Abstract
Heavy metal ion pollution is a serious threat for aquatic and terrestrial living beings. Adsorption is a facile process to encounter heavy metal pollution. Various types of adsorbents have been developed and used for environmental remediation. Activated carbon is one of the cheapest adsorbents derived from various biomass. In this work, the adsorption of cadmium ions (Cd (II)) with starch-based activated carbon (AC) having a specific surface area of 1600 m2 g−1 was investigated in a series of batch laboratory studies. The effective operating parameters, such as initial pH (pH0), initial concentration of metal ions, contact time, and temperature on the adsorption, were investigated. Validation of the kinetic study shows that the adsorption process is better predicted by the pseudo-second-order model. The extended Freundlich and Langmuir isotherms were applied to the study. The results show that the metal ion adsorption capacities of activated carbon increased with increasing pH, and it was found that maximum adsorption (284 mg g−1) of Cd (II) was achieved at pH solution of 5.5–6. The thermodynamic parameters, such as ∆G, ∆H, and ∆S, were found to be −17.42 kJ mol−1, 8.49 kJ mol−1, and 58.66 J mol−1 K−1, respectively, revealing that the adsorption mechanism is endothermic, spontaneous, and feasible. Furthermore, the density functional theory simulations demonstrated that the activated carbon strongly interacted with toxicity and mobility, so it is very urgent to remove this species from industrial wastewater before it is discharged into the environment. The adsorption energy calculated for all interactive sites was negative (−43.41 kJ mol−1 to −967.74 kJ mol−1), showing effective interaction between the adsorbate and adsorbent. The PDOS clearly shows that there is a stronger overlapping at the Femi level between the d orbital of the Cd ion and the p orbital of the O atom, showing a strong interaction and confirming the chemical bond formation between the Cd (II) ion and O atom.
1. Introduction
Due to rapid industrialization and urbanization, heavy metal concentrations have increased enormously in water reservoirs. The rate of depletion of water reservoirs is alarming and is a serious environmental problem [1,2,3]. Clean water vitality cannot be compromised in any case. The large number of pollutants have been identified from industrial and domestic sources and include antibiotics, dyes, phenols, insecticides, and heavy metals constituents [4,5]. Due to their non-degradable and accumulative nature, heavy metals are extremely harmful to living organisms. Among heavy metals, nontritive cadmium (II) metal is classified as a human teratogen, carcinogen, and the most dangerous priority water pollutant with a biological half-life of 10–30 years [6,7].
The main sources of Cd (II) ion contamination are industrial wastewater, combustion of oil, and coal waste incineration. Cd (II) is extensively used in different industrial processes, e.g., as a coloring pigment, anticorrosive agent, fabrication of batteries, and in nuclear power plants as an absorber of neutrons. It has been estimated that about 7000 tons/annum has been released on a global level [8]. According to the World Health Organization (WHO), the permissible level of Cd (II) is less than 0.003 mg dm−3 for drinking water [8]. Cd (II) has noxious effects on the lungs, liver, and kidneys, even in a very low concentration [9,10,11]. The inhalation of Cd (II) contaminated air can cause shortness of breath, damage of mucous membranes, and lung edema. The intake of food contaminated with Cd (II) can cause diarrhea and severe vomiting. Similarly, it can cause bone damage, infertility, prostate cancer, and tumors [12,13]. So, it is pertinent for human health and environmental safety to ascertain the concentration of Cd (II) on a regular basis and to develop techniques for the capturing of Cd (II) ion before it is discharged into bodies of water.
Several techniques, such as reverse osmosis, ion exchange, chemical precipitation, electrolytic extraction, redox method, and electrodialysis, have been designed and applied for the scavenging of heavy metals. However, these methods have their limitations, e.g., long processing times, high energy consumption, and poor sequestration of heavy metals [14].
The adsorption technique is as efficient as it is simple in operation, cost-effective, and environmentally friendly [1]. A variety of adsorbents, such as silica gel [15,16], clay [17,18], and bio-sorbents [19], have already been reported for the sequestration of heavy metals from wastewater. Theoretical analysis is a complementary tool to know the mechanism of the absorption process [20,21,22]. The efficiency of ACs as adsorbents for diverse types of pollutants is well reported [23]. It is well known that activated carbon has been found to be much more efficient for removing organic compounds than metals and other inorganic pollutants. Efforts are ongoing to substantially improve the potential of carbon surfaces by using different chemicals or suitable treatment methods, which will enable AC to enhance its potential for the removal of specific contaminants from the aqueous phase. The physical and chemical structure of carbon could be changed by various methods, i.e., activation conditions (different agents, temperature, and time of the process), precursor, and additives, etc.
Starch-based activated carbon was prepared and characterized to remove Cd (II) ions from spiked water. For the determination of the adsorption mechanism, the data were subjected to different adsorption models. The sorption obeys second-order kinetics, Freundlich and Langmuir isotherms, and was more favorable at pH 6. For the determination of thermodynamic parameters of the adsorption mechanism, the temperature study was carried out, which shows that the adsorption was spontaneous, feasible, and of endothermic nature. The DFT supported the experimental findings.
