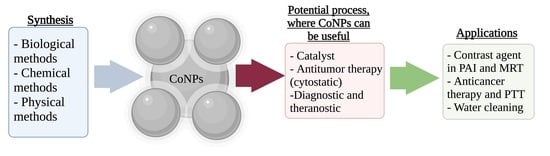
Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review
Abstract
:1. Introduction
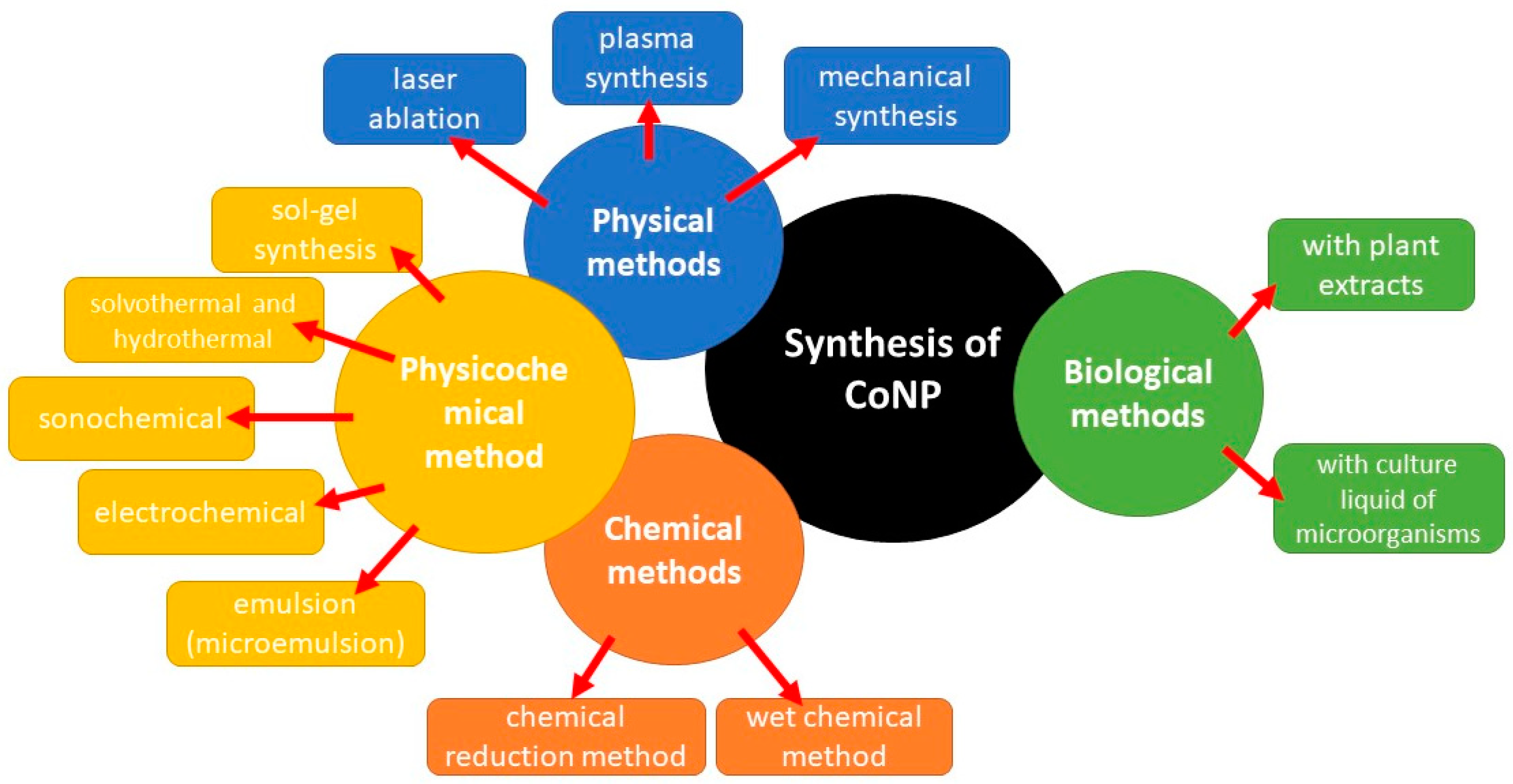
2. Synthesis Methods of CoNPs
- High yield of the target product,
- The possibility of obtaining nanoparticles of a given structure (crystallinity, size, shape
- Safety and practicality of the method of obtaining
- Environmentally friendly methods
- Scalability
2.1. Solution Method of CoNPs Synthesis
2.1.1. Hydrothermal Method of CoNPs Synthesis
2.1.2. The Solvothermal Method of CoNPs Synthesis
2.1.3. Microemulsion Method of CoNPs Synthesis
2.2. Green Methods of CoNPs Synthesis
2.3. Physical Methods of CoNPs Synthesis
2.3.1. Vapor Condensation Method
2.3.2. The Arc Plasma-Assisted Deposition Method
2.3.3. Liquid-Phase Plasma Method
2.3.4. The Ultrasonic Method
2.4. Preparation of Hybrid Materials Based on CoNPs
3. Applications of CoNPs
3.1. CoNPs in Catalysis
3.2. CoNPs as an Anticancer Drug
3.3. CoNPs as a Contrast Agent and Diagnostic/Theranostic Agent
3.4. Magnetic Applications of CoNPs
3.5. CoNPs Toxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szczypiński, F.T.; Bennett, S.; Jelfs, K.E. Can we predict materials that can be synthesised? Chem. Sci. 2021, 12, 830–840. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Y.; Pan, L.; Ouyang, J.; Zhang, Q. Recent developments in flexible thermoelectrics: From materials to devices. Renew. Sustain. Energy Rev. 2021, 137, 110448. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef] [PubMed]
- Manickam, S.; Ashokkumar, M. Cavitation: A Novel Energy-Efficient Technique for the Generation of Nanomaterials. In Cavitation: A Novel Energy-Efficient Technique for the Generation of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–433. [Google Scholar] [CrossRef]
- Gong, N.; Sheppard, N.C.; Billingsley, M.M.; June, C.H.; Mitchell, M.J. Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 2021, 16, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265, 120404. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Komal, P.; Gautam, P.; Sharma, A.; Kumar, N.; Jung, J. Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications. Metals 2021, 11, 329. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Kari, Z.A.; Razab, M.K.A.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium Nanoparticles as a Natural Antioxidant and Metabolic Regulator in Aquaculture: A Review. Antioxidants 2021, 10, 1364. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samorì, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2020, 50, 1269–1304. [Google Scholar] [CrossRef]
- Yang, W.; Pan, M.; Huanga, C.; Zhaoa, Z.; Wangb, J.; Zenga, H. Graphene oxide-based noble-metal nanoparticles composites for environmental application. Compos. Commun. 2021, 24, 100645. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Rizk, M.G.H.; Kezimana, P.; Kirichuk, A.A.; Stanishevskiy, Y.M. Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering 2021, 5, 69. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Zan, Y.; Salmon, L.; Bousseksou, A. Morphological Studies of Composite Spin Crossover@SiO2 Nanoparticles. Nanomaterials 2021, 11, 3169. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P. Occurrence, exposure, effects, recommended intake and possible dietary use of selected trace compounds (aluminium, bismuth, cobalt, gold, lithium, nickel, silver). Int. J. Food Sci. Nutr. 2014, 65, 911–924. [Google Scholar] [CrossRef]
- Debussche, L.; Couder, M.; Thibaut, D.; Cameron, B.; Crouzet, J.; Blanche, F. Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans. J. Bacteriol. 1992, 174, 7445–7451. [Google Scholar] [CrossRef] [Green Version]
- Neil, E.; Marsh, E.N. Coenzyme B12 (cobalamin)-dependent enzymes. Essays Biochem. 1999, 34, 139–154. [Google Scholar] [CrossRef]
- Dong, H.; Meininger, A.; Jiang, H.; Moon, K.-S.; Wong, C.P. Magnetic Nanocomposite for Potential Ultrahigh Frequency Microelectronic Application. J. Electron. Mater. 2007, 36, 593–597. [Google Scholar] [CrossRef]
- Jarestan, M.; Khalatbari, K.; Pouraei, A.; Shandiz, S.A.S.; Beigi, S.; Hedayati, M.; Majlesi, A.; Akbari, F.; Salehzadeh, A. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Puche, M.; Liu, L.; Concepción, P.; Sorribes, I.; Corma, A. Tuning the Catalytic Performance of Cobalt Nanoparticles by Tungsten Doping for Efficient and Selective Hydrogenation of Quinolines under Mild Conditions. ACS Catal. 2021, 11, 8197–8210. [Google Scholar] [CrossRef]
