scholar] [CrossRef]
- Utsumi, S.; Honda, H.; Hattori, Y.; Kanoh, H.; Takahashi, K.; Sakai, H.; Abe, M.; Yudasaka, M.; Iijima, S.; Kaneko, K. Direct Evidence on C−C Single Bonding in Single-Wall Carbon Nanohorn Aggregates. J. Phys. Chem. C 2007, 111, 5572–5575. [Google Scholar] [CrossRef]
- Siaj, M.; Maltais, C.; Zahidi, E.M.; Oudghiri-Hassani, H.; Wang, J.; Rosei, A.F.; McBreen, P.H. Carbon−Nitrogen Place Exchange on NO Exposed β-Mo2C. J. Phys. Chem. B 2005, 109, 15376–15382. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, S.B.; Faisal, M.; Rub, M.A.; Al-Youbi, A.O.; Asiri, A.M. Electrochemical determination of olmesartan medoxomil using hydrothermally prepared nanoparticles composed SnO2–Co3O4 nanocubes in tablet dosage forms. Talanta 2012, 99, 924–931. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alenazi, N.A.; Hussein, M.A.; Alam, M.M.; Alamry, K.A.; Asiri, A.M. Hybride ZnCdCrO embedded aminated polyethersulfone nanocomposites for the development of Hg2+ ionic sensor. Mater. Res. Express 2018, 5, 065019. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alamry, K.A.; Awual, R.; Mekky, A.E. Efficient Hg(II) ionic probe development based on one-step synthesized diethyl thieno[2,3-b]thiophene-2,5-dicarboxylate (DETTDC2) onto glassy carbon electrode. Microchem. J. 2020, 152, 104291. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Arshad, M.N.; Asiri, A.M. Hg2+ Sensor Development Based on (E)-N′-Nitrobenzylidene-Benzenesulfonohydrazide (NBBSH) Derivatives Fabricated on a Glassy Carbon Electrode with a Nafion Matrix. ACS Omega 2017, 2, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.N.; Rahman, M.M.; Asiri, A.M.; Sobahi, T.R.; Yu, S.-H. Development of Hg2+ sensor based on N′-[1-(pyridin-2-yl)ethylidene]benzenesulfono-hydrazide (PEBSH) fabricated silver electrode for environmental remediation. RSC Adv. 2015, 5, 81275–81281. [Google Scholar] [CrossRef]
- Awual, R.; Hasan, M.; Eldesoky, G.E.; Khaleque, A.; Rahman, M.M.; Naushad, M. Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem. Eng. J. 2016, 290, 243–251. [Google Scholar] [CrossRef]
- Katowah, D.F.; Alqarni, S.; Mohammed, G.I.; Al Sheheri, S.Z.; Alam, M.M.; Ismail, S.H.; Asiri, A.M.; Hussein, M.A.; Rahman, M.M. Selective Hg2+ sensor performance based various carbon-nanofillers into CuO-PMMA nanocomposites. Polym. Adv. Technol. 2020, 31, 1946–1962. [Google Scholar] [CrossRef]
- Soman, S.; PV, A. Covalently modified graphene quantum dot using a thiourea based imprinted polymer for the selective electrochemical sensing of Hg(II) ions. J. Polym. Res. 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Koshki, M.-S.; Baghayeri, M.; Fayazi, M. Application of sepiolite/FeS2 nanocomposite for highly selective detection of mercury(II) based on stripping voltammetric analysis. J. Food Meas. Charact. 2021, 15, 5318–5325. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Fox, D.; Stanberry, J.; Anagnostopoulos, V.; Zhai, L.; Lee, W. Direct Mercury Detection in Landfill Leachate Using a Novel AuNP-Biopolymer Carbon Screen-Printed Electrode Sensor. Micromachines 2021, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.-C.; Ke, X.-X.; Chen, X.; Weerasooriya, R.; Hong, Z.-Y.; Wang, L.-C.; Wu, Y.-C. Assembling reduced graphene oxide with sulfur/nitrogen-“hooks” for electrochemical determination of Hg(II). Anal. Chim. Acta 2020, 1100, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Raicopol, M.D.; Chira, N.A.; Pandele, A.M.; Hanganu, A.; Ivanov, A.A.; Tecuceanu, V.; Bugean, I.G.; Buica, G.-O. Electrodes modified with clickable thiosemicarbazone ligands for sensitive voltammetric detection of Hg(II) ions. Sens. Actuators B Chem. 2020, 313, 128030. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Li, J.; Chen, L. Highly sensitive and selective voltammetric detection of mercury(II) using an ITO electrode modified with 5-methyl-2-thiouracil, graphene oxide and gold nanoparticles. Mikrochim. Acta 2013, 180, 493–499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).