2. Experimental Section
2.1. Materials
The chemicals such as CdCl2, dithizone, NaOH, HCl, KOH, CH3COOH, CH3COONa, H3BO3, and NaOH of analytical grade were supplied by Sigma-Aldrich (Pvt.) Ltd. (Karachi, Pakistan) and were used without any further purification. The solutions of buffer 1–7 with the ionic strength of 10 mmol were prepared by taking the calculated amount of KCl and HCl for buffer 1–2, CH3COOH, and CH3COONa for buffer 3–6, and NaOH and H3BO3 for buffer 7. CdCl2 was mixed with dithizone in 2 M NaOH to obtain orange-colored Cd-dithizone complex (Figure 1), and its absorbance was studied at 549 nm wavelength as compared to the standard [24].
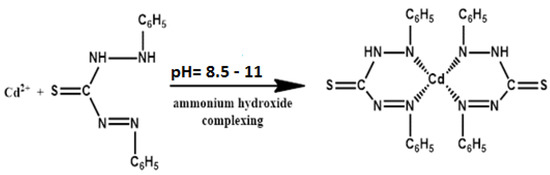
Figure 1.
Cadmium dithizone complex.
The concentration of Cadmium (II) ions was determined from its complex with dithizone in the presence of non-ionic surfactant, e.g., CTAB. The molar absorptivity of the complex becomes almost double as compared to the standard method, and a very small amount of Cd+2 can be determined spectrometrically at a wavelength of 549 nm. The increase in the molar absorptivity and rate constant are due to the micelle, which favors the formation of a Cd (II) ion and dithizone complex.
2.2. Synthesis of Adsorbent
The starch sample was sieved by 300 mesh size and mixed with potassium hydroxide in a 1:4 weight ratio and activated at 850 °C for 90 min with argon at 3 °C/min rise. The product obtained was thoroughly washed again and again with 5 wt. % HCl followed by washing with double-distilled water until the product was neutralized and dried at 120 °C overnight. The product thus obtained was named starch-based activated carbon (AC) and characterized by BET, SEM, TEM, XRD, FT-IR, TGA, and zeta potential [25].
2.3. Adsorption Experiment
Glass culture tubes with a given amount of adsorbent and a specific volume of sorbate with a known concentration were used in the batch mode experiments at room temperature or specified otherwise. The culture tubes were shaken on a wrist-action shaker (Model Burrell 75, Burrell Scientific, Pittsburgh, PA, USA). After shaking, the concentration of Cd(II) was recorded by using a double-beam UV-Visible spectrophotometer (Labomed, Inc. UVD 2960, Los Angeles, CA, USA). The adsorbed amount of Cd(II) and the distribution coefficient (Kd) were determined through the following equations:
where Ci is the initial Cd (II) concentration, Ce is the equilibrium concentration, W is weight of the sorbent, and V is the volume of Cd (II) ions solution.
All the experiments were carried out in triplicate at room temperature or described otherwise. The linear regression of the data was carried out and was in the range of 1–0.997 for all analyses.
3. Results and Discussion
3.1. Material Characterization
The activated carbon derived from starch was used for the adsorption of Cd (II) ions from industrial wastewater. The material was characterized through the following techniques.
3.1.1. SEM and TEM Analysis
The SEM images of the AC derived from starch at different resolutions illustrate the sheet-type morphology that ranges from nanometers to several micrometers (Figure 2a,b), whereas the TEM images at low and high magnification further confirm the multilayer nature of the individual sheet for the obtained activated carbon (Figure 2c,d) [26].
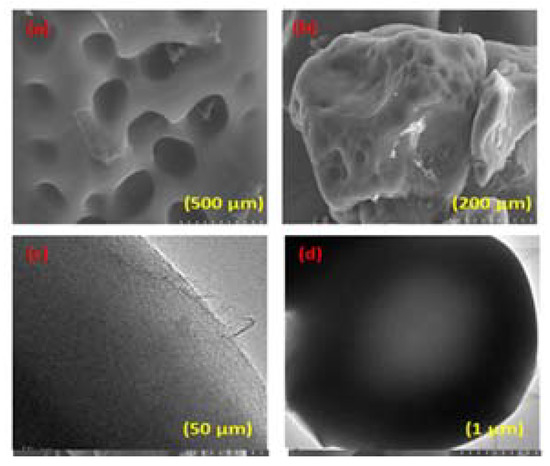
Figure 2.
(a,b) SEM images of the starch-based AC and (c,d) TEM images of starch-based AC.
3.1.2. AFM Analysis
An AFM technique was performed further to investigate the average and maximum height of the AC. Figure 3a,b shows 3-dimensional (3D) patterns of starch-based AC with particle heights that varied from 5 to 40 nm [27]. Thus, through the AFM technique, we could guess the maximum height (40 nm) of the multilayer AC sheets, which makes it an attractive choice for toxic Cd (II) ion adsorption [23].
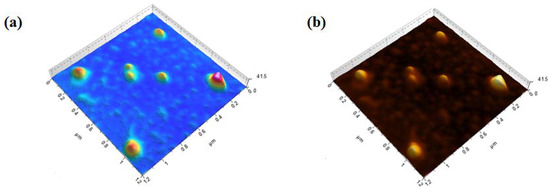
Figure 3.
AFM (a,b) 3D images of the starch-based AC.
3.1.3. FT-IR Analysis
FT-IR analysis is used to confirm different groups on the surface of AC (Figure 4). The broader peak at 3450 cm−1 corresponds to the hydroxyl group [28]. The absorption peak at 2920 is due to –CH3, and the peak at 1630 cm−1 is due to –C=O vibration [29]. The peaks on 1550 and 1120 cm−1 represent –NO2 and –SO3H groups, respectively [30]. The spectra indicate that the starch-based AC contains aromatic rings [31].