- Parkes, L.M.; Hodgson, R.; Lu, L.T.; Tung, L.D.; Robinson, I.; Fernig, D.G.; Thanh, N.T.K. Cobalt nanoparticles as a novel magnetic resonance contrast agent-relaxivities at 1.5 and 3 Tesla. Contrast Media Mol. Imaging 2008, 3, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Farkaš, B.; Terranova, U.; De Leeuw, N.H. The mechanism underlying the functionalisation of cobalt nanoparticles by carboxylic acids: A first-principles computational study. J. Mater. Chem. B 2021, 9, 4915–4928. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Upadhyay, P.; Das, A.; Ghosh, A.; Adhikary, A.; Goswami, M.M. Studies on cancer cell death through delivery of dopamine as anti-cancer drug by a newly functionalized cobalt ferrite nano-carrier. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 627, 127202. [Google Scholar] [CrossRef]
- Jincy, C.; Meena, P. Synthesis, characterization, and NH3 gas sensing application of Zn doped cobalt oxide nanoparticles. Inorg. Chem. Commun. 2020, 120, 108145. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020, 22, 2643–2661. [Google Scholar] [CrossRef]
- Haq, S.; Abbasi, F.; Ben Ali, M.; Hedfi, A.; Mezni, A.; Rehman, W.; Waseem, M.; Khan, A.R.; Shaheen, H. Green synthesis of cobalt oxide nanoparticles and the effect of annealing temperature on their physiochemical and biological properties. Mater. Res. Express 2021, 8, 075009. [Google Scholar] [CrossRef]
- Mondal, A.; Adhikary, B.; Mukherjee, D. Room-temperature synthesis of air stable cobalt nanoparticles and their use as catalyst for methyl orange dye degradation. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 248–257. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, L. Room-temperature synthesis of air-stable cobalt nanoparticles and their highly efficient adsorption ability for Congo red. RSC Adv. 2012, 2, 5485–5487. [Google Scholar] [CrossRef]
- Clifford, D.; El-Gendy, A.A.; Lu, A.J.; Pestov, D.; Carpenter, E.E. Room Temperature Synthesis of Highly Magnetic Cobalt Nanoparticles by Continuous Flow in a Microfluidic Reactor. J. Flow Chem. 2014, 4, 148–152. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, H.; Yang, Z.; Qian, Y. Synthesis of cobalt nanoparticles in ethanol hydrazine alkaline system (EHAS) at room temperature. Mater. Lett. 2002, 56, 906–909. [Google Scholar] [CrossRef]
- Zola, A.S.; Ribeiro, R.U.; Bueno, J.M.; Zanchet, D.; Arroyo, P. Cobalt nanoparticles prepared by three different methods. J. Exp. Nanosci. 2012, 9, 398–405. [Google Scholar] [CrossRef]
- Ansari, S.; Bhor, R.; Pai, K.; Sen, D.; Mazumder, S.; Ghosh, K.; Kolekar, Y.; Ramana, C. Cobalt nanoparticles for biomedical applications: Facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl. Surf. Sci. 2017, 414, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Seong, G.; Takami, S.; Arita, T.; Minami, K.; Hojo, D.; Yavari, A.R.; Adschiri, T. Supercritical hydrothermal synthesis of metallic cobalt nanoparticles and its thermodynamic analysis. J. Supercrit. Fluids 2011, 60, 113–120. [Google Scholar] [CrossRef]
- Kim, M.; Son, W.-S.; Ahn, K.H.; Kim, D.S.; Lee, H.-S.; Lee, Y.-W. Hydrothermal synthesis of metal nanoparticles using glycerol as a reducing agent. J. Supercrit. Fluids 2014, 90, 53–59. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, G.; Li, X. Shape-controlled synthesis and properties of uniform spinel cobalt oxide nanocubes. Nanotechnology 2005, 16, 3035–3040. [Google Scholar] [CrossRef]
- Xie, B.; Qian, Y.; Zhang, S.; Fu, S.; Yu, W. A Hydrothermal Reduction Route to Single-Crystalline Hexagonal Cobalt Nanowires. Eur. J. Inorg. Chem. 2006, 2006, 2454–2459. [Google Scholar] [CrossRef]
- Alagiri, M.; Muthamizhchelvan, C.; Hamid, S.B.A. Synthesis of superparamagnetic cobalt nanoparticles through solvothermal process. J. Mater. Sci. Mater. Electron. 2013, 24, 4157–4160. [Google Scholar] [CrossRef]
- Xu, R.; Xie, T.; Zhao, Y.; Li, Y. Quasi-homogeneous catalytic hydrogenation over monodisperse nickel and cobalt nanoparticles. Nanotechnology 2007, 18, 55602. [Google Scholar] [CrossRef]
- Shin, N.C.; Lee, Y.-H.; Shin, Y.H.; Kim, J.; Lee, Y.-W. Synthesis of cobalt nanoparticles in supercritical methanol. Mater. Chem. Phys. 2010, 124, 140–144. [Google Scholar] [CrossRef]
- Dumestre, F.; Chaudret, B.; Amiens, C.; Fromen, M.-C.; Casanove, M.-J.; Renaud, P.; Zurcher, P. Shape Control of Thermodynamically Stable Cobalt Nanorods through Organometallic Chemistry. Angew. Chem. Int. Ed. 2002, 41, 4286–4289. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Zhang, X.; Shi, C. Synthesis and magnetic properties of nickel and cobalt nanoparticles obtained in DMF solution. Solid State Commun. 2006, 139, 403–405. [Google Scholar] [CrossRef]
- Osuna, J.; de Caro, D.; Amiens, C.; Chaudret, B.; Snoeck, E.; Respaud, M.; Broto, J.-M.; Fert, A. Synthesis, Characterization, and Magnetic Properties of Cobalt Nanoparticles from an Organometallic Precursor. J. Phys. Chem. 1996, 100, 14571–14574. [Google Scholar] [CrossRef]