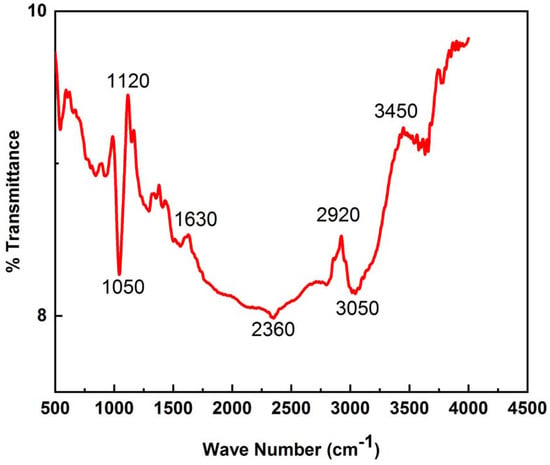
Figure 4.
FTIR spectra of starch-based AC.
3.1.4. TGA Study
The thermal stability of the starch-based AC was studied at temperatures up to 1000 °C. The total 14% weight loss was observed from the total mass of the AC, and this may be due to the conversion of the starch-based AC into CO and CO2 [32] and some other compounds at various temperatures [33]. It remained stable, it can be concluded that the starch-based AC was stable at a very high temperature up to 1000 °C (Figure 5).
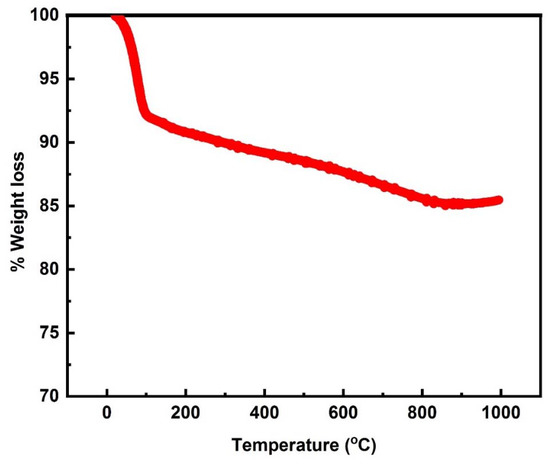
Figure 5.
TGA plot of the starch-based AC.
3.1.5. Zeta Potential and Zeta Sizer Analysis
Particle size distribution is a useful parameter to elucidate the arrangement of particle size in the adsorbents, while its zeta potential determines the surface charge required for dispersion. Figure 6 shows the size distribution and zeta potential plots of the AC, which reveals that the average particle size was 575 nm and polydispersity index (PDI) of 0.635, representing sufficient variation in sizes. Its surface charge was −16.9 mV, which is sufficient to remain suspended [34].
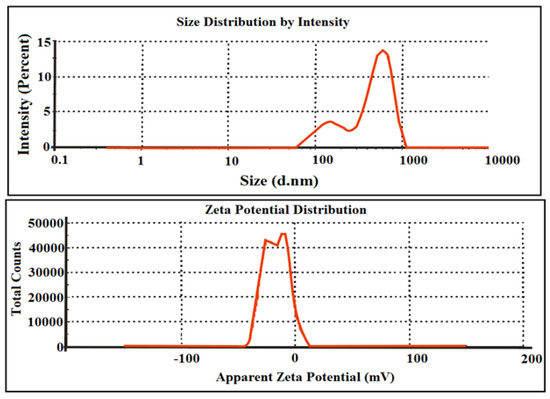
Figure 6.
Zeta sizer and zeta potential of the starch-based AC.
3.1.6. BET and BJH Analysis
The surface area was found to be 1600 m2 g−1 of starch-derived AC, as determined from a BET isotherm with a porous structure (Figure 7). The BJH analysis shows that AC has microspores and mesopores on its surface (inset of Figure 7a). The BET isotherm (Figure 7b) shows the surface area of Cd(II) ions loaded AC, which is only 13.35 m2 g−1 against the previous prestine AC surface area, which was 1600 m2 g−1, reflecting that most of the surface has been covered by the metal ions.
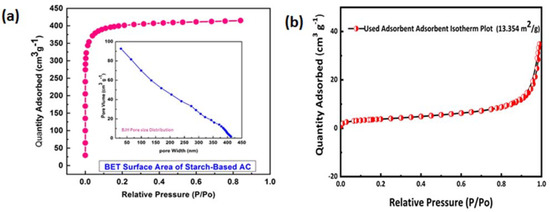
Figure 7.
(a) Nitrogen sorption isotherm of starch-based AC at 77.5 K with inset of BJH pore size distribution and (b) BET isotherm of the Cd (II)-ion-loaded AC.
3.2. Adsorption Experiments
3.2.1. Effect of pH on Sorption
The sorption capacity is greatly influenced by the medium [2]. The effect of pH on the adsorption of Cd (II) was investigated at pH 1.0–9.0. The concentration of sorbate of 100 mg L−1, sorbent amount of 30 mg, and shaking time of 120 min were chosen arbitrarily. The equilibrium uptake of Cd (II) increased notably up to pH 6 and then decreased continuously. The maximum uptake (98.58%) was observed at pH 6 and was selected for further experiments. At acidic pH, the electrostatic repulsion is created between the protonated surface of AC and Cd (II) metal ions. These observations also have been reported by other researchers [6,35]. Beyond pH 6, the adsorption capacity decreased due to the formation of Cd(OH)2 (cadmium hydroxide) [36]. The reaction mechanism involved is written as below.