- Scariot, M.; Silva, D.O.; Scholten, J.D.; Machado, G.; Teixeira, S.R.; Novak, M.A.; Ebeling, G.; Dupont, J. Cobalt Nanocubes in Ionic Liquids: Synthesis and Properties. Angew. Chem. Int. Ed. 2008, 47, 9075–9078. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Mitan, M.; Kim, H.; Vaya, D. Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. J. Phys. Chem. Solids 2021, 155, 110125. [Google Scholar] [CrossRef]
- Nam, K.M.; Shim, J.H.; Ki, H.; Choi, S.-I.; Lee, G.; Jang, J.K.; Jo, Y.; Jung, M.-H.; Song, H.; Park, J.T. Single-Crystalline Hollow Face-Centered-Cubic Cobalt Nanoparticles from Solid Face-Centered-Cubic Cobalt Oxide Nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 9504–9508. [Google Scholar] [CrossRef]
- Song, Y.; Henry, L.L.; Yang, W. Stable Amorphous Cobalt Nanoparticles Formed by an in Situ Rapidly Cooling Microfluidic Process. Langmuir 2009, 25, 10209–10217. [Google Scholar] [CrossRef]
- Salman, S.A.; Usami, T.; Kuroda, K.; Okido, M. Synthesis and Characterization of Cobalt Nanoparticles Using Hydrazine and Citric Acid. J. Nanotechnol. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cruz, J.C.; Nascimento, M.A.; Amaral, H.A.; Lima, D.S.; Teixeira, A.P.C.; Lopes, R.P. Synthesis and characterization of cobalt nanoparticles for application in the removal of textile dye. J. Environ. Manag. 2019, 242, 220–228. [Google Scholar] [CrossRef]
- Song, Y.; Modrow, H.; Henry, L.L.; Saw, C.K.; Doomes, E.E.; Palshin, V.; Hormes, A.J.; Kumar, C.S.S.R.; Bonn, D. Microfluidic Synthesis of Cobalt Nanoparticles. Chem. Mater. 2006, 18, 2817–2827. [Google Scholar] [CrossRef]
- Duggan, J.N.; Bozack, M.J.; Roberts, C.B. The synthesis and arrested oxidation of amorphous cobalt nanoparticles using DMSO as a functional solvent. J. Nanopart. Res. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Balela, M.D.L.; Yagi, S.; Matsubara, E. Room-Temperature Synthesis of Cobalt Nanoparticles by Electroless Deposition in Aqueous Solution. Electrochem. Solid-State Lett. 2010, 13, D4–D6. [Google Scholar] [CrossRef]
- Li, H.; Liao, S. Organic colloid method to prepare ultrafine cobalt nanoparticles with the size of 2 nm. Solid State Commun. 2008, 145, 118–121. [Google Scholar] [CrossRef]
- Yanilkin, V.V.; Nasretdinova, G.R.; Osin, Y.; Salnikov, V.V. Anthracene mediated electrochemical synthesis of metallic cobalt nanoparticles in solution. Electrochim. Acta 2015, 168, 82–88. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Lee, H.; Kim, B.H.; Kim, S.-J.; Lee, J.-M.; Jung, S.-C. Synthesis Process of Cobalt Nanoparticles in Liquid-Phase Plasma. Jpn. J. Appl. Phys. 2013, 52, 01AN03. [Google Scholar] [CrossRef]
- Sergiienko, R.; Shibata, E.; Zentaro, A.; Shindo, D.; Nakamura, T.; Qin, G. Formation and characterization of graphite-encapsulated cobalt nanoparticles synthesized by electric discharge in an ultrasonic cavitation field of liquid ethanol. Acta Mater. 2007, 55, 3671–3680. [Google Scholar] [CrossRef]
- Aref’eva, L.P.; Kravtsov, A.A.; Blinov, A.V.; Kharchenko, S.V.; Serov, A.V.; Solov’ev, I.E. Synthesis and Investigation of Cobalt Containing Nanoparticles Morphology. Her. Bauman Mosc. State Tech. Univ. Ser. Nat. Sci. 2017, 71, 85–95. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B. Synthesis of monodisperse cobalt nanocrystals and their assembly into magnetic superlattices (invited). J. Appl. Phys. 1999, 85, 4325–4330. [Google Scholar] [CrossRef]
- Khusnuriyalova, A.F.; Caporali, M.; Hey-Hawkins, E.; Sinyashin, O.G.; Yakhvarov, D.G. Preparation of Cobalt Nanoparticles. Eur. J. Inorg. Chem. 2021, 2021, 3023–3047. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M.; Shaterian, M. Preparation of cobalt nanoparticles from [bis(salicylidene)cobalt(II)]–oleylamine complex by thermal decomposition. J. Magn. Magn. Mater. 2008, 320, 575–578. [Google Scholar] [CrossRef]
- Hertrich, M.F.; Scharnagl, F.K.; Pews-Davtyan, A.; Kreyenschulte, C.R.; Lund, H.; Bartling, S.; Jackstell, R.; Beller, M. Supported Cobalt Nanoparticles for Hydroformylation Reactions. Chem.—A Eur. J. 2019, 25, 5534–5538. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Natarajan, K.; Tiwari, S.; Ganguli, A.K. Nanostructures synthesized by the reverse microemulsion method and their magnetic properties. Mater. Res. Express 2020, 7, 104001. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liang, D.; Zhang, X.; Lin, Q.; Hao, L. Preparation and Antibacterial Performances of Electrocatalytic Zinc Oxide Nanoparticles with Diverse Morphologies. J. Biomed. Nanotechnol. 2021, 17, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Rehman, W.; Waseem, M.; Din, S.U. Antibacterial, antioxidant and physicochemical investigations of tin dioxide nanoparticles synthesized via microemulsion method. Mater. Res. Express 2021, 8, 035013. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Kale, A.R.; Barai, D.P.; Bhanvase, B.A.; Sonawane, S.H. An Ultrasound-Assisted Minireactor System for Continuous Production of TiO2 Nanoparticles in a Water-in-Oil Emulsion. Ind. Eng. Chem. Res. 2021, 60, 14747–14757. [Google Scholar] [CrossRef]
- Sopoušek, J.; Pinkas, J.; Buršík, J.; Svoboda, M.; Krásenský, P. Continuous Flow Synthesis of Iron Oxide Nanoparticles Using Water-in-Oil Microemulsion. Colloid J. 2020, 82, 727–734. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Zhu, B.; Zhou, Y.; Liu, X.; Yang, C. Temperature-Switchable Surfactant-Free Microemulsion. Langmuir 2020, 36, 7356–7364. [Google Scholar] [CrossRef]
- Sandhu, R.K.; Kaur, A.; Kaur, P.; Rajput, J.K.; Khullar, P.; Bakshi, M.S. Solubilization of surfactant stabilized gold nanoparticles in oil-in-water and water-in-oil microemulsions. J. Mol. Liq. 2021, 336, 116305. [Google Scholar] [CrossRef]
- Kamal, S.K.; Sahoo, P.; Premkumar, M.; Rao, N.R.; Kumar, T.J.; Sreedhar, B.; Singh, A.; Ram, S.; Sekhar, K.C. Synthesis of cobalt nanoparticles by a modified polyol process using cobalt hydrazine complex. J. Alloys Compd. 2009, 474, 214–218. [Google Scholar] [CrossRef]
- Khan, J.; Ullah, H.; Sajjad, M.; Ali, A.; Thebo, K.H. Synthesis, characterization and electrochemical performance of cobalt fluoride nanoparticles by reverse micro-emulsion method. Inorg. Chem. Commun. 2018, 98, 132–140. [Google Scholar] [CrossRef]
- Meydan, E.; Demirci, S.; Aktaş, N.; Sahiner, N.; Ozturk, O.F. Boron-containing magnetic nanoparticles from Co, Ni, and Fe chloride salts and their catalytic performances on 4-nitrophenol reduction. Inorg. Chem. Commun. 2020, 116, 107930. [Google Scholar] [CrossRef]
- Nilavukkarasi, M.; Vijayakumar, S.; Kumar, S.P. Biological synthesis and characterization of silver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and anti proliferation efficiency. Mater. Sci. Energy Technol. 2020, 3, 371–376. [Google Scholar] [CrossRef]
- Maheshwaran, G.; Bharathi, A.N.; Selvi, M.M.; Kumar, M.K.; Kumar, R.M.; Sudhahar, S. Green synthesis of Silver oxide nanoparticles using Zephyranthes Rosea flower extract and evaluation of biological activities. J. Environ. Chem. Eng. 2020, 8, 104137. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef] [PubMed]
- Esa, Y.A.M.; Sapawe, N. A short review on biosynthesis of cobalt metal nanoparticles. Mater. Today Proc. 2020, 31, 378–385. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Mathimani, T.; Santhanam, N.; Phuong, T.N.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J. Photochem. Photobiol. B Biol. 2020, 211, 112011. [Google Scholar] [CrossRef]
- Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Taher, M.M.; Khalaf, M. Benign Synthesis of Cobalt Oxide Nanoparticles Containing Red Algae Extract: Antioxidant, Antimicrobial, Anticancer, and Anticoagulant Activity. J. Clust. Sci. 2021, 1–12. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Iqbal, M.; Kamal, S.; Nawaz, H.; Nouren, S.; Safa, Y.; Jilani, K.; Sultan, M.; Ata, S.; et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder Technol. 2017, 28, 2035–2043. [Google Scholar] [CrossRef]
- Jang, E.; Ryu, B.H.; Shim, H.-W.; Ju, H.; Kim, D.-W.; Kim, T.D. Adsorption of microbial esterases on Bacillus subtilis-templated cobalt oxide nanoparticles. Int. J. Biol. Macromol. 2014, 65, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Nassar, H.; AliYounis, S.; El-Salamony, R.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404—Optimization, characterization and antimicrobial activity. J. Appl. Microbiol. 2019, 128, 438–457. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, J.; Zhang, J.; Cai, J.; Pi, J.; Xu, J.-F. Inspirations of Cobalt Oxide Nanoparticle Based Anticancer Therapeutics. Pharmaceutics 2021, 13, 1599. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, P.; Das, A.K. Unconventional Physical Methods for Synthesis of Metal and Non-metal Nanoparticles: A Review. Proc. Natl. Acad. Sci. USA 2018, 89, 199–221. [Google Scholar] [CrossRef]
- Dong, X.; Choi, C.; Kim, B. Chemical synthesis of Co nanoparticles by chemical vapor condensation. Scr. Mater. 2002, 47, 857–861. [Google Scholar] [CrossRef]
- Wang, Z.; Choi, C.; Kim, B.; Kim, J.; Zhang, Z. Characterization and magnetic properties of carbon-coated cobalt nanocapsules synthesized by the chemical vapor-condensation process. Carbon 2003, 41, 1751–1758. [Google Scholar] [CrossRef]