3.2.2. Influence of the AC Dose
The influence of the dose of the AC on the adsorption of Cd (II) ion was tested at AC dose ranges of 0.01–0.035 g Figure 8a. The rate of adsorption increased at the AC dose up to 0.025 g, and then it remained constant. Thus, 0.025 g was used as the standard for further experiments.
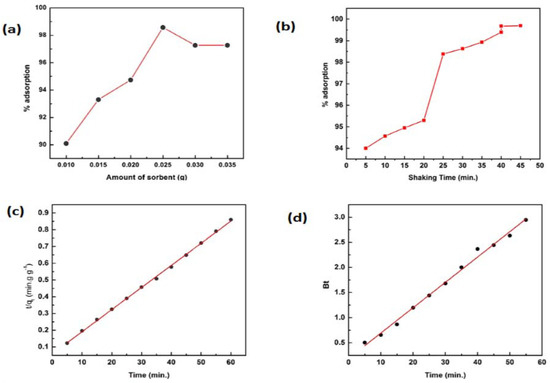
Figure 8.
(a) Effect of amount of AC, (b) effect of shaking time, (c) pseudo-second-order kinetic model, and (d) Reichenberg model.
3.2.3. Influence of Shaking Time
The influence of shaking time on Cd (II) ion removal was investigated between 0 and 50 min. The equilibrium was attained within 40 min and was selected for further investigations (Figure 8b). The adsorption rate of Cd (II) ions was high initially as abundant vacant sites were available, and after that, the movement of the ions was intraparticle, which was a relatively slow process.
The mass transfer during the process in the rate-determining step can be determined by using kinetic models [2,37,38]. The pseudo-second-order equation applied in the following linear form was the best-fitted:
where k2 rate constant and qe can be determined from the slope Figure 8c, and their magnitudes are calculated and listed in Table 1. The experimental and theoretical values of qe are in close agreement with each other, indicating the applicability of the model.

Table 1.
Kinetic parameters of Cd (II) ion adsorption onto starch-based AC.
The Reichenberg equation was used in the following form in order to further explore the mechanism [39]:
where F is the ratio of qt and qe and BT is a constant (Figure 8d), showing that both film diffusion and intraparticle diffusion are involved in the mechanism.
3.2.4. Effect of Cd (II) Ion Solution Concentration
The effect of Cd (II) ion solution concentration was studied at 10–160 mg L−1 (Figure 9b). The maximum rate of removal of adsorbate was at 80 mg L−1, and then it remained constant [40,41], thus, 80 mg L−1 was used as the standard for further experiments.
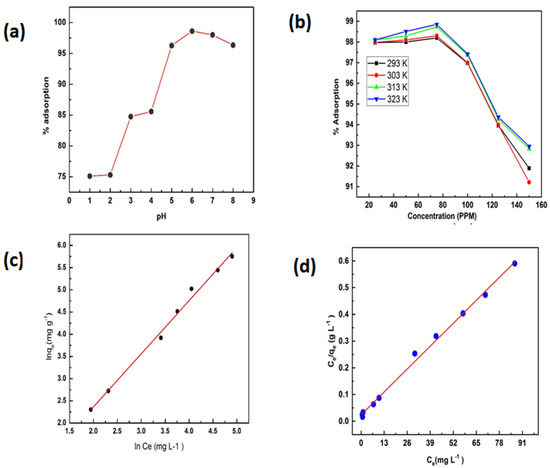
Figure 9.
(a) Effect of pH, (b) effect of concentration, (c) Freundlich, and (d) Langmuir isotherms.
The Langmuir equation was used in the following form [42]:
where Qmax. is the amount adsorbed, KL, qe, and Ce were calculated from the slope and intercept of the graph(Figure 9d), and their magnitudes are listed in Table 1. The adsorbate affinity (RL) is represented below:
The RL (0.0011) indicating favorable adsorption [43,44].
The Freundlich equation was used in the following form [45]:
where the adsorption capacity (KF mmol1−1/n kg−1 L1/n) and 1/n represented the surface heterogeneity and can be calculated from the slope and intercept of the graph (Figure 9c). The Freundlich parameters are tabulated in Table 2 [46,47].

Table 2.
Langmuir and Freundlich parameters along with correlation coefficients for the adsorption of Cd (II) ion onto AC at pH 6.
4. The Thermodynamic Study
The effect of temperature on sorption was investigated in the temperature range of 283–313 K. It was observed that the adsorption increased with the increases in temperature (Figure 10a), which reveals that the process may be chemisorption, and the reverse may be true for physisorption. This increase in the rate of adsorption with temperature may be due to the efficient flow of sorbate molecules against the concentration gradient or the transport of sorbate through the energy barrier. This may also create some new sites on the surface of the sorbent [48,49]. The thermodynamic parameters, such as Gibbs free energy change (ΔG), enthalpy (ΔH), and entropy (ΔS), were determined by using the relations below [50,51].
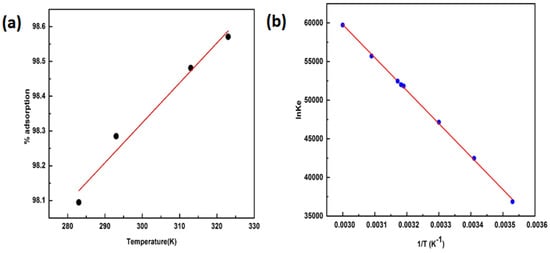
Figure 10.