- Choi, C.; Dong, X.; Kim, B. Characterization of Fe and Co nanoparticles synthesized by chemical vapor condensation. Scr. Mater. 2001, 44, 2225–2229. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, F.; Zhang, Z. Preparation of cobalt nanoparticles by direct current arc plasma evaporation method. Int. J. Refract. Met. Hard Mater. 2012, 31, 224–229. [Google Scholar] [CrossRef]
- Song, S.; Zhou, X.; Li, L.; Ma, W. Numerical simulation and experimental validation of SiC nanoparticle distribution in magnesium melts during ultrasonic cavitation based processing of magnesium matrix nanocomposites. Ultrason. Sonochem. 2015, 24, 43–54. [Google Scholar] [CrossRef]
- Ohayon, E.; Gedanken, A. The application of ultrasound radiation to the synthesis of nanocrystalline metal oxide in a non-aqueous solvent. Ultrason. Sonochem. 2010, 17, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhuang, T.; Dong, J.; Wang, L.; Xia, J.; Wang, H.; Cui, X.; Wang, Z. Sonochemical fabrication of inorganic nanoparticles for applications in catalysis. Ultrason. Sonochem. 2021, 71, 105384. [Google Scholar] [CrossRef] [PubMed]
- Dabalà, M.; Pollet, B.G.; Zin, V.; Campadello, E.; Mason, T.J. Sonoelectrochemical (20 kHz) production of Co65Fe35 alloy nanoparticles from Aotani solutions. J. Appl. Electrochem. 2008, 38, 395–402. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, K.-Y. Microemulsion synthesis and electrocatalytic properties of platinum–cobalt nanoparticles. J. Mater. Chem. 2002, 12, 1203–1206. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Dong, Y.; Wang, L.; Jin, C.; Zhou, N.; Chen, M.; Dong, Y.; Xie, Z.; Zhang, C. Porous cobalt oxides/carbon foam hybrid materials for high supercapacitive performance. J. Colloid Interface Sci. 2019, 542, 102–111. [Google Scholar] [CrossRef]
- Lai, F.; Huang, Y.; Miao, Y.-E.; Liu, T. Controllable preparation of multi-dimensional hybrid materials of nickel-cobalt layered double hydroxide nanorods/nanosheets on electrospun carbon nanofibers for high-performance supercapacitors. Electrochim. Acta 2015, 174, 456–463. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabe, A.; García-Aguilar, J.; Berenguer-Murcia, Á.; Morallón, E.; Cazorla-Amorós, D. Key factors improving oxygen reduction reaction activity in cobalt nanoparticles modified carbon nanotubes. Appl. Catal. B Environ. 2017, 217, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Chen, S.; Bandal, H.A.; Appiah-Ntiamoah, R.; Jadhav, A.R.; Kim, H. Cobalt nanoparticles supported on magnetic core-shell structured carbon as a highly efficient catalyst for hydrogen generation from NaBH4 hydrolysis. Int. J. Hydrogen Energy 2018, 43, 9296–9306. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, W.; Wang, Y.; Gao, Y.; Qi, K.; Zhang, K. Fast hydrogen generation from NaBH4 hydrolysis catalyzed by nanostructured Co–Ni–B catalysts. Int. J. Hydrogen Energy 2017, 42, 6072–6079. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, C.; Wang, J.; Ding, G.; Xue, Y.; Zhang, Y.; Zhang, K.; Liu, P.; Gao, X. Cobalt nanoparticles packaged into nitrogen-doped porous carbon derived from metal-organic framework nanocrystals for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 8365–8375. [Google Scholar] [CrossRef]
- Wang, J.; Ke, D.; Li, Y.; Zhang, H.; Wang, C.; Zhao, X.; Yuan, Y.; Han, S. Efficient hydrolysis of alkaline sodium borohydride catalyzed by cobalt nanoparticles supported on three–dimensional graphene oxide. Mater. Res. Bull. 2017, 95, 204–210. [Google Scholar] [CrossRef]
- Li, J.; Hong, X.; Wang, Y.; Luo, Y.; Huang, P.; Li, B.; Zhang, K.; Zou, Y.; Sun, L.; Xu, F.; et al. Encapsulated cobalt nanoparticles as a recoverable catalyst for the hydrolysis of sodium borohydride. Energy Storage Mater. 2020, 27, 187–197. [Google Scholar] [CrossRef]
- Makiabadi, M.; Shamspur, T.; Mostafavi, A. Performance improvement of oxygen on the carbon substrate surface for dispersion of cobalt nanoparticles and its effect on hydrogen generation rate via NaBH4 hydrolysis. Int. J. Hydrogen Energy 2020, 45, 1706–1718. [Google Scholar] [CrossRef]
- Xu, Y.; Shan, W.; Liang, X.; Gao, X.; Li, W.; Li, H.; Qiu, X. Cobalt Nanoparticles Encapsulated in Nitrogen-Doped Carbon Shells: Efficient and Stable Catalyst for Nitrobenzene Reduction. Ind. Eng. Chem. Res. 2020, 59, 4367–4376. [Google Scholar] [CrossRef]
- Shu, H.; Lu, L.; Zhu, S.; Liu, M.; Zhu, Y.; Ni, J.; Ruan, Z.; Liu, Y. Ultra small cobalt nanoparticles supported on MCM41: One-pot synthesis and catalytic hydrogen production from alkaline borohydride. Catal. Commun. 2019, 118, 30–34. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Wang, J.; Feng, X.; Cheng, L.; Zhang, H.; Han, S. Co-Mo nanoparticles loaded on three–dimensional graphene oxide as efficient catalysts for hydrogen generation from catalytic hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 29075–29082. [Google Scholar] [CrossRef]
- Rakap, M. Catalytic hydrolysis of hydrazine borane to release hydrogen by cobalt-ruthenium nanoclusters. Int. J. Hydrogen Energy 2020, 45, 15611–15617. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Zhang, D.; Cao, Z.; Zhang, K.; Xie, Y.; Zhou, G.; Li, G.; Bai, S. Cobalt–copper–boron nanoparticles as catalysts for the efficient hydrolysis of alkaline sodium borohydride solution. Int. J. Hydrogen Energy 2020, 45, 9845–9853. [Google Scholar] [CrossRef]
- Wu, D.; Ye, P.; Wang, M.; Wei, Y.; Li, X.; Xu, A. Cobalt nanoparticles encapsulated in nitrogen-rich carbon nanotubes as efficient catalysts for organic pollutants degradation via sulfite activation. J. Hazard. Mater. 2018, 352, 148–156. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Iqbal, H.M. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal. Agric. Biotechnol. 2019, 19, 101154. [Google Scholar] [CrossRef]
- Zeng, Q.-X.; Xu, G.-C.; Zhang, L.; Lv, Y. Porous Cu2O microcubes derived from a metal-formate framework as photocatalyst for degradation of methyl orange. Mater. Res. Bull. 2019, 119, 110537. [Google Scholar] [CrossRef]
- Mapukata, S.; Kobayashi, N.; Kimura, M.; Nyokong, T. Asymmetrical and symmetrical zinc phthalocyanine-cobalt ferrite conjugates embedded in electrospun fibers for dual photocatalytic degradation of azo dyes: Methyl Orange and Orange G. J. Photochem. Photobiol. A Chem. 2019, 379, 112–122. [Google Scholar] [CrossRef]
- Kaur, J.; Singhal, S. Facile synthesis of ZnO and transition metal doped ZnO nanoparticles for the photocatalytic degradation of Methyl Orange. Ceram. Int. 2014, 40, 7417–7424. [Google Scholar] [CrossRef]
- Dey, P.C.; Das, R. Enhanced photocatalytic degradation of methyl orange dye on interaction with synthesized ligand free CdS nanocrystals under visible light illumination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118122. [Google Scholar] [CrossRef]
- Naraginti, S.; Stephen, F.B.; Radhakrishnan, A.; Sivakumar, A. Zirconium and silver co-doped TiO2 nanoparticles as visible light catalyst for reduction of 4-nitrophenol, degradation of methyl orange and methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 814–819. [Google Scholar] [CrossRef]
- Ahmad, I. Inexpensive and quick photocatalytic activity of rare earth (Er, Yb) co-doped ZnO nanoparticles for degradation of methyl orange dye. Sep. Purif. Technol. 2019, 227, 115726. [Google Scholar] [CrossRef]
- Dhas, C.R.; Venkatesh, R.; Jothivenkatachalam, K.; Nithya, A.; Benjamin, B.S.; Raj, A.M.E.; Jeyadheepan, K.; Sanjeeviraja, C. Visible light driven photocatalytic degradation of Rhodamine B and Direct Red using cobalt oxide nanoparticles. Ceram. Int. 2015, 41, 9301–9313. [Google Scholar] [CrossRef]
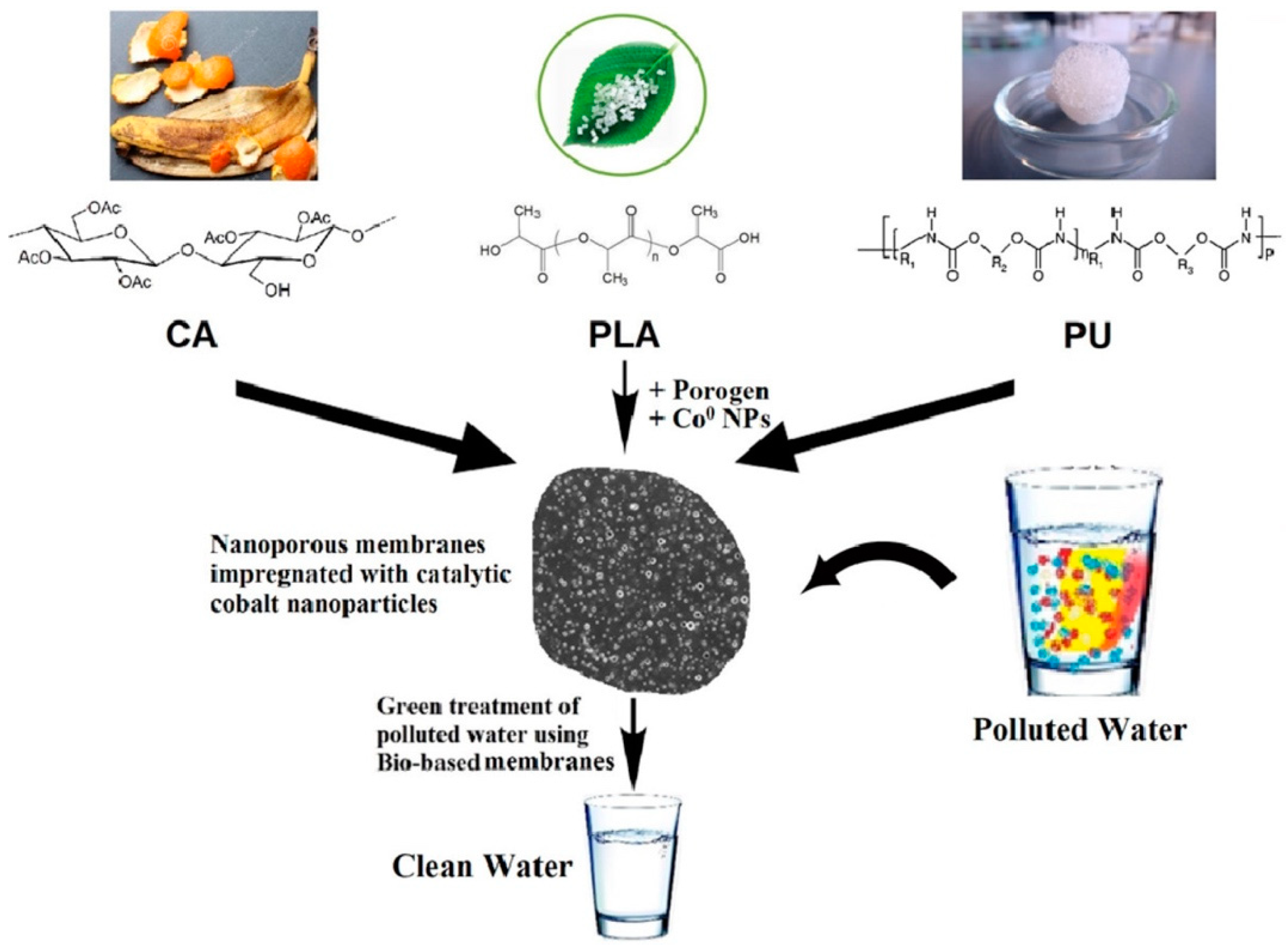
- El-Sayed, M.M.; Elsayed, R.E.; Attia, A.; Farghal, H.H.; Azzam, R.A.; Madkour, T.M. Novel nanoporous membranes of bio-based cellulose acetate, poly(lactic acid) and biodegradable polyurethane in-situ impregnated with catalytic cobalt nanoparticles for the removal of Methylene Blue and Congo Red dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L.; Zhang, S.; Su, J.; Somorjai, G.A. A mini review of cobalt-based nanocatalyst in Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2020, 602, 117701. [Google Scholar] [CrossRef]
- Ralston, W.T.; Melaet, G.; Saephan, T.; Somorjai, G.A. Evidence of Structure Sensitivity in the Fischer-Tropsch Reaction on Model Cobalt Nanoparticles by Time-Resolved Chemical Transient Kinetics. Angew. Chem. Int. Ed. 2017, 56, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-X.; Guo, L.-P.; Yao, S.-Y.; Bao, J.; Liu, Z.-T.; Liu, Z.-W. Cobalt nanoparticles confined in carbon matrix for probing the size dependence in Fischer-Tropsch synthesis. J. Catal. 2019, 369, 143–156. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Bio-Catalysis and Biomedical Perspectives of Magnetic Nanoparticles as Versatile Carriers. Magnetochemistry 2019, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.A.; Iqbal, J.; Khan, Z.; Ahmad, R.; Uddin, S.; Shahbaz, A.; Zahra, S.A.; Shaukat, M.; Kiran, F.; Kanwal, S.; et al. Phytofabrication of cobalt oxide nanoparticles from Rhamnus virgata leaves extract and investigation of different bioactivities. Microsc. Res. Tech. 2021, 84, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Alijani, H.Q.; Peydayesh, M.; Khatami, M.; Baravati, F.B.; Borhani, F.; Šlouf, M.; Soltaninezhad, S. Magnetic cobalt oxide nanosheets: Green synthesis and in vitro cytotoxicity. Bioprocess Biosyst. Eng. 2021, 44, 1423–1432. [Google Scholar] [CrossRef]