(a) Effect of temperature on adsorption, and (b) determination of thermodynamic parameters by the Van ’t Hoff equation.
The calculated value of ΔG was −15.75 kJ mol−1, revealing that the adsorption of the Cd(II) ion onto AC is feasible and spontaneous [52]. The magnitude of ΔH was 16.24 kJ mol−1, showing that the sorption mechanism is endothermic [53,54]. The ΔS as determined from the intercept of the graph of lnke against 1/T was 58.66 J mol−1 (Figure 10b), reflecting the sorbate/sorbent complex stability [51,55]. The thermodynamic results are summarized in Table 3 and were calculated by using a second-order polynomial relationship.

Table 3.
Thermodynamic parameters of adsorption of Cd (II) ion onto AC.
5. DFT Study
To gain deeper insights into the interactions of the Cd(II) ion with the activated carbon, density functional theory (DFT) simulations were performed, which were recently frequently utilized in combination with experimental studies to understand the mechanism of the adsorption process [42,56,57]. The DFT simulations were performed by DMol3 code [58,59] using the spin unrestricted density functional theory along with the Perdew–Burke–Ernzerhof (PBE) formulation in combination with double numerical basis sets involving polarization functions (DNP). The Hirshfeld charge density method was used for the population analysis. The adsorption energies were calculated using the formula:
where Ecomplex is the total electronic energy of the complex system (Cd (II) ion adsorbed over activated carbon), EAC and ECd(II) are the energies of activated carbon and Cd (II) ion separately.
The optimized geometries are represented in Figure 11, and values of adsorption energies, intermolecular distances, and charge transfers are collected in Table 4. Four different adsorption modes of Cd (II) ion onto activated carbon (AC) are simulated, which are named CMP-1, CMP-2, CMP-3, and CMP-4, as illustrated in Figure 11. In CMP-1, the Cd (II) ion interacted with the O atom of C=O bond present on the surface of AC. The geometry optimization revealed that the Cd (II) ion formed an intermolecular bond of a bond distance of 2.39 Å, while the adsorption energy (Ead) showed that the Ead value for this complex was −9.20 eV, which showed the stronger interaction. The Hirshfeld charge analysis depicts that a larger charge transfer (0.59 e) occurred from the Cd (II) ion to the O atom of the surface, which resulted in a stronger intermolecular bond. In CMP-2, the Cd (II) ion interacted with the O atoms of the NO2 group and the O atom of the C=O group at the edge of the AC. The optimization shows that the Cd (II) ion formed intermolecular bonds with one O atom of the NO2 group and with the O atom of the C=O group. The bond distances noticed for this interaction were 2.25 and 2.41 Å, respectively. The Ead energy and Hirshfeld charge transfer value obtained for this complex was −10.03 eV and 0.70 e. This larger Ead value and higher charge transfer value demonstrate that the formation of this complex is highly thermodynamically stable. In CMP-3, the Cd (II) ion interacted with the SO3H group at the edge of AC. The intermolecular bond distances calculated for this complex were 2.98 and 2.92 Å, respectively. The Ead and charge transfer values noticed for this complex were −9.54 eV and 0.59 e. In CMP-4, the Cd (II) ion was placed over the top of the hexagonal ring of the AC. The geometry optimization reveals that the Cd (II) ion formed weak intermolecular bonds in this complex with binding distances up to 3.45 Å. Smaller Ead and charge transfer values obtained for this complex was −0.45 eV and 0.23 e. In summary, the DFT simulations showed that the AC has a higher affinity for the adsorption of Cd (II) ions due to the presence of higher negative active sites, i.e., N, O, and S atoms. The Cd formed stronger bonds with these active sites, which resulted in stronger adsorption. The larger negative values depict that the adsorption process is spontaneous and thermodynamically stable. In CMP-4, the Cd (II) ion was placed above the aromatic ring of the activated carbon. The bond lengths mentioned in Table 4 for CMP-4 are weak intermolecular bonds between the C atoms of the aromatic ring and the Cd ion. The top view and the side view of CMP-4 are illustrated in Figure 12, where the bond lengths are shown with a dotted line.
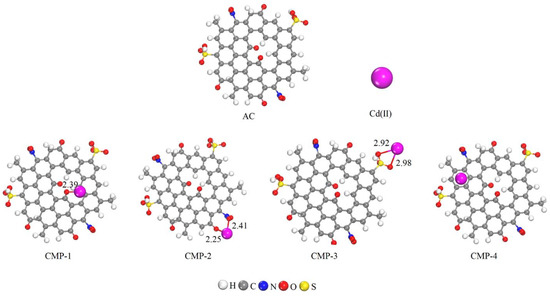
Figure 11.
Graphical representation of optimized geometry of AC and Cd (II)@AC complexes. Bond distances are in Å.

Table 4.
Computed bond distance (Å), adsorption energy (Ead, eV), and Hirshfeld charge transfer (ΔQCT, e).
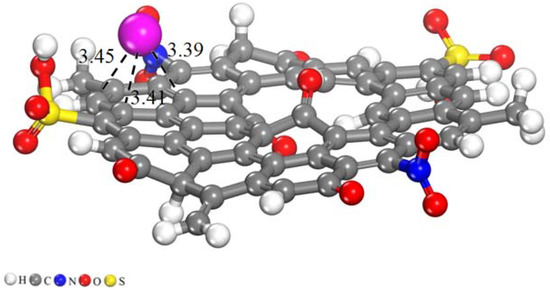
Figure 12.
Graphical representation of optimized geometry of AC and Cd (II)@AC complexes. Bond distances are in Å (dotted line).
In CMP-1, -2, and -3, chemical bonds formed between the O atoms and the Cd ion, which is justified by charge transfers as shown in Table 4. Larger charge transfers occurred between the O atoms and the Cd ion during complexation, and the value above 0.25 e clearly indicates the chemical bond formation. To further verify this statement, we computed the partial density of states analysis for the complex-1, as depicted in Figure 13. The PDOS clearly shows that there is stronger overlapping at the Femi level between the d orbital of the Cd ion and the p orbital of the O atom, evidenced by the strong interaction and confirming the chemical bond formation between the Cd ion and the O atom.
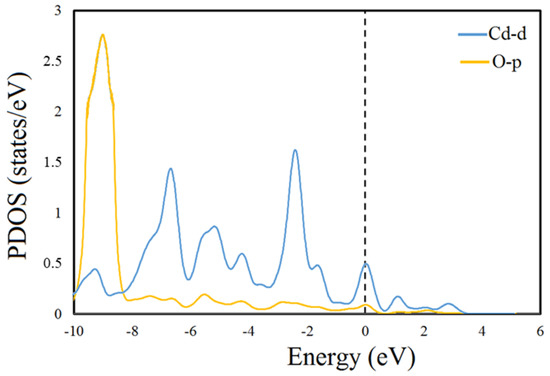
Figure 13.
Graphical representation of partial density of state (PDOS) of optimized geometry of AC and Cd (II)@AC complexes.
6. Conclusions
The starch-based activated carbon was proven to be an efficient adsorbent for remediation of cadmium ions from the water system. The results show that the metal ion adsorption capacity onto activated carbon increased with increasing pH, and it was found that maximum adsorption (284 mg g−1) of Cd (II) was achieved at a pH solution of about 6. In thermodynamic studies, the negative values of ΔG at all temperatures exhibited the spontaneous nature of Cd2+ adsorption on AC. The values of Qmax. calculated from Langmuir isotherms were also increased with increasing temperature, which also confirmed the endothermic nature of cadmium adsorption. Moreover, the DFT study shows that the AC has a stronger affinity for the adsorption of Cd (II) ions due to the presence of highly active sites. The shorter bond distances in CMP-1, CMP-2, and CMP-4 reveal that the Cd (II) strongly interacts with various active sites of the AC. Therefore, the Ead values are highly negative, which ranges from −43.41 kJ mol−1 to −967.74 kJ mol−1, and indicates that the adsorption process is spontaneous and thermodynamically feasible. The larger charge transfer (0.23 e to 0.70 e) provides evidence of the presence of a stronger intermolecular bond between the Cd (II) ions and AC.
Author Contributions
Conceptualization, S.M.; methodology, S.U.J.; software, A.A.K.; validation, S.U.; formal analysis, K.B.; investigation, B.B.; resources, S.U.J.; data curation, S.U.J.; writing—original draft preparation, S.U.J.; writing—review and editing, S.U.J.; visualization, Z.S.; supervision, S.U.J.; project administration, S.U.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors have no conflict of interest.
References
- Ali, Z.; Ahmad, R.; Khan, A. Functionalized nanospheres for efficient sequestration of cadmium ions. RSC Adv. 2014, 4, 50056–50063. [Google Scholar] [CrossRef]
- Hasany, S.M.; Ahmad, R. The potential of cost-effective coconut husk for the removal of toxic metal ions for environmen-tal protection. J. Environ. Manage. 2006, 81, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Fiberg, L.; Nordberg, G.; Vouk, V. Handbook of the Toxicology of Metals; Elsevier: New York, NY, USA, 1986; Volume 2. [Google Scholar]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; López, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treat-ment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Wen, X.; Wu, P.; Xu, K.; Wang, J.; Hou, X. On-line precipitation–dissolution in knotted reactor for thermospray flame fur-nace AAS for determination of ultratrace cadmium. Microchem. J. 2009, 91, 193–196. [Google Scholar] [CrossRef]
- Arnold, R.; Augier, C.; Baker, J.; Barabash, A.; Basharina-Freshville, A.; Blondel, S.; Blot, S.; Bongrand, M.; Boursette, D.; Brudanin, V. Measurement of the 2 ν β β decay half-life and search for the 0 ν β β decay of Cd 116 with the NEMO-3 detector. Phys. Rev. D. 2017, 95, 012007. [Google Scholar] [CrossRef] [Green Version]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.C.; Wu, P.; Zhang, X.; Hou, X.; Jones, B.T. Determination of Cadmium in Biological Samples. Appl. Spectrosc. Rev. 2006, 41, 35–75. [Google Scholar] [CrossRef]
- Wen, X.; Yang, Q.; Yan, Z.; Deng, Q. Determination of cadmium and copper in water and food samples by dispersive liquid–liquid microextraction combined with UV–vis spectrophotometry. Microchem. J. 2011, 97, 249–254. [Google Scholar] [CrossRef]
- Ma, W.; Song, X.; Pan, Y.; Cheng, Z.; Xin, G.; Wang, B.; Wang, X. Adsorption behavior of crystal violet onto opal and reuse feasibility of opal-dye sludge for binding heavy metals from aqueous solutions. Chem. Eng. J. 2012, 193–194, 381–390. [Google Scholar] [CrossRef]