- Verma, S.K.; Panda, P.K.; Kumari, P.; Patel, P.; Arunima, A.; Jha, E.; Husain, S.; Prakash, R.; Hergenröder, R.; Mishra, Y.K.; et al. Determining factors for the nano-biocompatibility of cobalt oxide nanoparticles: Proximal discrepancy in intrinsic atomic interactions at differential vicinage. Green Chem. 2021, 23, 3439–3458. [Google Scholar] [CrossRef]
- Kgosiemang, I.K.; Lefojane, R.; Direko, P.; Madlanga, Z.; Mashele, S.; Sekhoacha, M. Green synthesis of magnesium and cobalt oxide nanoparticles using Euphorbia tirucalli: Characterization and potential application for breast cancer inhibition. Inorg. Nano-Metal Chem. 2020, 50, 1070–1080. [Google Scholar] [CrossRef]
- Farkas, B.; Santos-Carballal, D.; Cadi-Essadek, A.; de Leeuw, N.H. A DFT+U study of the oxidation of cobalt nanoparticles: Implications for biomedical applications. Materialia 2019, 7, 100381. [Google Scholar] [CrossRef]
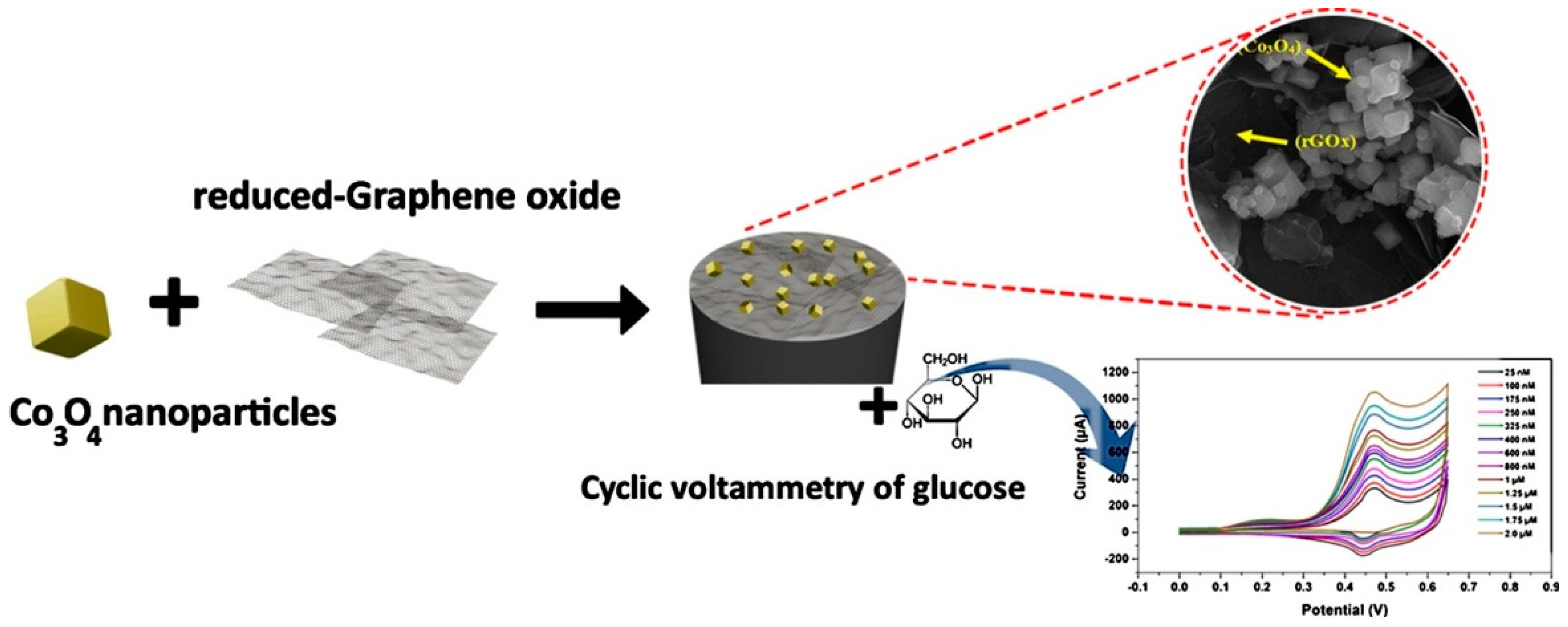
- Maghsoudi, S.; Mohammadi, A. Reduced graphene oxide nanosheets decorated with cobalt oxide nanoparticles: A nonenzymatic electrochemical approach for glucose detection. Synth. Met. 2020, 269, 116543. [Google Scholar] [CrossRef]
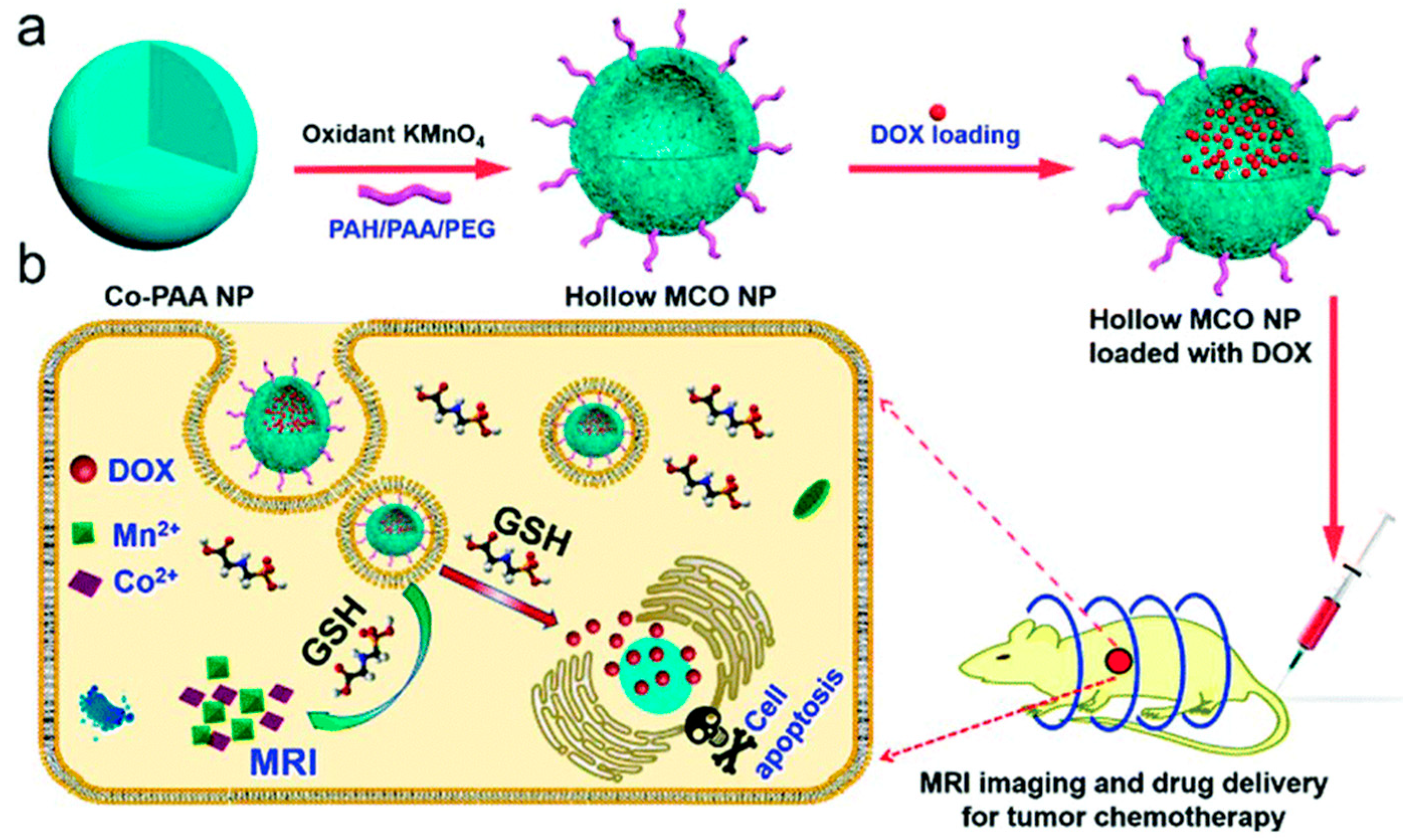
- Ren, Q.; Yang, K.; Zou, R.; Wan, Z.; Shen, Z.; Wu, G.; Zhou, Z.; Ni, Q.; Fan, W.; Hu, J.; et al. Biodegradable hollow manganese/cobalt oxide nanoparticles for tumor theranostics. Nanoscale 2019, 11, 23021–23026. [Google Scholar] [CrossRef]
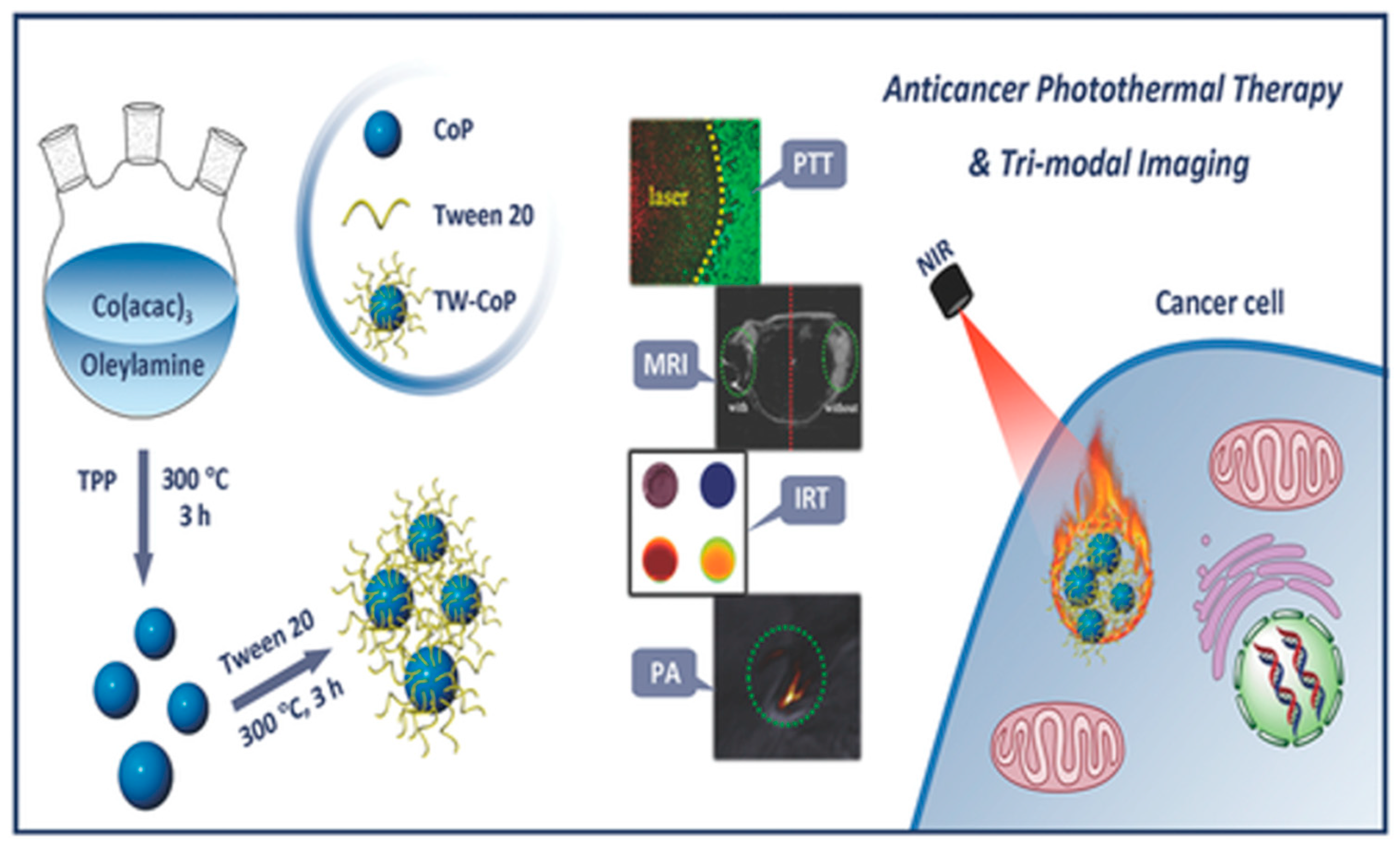
- Li, Z.; Hu, S.; Liu, J.; Hu, Y.; Chen, L.; Jiang, T.; Sun, L.; Sun, Y.; Besenbacher, F.; Chen, C.; et al. Cobalt Phosphide Nanoparticles Applied as a Theranostic Agent for Multimodal Imaging and Anticancer Photothermal Therapy. Part. Part. Syst. Charact. 2018, 35, 1800127. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Chen, L.; Hu, Y.; Hu, S.; Miao, Z.; Sun, Y.; Besenbacher, F.; Yu, M. Polyethylene glycol-modified cobalt sulfide nanosheets for high-performance photothermal conversion and photoacoustic/magnetic resonance imaging. Nano Res. 2018, 11, 2436–2449. [Google Scholar] [CrossRef]
- Dhawan, U.; Tseng, C.-L.; Wang, H.-Y.; Hsu, S.-Y.; Tsai, M.-T.; Chung, R.-J. Assessing Suitability of Co@Au Core/Shell Nanoparticle Geometry for Improved Theranostics in Colon Carcinoma. Nanomaterials 2021, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M.; Oza, G.; Velumani, S.; Ramirez, J.T.; Garcia-Sierra, F.; Andrade, N.B.; Vera, A.; Leija, L.; Garza-Navarro, M.A. Plasmonic/Magnetic Multifunctional nanoplatform for Cancer Theranostics. Sci. Rep. 2016, 6, 34874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Cai, H.; Zhou, H.; Li, T.; Jin, H.; Evans, C.; Cai, J.; Pi, J. Cobalt oxide nanoparticle-synergized protein degradation and phototherapy for enhanced anticancer therapeutics. Acta Biomater. 2021, 121, 605–620. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, H.; Chen, J.; Zheng, X.T.; Duan, H.; Pu, K.; Chen, P. Cobalt Phosphide Double-Shelled Nanocages: Broadband Light-Harvesting Nanostructures for Efficient Photothermal Therapy and Self-Powered Photoelectrochemical Biosensing. Small 2017, 13, 1700798. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater. Sci. Eng. C 2013, 33, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, C.; Jiang, J.; Ai, L. Magnetic cobalt nanoparticles embedded in hierarchically porous nitrogen-doped carbon frameworks for highly efficient and well-recyclable catalysis. J. Mater. Chem. A 2016, 4, 7476–7482. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, C.; Qin, J.; Wei, F.; Rao, M.; Wang, S. Synthesis of Magnetic Cobalt Nanoparticles Anchored on Graphene Nanosheets and Catalytic Decomposition of Orange II. Ind. Eng. Chem. Res. 2013, 52, 17341–17350. [Google Scholar] [CrossRef]
- Michalek, F.; Lagunas, A.; Jimeno, C.; Pericàs, M.A. Synthesis of functional cobalt nanoparticles for catalytic applications. Use in asymmetric transfer hydrogenation of ketones. J. Mater. Chem. 2008, 18, 4692–4697. [Google Scholar] [CrossRef]
- Liu, T.; Pang, Y.; Zhu, M.; Kobayashi, S. Microporous Co@CoO nanoparticles with superior microwave absorption properties. Nanoscale 2014, 6, 2447–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, S.Z.; Darijani, Z.; Karimi, M.A. Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles. J. Alloys Compd. 2020, 832, 154942. [Google Scholar] [CrossRef]
- Roushani, M.; Baghelani, Y.M.; Mavaei, M.; Abbasi, S.; Mohammadi, S.Z. Preparation of Modified Magnetic Cobalt Nanoparticles as a New Magnetic Sorbent for the Preconcentration and Determination of Trace Amounts of Lead Ions in Environmental Water and Soil (Air-Dust) Samples. Commun. Soil Sci. Plant Anal. 2018, 49, 645–657. [Google Scholar] [CrossRef]