- Waalkes, M.P.; Rehm, S.; Riggs, C.W.; Bare, R.M.; DeVor, D.E.; Poirier, L.A.; Wenk, M.L.; Henneman, J.R.; Balaschak, M.S. Cadmium carcinogenesis in male Wistar [Crl:(WI)BR] rats: Dose-response analysis of tumor induction in the prostate and testes and at the injection site. Cancer Res. 1988, 48, 4656–4663. [Google Scholar] [PubMed]
- Seidal, K.; Jorgensen, N.; Elinder, C.; Sjögren, B.; Vahter, M. Fatal cadmium-induced pneumonitis. Scand. J. Work. Environ. Health 1993, 19, 429–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Gao, P.; Cui, J.; Zhang, F.; Wang, F.; Cheng, J. Preparation and Cr (VI) removal performance of corncob activated carbon. Environ. Sci. Pollut. Res. 2018, 25, 20743–20755. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.B.; Valle-Vigón, P.; Sevilla, M. One-step synthesis of silica@ resorcinol–formaldehyde spheres and their applica-tion for the fabrication of polymer and carbon capsules. Chem. Commun. 2012, 48, 6124–6126. [Google Scholar] [CrossRef] [Green Version]
- Lo, K.-H.; Lu, C.-W.; Lin, W.-H.; Chien, C.-C.; Chen, S.-C.; Kao, C.-M. Enhanced reductive dechlorination of trichloroe-thene with immobilized Clostridium butyricum in silica gel. Chemosphere 2020, 238, 124596. [Google Scholar] [CrossRef]
- Chung, K.-T. The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes. Mutat. Res. Rev. Genet. Toxicology. 1983, 114, 269–281. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Khan, A.A.; Esrafili, M.D.; Ahmad, A.; Hull, E.; Ahmad, R.; Jan, S.U.; Ahmad, I. A computational study on the characteris-tics of open-shell H-bonding interaction between carbamic acid (NH2COOH) and HO2, HOS or HSO radicals. J. Mol. Model. 2019, 25, 189. [Google Scholar] [CrossRef]
- Allen, N.; Dai, C.; Hu, Y.; Kubicki, J.D.; Kabengi, N. Adsorption Study of Al3+, Cr3+, and Mn2+ onto Quartz and Corun-dum using Flow Microcalorimetry, Quartz Crystal Microbalance, and Density Functional Theory. ACS Earth Space Chem. 2019, 3, 432–441. [Google Scholar] [CrossRef]
- Acelas, N.Y.; Flórez, E. Density functional theory studies of the adsorption of Cr (VI) on Fe-(hydr) oxide: Gibbs free energies and pH effect. J. Physics: Conf. Ser. 2019, 1247, 012051. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents: Fundamentals and Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2003. [Google Scholar]
- Tayone, J. Spectrophotometric determination of chromium (VI) in canned fruit juices. Int. J. Sci. Basic Appl. Researchm. 2015, 19, 426–432. [Google Scholar]
- Shirazani, M.T.; Bakhshi, H.; Rashidi, A.; Taghizadeh, M. Starch-based activated carbon micro-spheres for adsorption of methane with superior performance in ANG technology. J. Environ. Chem. Eng. 2020, 8, 103910. [Google Scholar] [CrossRef]
- Yang, K.; Pan, T.; Zhao, Q.; Chen, C.; Zhu, X.; Wang, P.; Chen, B. Dual-function ultrafiltration membrane constructed from pure activated carbon particles via facile nanostructure reconstruction for high-efficient water purification. Carbon 2020, 168, 254–263. [Google Scholar] [CrossRef]
- Tyagi, A.; Banerjee, S.; Singh, S.; Kar, K.K. Biowaste derived activated carbon electrocatalyst for oxygen reduction reac-tion: Effect of chemical activation. Int. J. Hydrog. Energy 2020, 45, 16930–16943. [Google Scholar] [CrossRef]
- Singh, G.; Dwivedi, S. Decolorization and degradation of Direct Blue-1 (Azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environ. Technol. Innov. 2020, 18, 100751. [Google Scholar] [CrossRef]
- Karim, S.A.; Al-Gubury, H.Y.; Alrazzak, N.A. The Synthesis of a Novel Azo Dyes and Study of Photocatalytic Degradation. J. Phys. Conf. Ser. 2019, 1294, 052054. [Google Scholar] [CrossRef]
- Banerjee, M.; Basu, R.K.; Das, S.K. Cu (II) removal using green adsorbents: Kinetic modeling and plant scale-up design. Environ. Sci. Pollut. Res. 2019, 26, 11542–11557. [Google Scholar] [CrossRef]
- Maleki, B.; Reiser, O.; Esmaeilnezhad, E.; Choi, H.J. SO3H-dendrimer functionalized magnetic nanoparticles (Fe3O4@DNH (CH2)4SO3H): Synthesis, characterization and its application as a novel and heterogeneous catalyst for the one-pot syn-thesis of polyfunctionalized pyrans and polyhydroquinolines. Polyhedron 2019, 162, 129–141. [Google Scholar] [CrossRef]
- Rafatullah, M.; Ismail, S.; Ahmad, A. Optimization Study for the Desorption of Methylene Blue Dye from Clay Based Adsorbent Coating. Water 2019, 11, 1304. [Google Scholar]
- Naushad, M.; Alqadami, A.A.; AlOthman, Z.A.; Alsohaimi, I.H.; Algamdi, M.S.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated car-bon. J. Mol. Liquids. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, L.; Ma, A.; Xia, H.; Peng, J.; Li, C.; Shu, J. Comparison of activated carbon and iron/cerium modified activated carbon to remove methylene blue from wastewater. J. Environ. Sci. 2018, 65, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, S.; Toyohara, Y.; Saha, G.C.; Awual, R.; Kuba, T. Development of synthetic zeolites from bio-slag for cesium adsorption: Kinetic, isotherm and thermodynamic studies. J. Water Process Eng. 2020, 33, 101055. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated car-bon/zirconium oxide composite: Isotherm and kinetic modelling. J. Mol. Liq. 2020, 310, 113025. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Brito, S.M.d.; Cordeiro, J.L.C.; Ramalho, L.d.; Oliveira, J.F.R. Eriochrome black adsorption on yellow passion fruit peel (Passiflora edulis f. Flavicarpa) treated with sodium hydroxide and nitric acid: Study of adsorption isotherms, kinetic models and thermodynamic parameters. SN Appl. Sci. 2019, 1, 1226. [Google Scholar] [CrossRef] [Green Version]
- Kazeem, T.S.; Zubair, M.; Daud, M.; Mu’azu, N.D.; Al-Harthi, M.A. Graphene/ternary layered double hydroxide compo-sites: Efficient removal of anionic dye from aqueous phase. Korean J. Chem. Eng. 2019, 36, 1057–1068. [Google Scholar] [CrossRef]
- Kim, D.-W.; Wee, J.-H.; Yang, C.-M.; Yang, K.S. Efficient removals of Hg and Cd in aqueous solution through NaOH-modified activated carbon fiber. Chem. Eng. J. 2020, 392, 123768. [Google Scholar] [CrossRef]
- Jan, S.U.; Ahmad, A.; Khan, A.A.; Melhi, S.; Ahmad, I.; Sun, G.; Chen, C.-M.; Ahmad, R. Removal of azo dye from aqueous solution by a low-cost activated carbon prepared from coal: Adsorption kinetics, isotherms study, and DFT simulation. Environ. Sci. Pollut. Res. 2021, 28, 10234–10247. [Google Scholar] [CrossRef]
- Desta, M.B. Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J. Thermodyn. 2013, 2013, 375830. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.-J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Jin, Q.; Li, Z.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, H. Sludge-based biochar activation to enhance Pb(II) adsorption. Fuel 2019, 252, 101–108. [Google Scholar] [CrossRef]
- Fajarwati, F.I.; Anugrahwati, M.; Yanti, I.; Safitri, R.A.; Yeni; Yuanita, E. Adsorption Study of Methylene Blue and Eriochrome Black T Dyes on Activated Carbon and Magnetic Carbon Composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 599, 012025. [Google Scholar] [CrossRef]
- Alkherraz, A.M.; Ali, A.K.; Elsherif, K.M. Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by ad-sorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2020, 6, 11–20. [Google Scholar]
- Ahmad, R.; Saeed, M.M.; Ali, A.; Zaidi, J.H. Removal of Tm(III) ions from aqueous solution using PAN-incorporated sol–gel matrices. Radiochim. Acta 2007, 95, 451–457. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H. Equilibrium, thermodynamics and mechanisms of Ni2+ biosorption by aerobic granules. Biochem. Eng. J. 2007, 35, 174–182. [Google Scholar] [CrossRef]
- Ali, Z.; Ahmad, R.; Khan, A.; Adalat, B. Organic-inorganic hybrids: An efficient extractant of environmental mercury ions. Mater. Res. Express 2018, 5, 075007. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calcu-lation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Chang, Y.; Lai, J.-Y.; Lee, D.-J. Thermodynamic parameters for adsorption equilibrium of heavy metals and dyes from wastewaters: Research updated. Bioresour. Technol. 2016, 222, 513–516. [Google Scholar] [CrossRef]
- Kim, H.; Cho, H.J.; Narayanan, S.; Yang, S.; Furukawa, H.; Schiffres, S.; Li, X.; Zhang, Y.-B.; Jiang, J.; Yaghi, O.M. Character-ization of adsorption enthalpy of novel water-stable zeolites and metal-organic frameworks. Sci. Rep. 2016, 6, 19097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the Binding Performance of Amyloid–Carbon Mem-branes toward Heavy Metal Ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, Z.A.; Khattak, A.M.; Iqbal, R.; Ahmad, R.; Khan, A.A.; Usman, M.; Nawaz, F.; Ali, W.; Felegari, Z.; Jan, S.U. Adsorptive removal of Cd2+ from aqueous solutions by a highly stable covalent triazine-based framework. N. J. Chem. 2018, 42, 10234–10242. [Google Scholar] [CrossRef]
- Hussain, M.; Khaliq, M.N.; Nisar, A.; Khan, P.D.M.; Karim, S.; Khan, A.A.; Yi, P.D.X.; Maqbool, M.; Ali, G. TiO2 nanotube array-modified electrodes for L-cysteine biosensing: Experimental and density-functional theory study. Nanotechnology 2020, 31, 505501. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).