- Zeisberger, M.; Dutz, S.; Müller, R.; Hergt, R.; Matoussevitch, N.; Bönnemann, H. Metallic cobalt nanoparticles for heating applications. J. Magn. Magn. Mater. 2007, 311, 224–227. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, A.K.; O’Neill, E.S.; Hambley, T.; New, E.J. Harnessing the properties of cobalt coordination complexes for biological application. Coord. Chem. Rev. 2018, 375, 221–233. [Google Scholar] [CrossRef]
- Nouri, M.; Esfahanizadeh, N.; Shahpar, M.G.; Attar, F.; Sartipnia, N.; Akhtari, K.; Saboury, A.A.; Falahati, M. Cobalt oxide nanoparticles mediate tau denaturation and cytotoxicity against PC-12 cell line. Int. J. Biol. Macromol. 2018, 118, 1763–1772. [Google Scholar] [CrossRef]
| No. | Capping Agent | Size | Chemical Formula | Method of Analysis | Shape | References |
|---|---|---|---|---|---|---|
| Wet Chemical Method | ||||||
| 1 | Tetrabutyl ammonium bromide | 80–100 nm | Co(0) | XRD | Spherical | [28] |
| 2 | Without use capping agent | 10–20 nm | Co(0) | XRD | Spherical | [29] |
| 3 | Without use capping agent | 20–40 nm | CoO | XPS | Spherical | [30] |
| 4 | Without use capping agent | 20 nm | Co(0) | XRD | Hexagonal and quadrilateral | [31] |
| 5 | Oleic acid | 3–4 nm | Co(0) | - | Spherical, uniform shape | [32] |
| Hydrothermal Method | ||||||
| 6 | Oleic acid | 1–10 nm | Co(0), CoO < 5% | XRD | Spherical | [33] |
| 7 | Without use capping agent | 20–400 nm | CoO | XRD | Spherical | [34] |
| 8 | Without use capping agent | 3–4 nm | CoO, Co3O4 | XRD | Hexagonal nanocrystals | [35] |
| 9 | Sodium dodecyl sulfate | 20 nm | Co3O4 | XRD | Cubic shape | [36] |
| 10 | Trioctylphosphane, oleic acid | 150–300 nm | Co(0) | XRD | Nanowire | [37] |
| Solvothermal Method | ||||||
| 11 | Without use capping agent | 2 nm | Co(0) | XRD | Spherical | [38] |
| 12 | Oleylamine | 100–120 nm | Co(0) | XRD | Spherical, hexagonal nanocrystals | [39] |
| 13 | Without use capping agent | 10–30 nm | CoO, Co3O4 | XRD | Cubic-shape, spherical | [40] |
| 14 | Oleylamine, oleic acid | 5–30 nm | Co(0) | WAXS, HREM | Nanorods, nanowires | [41] |
| 15 | Without use capping agent | 50–75 nm | Co(0) | XRD | Spherical | [42] |
| 16 | Without use capping agent | 1.5 nm | Co(0) | HREM | Spherical | [43] |
| 17 | Without use capping agent | 79 ± 17 nm 11 ± 3 nm | Co(0) | XRD | Cubic shape spherical | [44] |
| Sol-gel method | ||||||
| 18 | Citric acid and oleic acid | 8–12 nm | Co3O4 | XRD | Cubic crystal | [45] |
| 19 | Oleylamine | 20 ± 3 | CoO | XRD | Hollow nanoparallelepipeds | [46] |
| Electroless Deposition (Chemical Reduction) in Solution | ||||||
| 20 | 3- (N, N-dimethyldodecylammonia) propanesulfonate | 3–5 nm | Co(0) | XRD | Hexagonal | [47] |
| 21 | Sodium citrate dihydrate | 400 nm | Co(0) | XRD | Hexagonal | [48] |
| 22 | Without use capping agent | 100–120 nm | Co, CoO, Co3O4 | XRD | Spherical | [49] |
| 23 | 3- (N, N-dimethyldodecylammonia) propanesulfonate | 3–5 nm | Co(0) | XRD | Hexagonal | [50] |
| 24 | Without use capping agent | 2.2–4.2 nm | Co(0) | XPS | Spherical | [51] |
| 25 | Chloroplatinic acid hexahydrate | 24–110 nm | CoO | XRD | Hexagonal | [52] |
| Electrochemical Method | ||||||
| 26 | Sodium formate | 2 nm | CoO | XRD | Spherical | [53] |
| Electrosynthesis of Metal in a Liquid Phase Reduction of Their Ions or Complexes | ||||||
| 27 | Tetrabutylammonium chloride | 5–100 nm | CoO, Co3O4 | EDX, XRD | Spherical | [54] |
| Galvanic Replacement Method | ||||||
| 28 | Sodium citrate dihydrate | 100–120 nm | Co(0) | EDX | Spherical, hollow nanoparticles | [55] |
| Liquid Phase Plasma Reduction Method | ||||||
| 29 | Chloroplatinic acid hexahydrate | 24–112 nm | Co(0), CoO, Co3O4 | EDX, XRD | Spherical | [56] |
| 30 | Without use capping agent | 10 nm | CoO | XRD | Spherical | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodyashkin, A.A.; Kezimana, P.; Prokonov, F.Y.; Vasilenko, I.A.; Stanishevskiy, Y.M. Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review. Crystals 2022, 12, 272. https://doi.org/10.3390/cryst12020272
Vodyashkin AA, Kezimana P, Prokonov FY, Vasilenko IA, Stanishevskiy YM. Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review. Crystals. 2022; 12(2):272. https://doi.org/10.3390/cryst12020272
Chicago/Turabian StyleVodyashkin, Andrey A., Parfait Kezimana, Fedor Y. Prokonov, Ivan A. Vasilenko, and Yaroslav M. Stanishevskiy. 2022. "Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review" Crystals 12, no. 2: 272. https://doi.org/10.3390/cryst12020272
APA StyleVodyashkin, A. A., Kezimana, P., Prokonov, F. Y., Vasilenko, I. A., & Stanishevskiy, Y. M. (2022). Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review. Crystals, 12(2), 272. https://doi.org/10.3390/cryst12020272