Electrical Conductivity and Dielectric Relaxation in Ag1−xLixNbO3
Abstract
:1. Introduction
2. Materials and Methods
3. Results
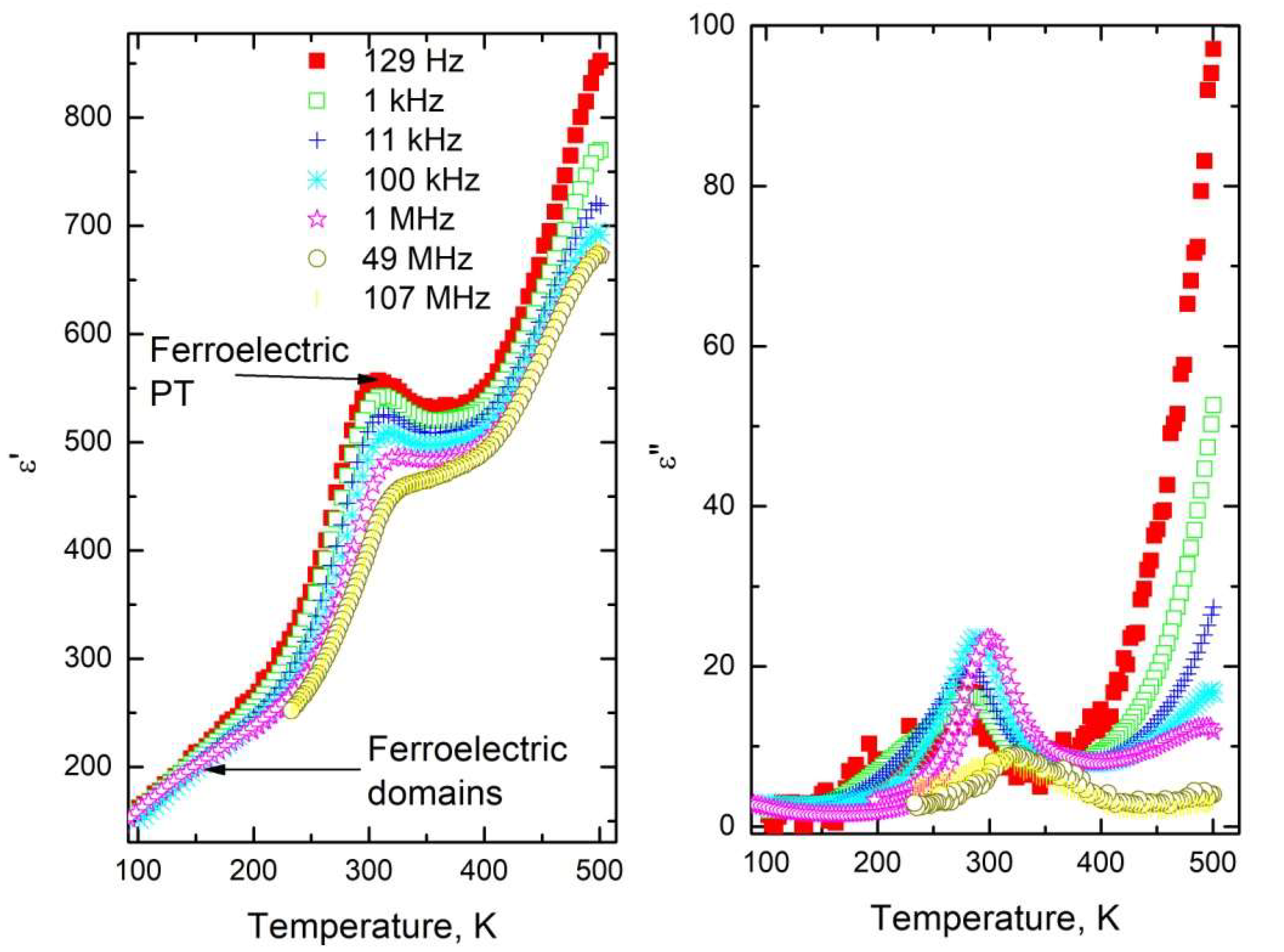
3.1. Phase Transitions in ALN Ceramics
3.2. Electrical Conductivity in ALN Ceramics
3.3. Dielectric Anomaly at Low Temperaures in ALN Ceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 7548. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.S.; Lee, M.K.; Kim, B.H.; Park, K.H.; Park, J.J.; Lee, S.H.; Jeong, Y.G.; Park, K.; Jeong, C.K.; Lee, G.J. Role of oxygen vacancy defects in piezoelectric thermal stability characteristics of Mn-doped (K, Na, Li)NbO3 piezoceramics. Ceram. Int. 2021, 47, 27803. [Google Scholar] [CrossRef]
- Kania, A.; Roleder, K.; Lukaszewki, M. The ferroelectric phase in AgNbO3. Ferroelectrics 1984, 265, 52. [Google Scholar] [CrossRef]
- Fu, D.; Endo, M.; Taniguchi, H.; Tanijama, T.; Itoh, M. AgNbO3: A lead-free material with large polarization and electromechanical response. Appl. Phys. Lett. 2007, 90, 252907. [Google Scholar] [CrossRef]
- Wada, S.; Saito, A.; Hoshina, T.; Kakemoto, H.; Tsurumi, T.; Moriyoshi, C.; Kuroiwa, Y. Growth of Silver Lithium Niobate Single Crystals and Their Piezoelectric Properties. Ferroelectrics 2007, 346, 64. [Google Scholar] [CrossRef]
- Sciau, P.; Kania, A.; Dkhil, B.; Suard, E.; Ratuszna, A. Structural investigation of AgNbO3 phases using x-ray and neutron diffraction. J. Phys. Condens. Mater 2004, 16, 2795–2810. [Google Scholar] [CrossRef]
- Miga, S.; Kania, A.; Dec, J. Freezing of Nb5+ ions dynamics in AgNbO3 studied by linear and nonlinear dielectric response. J. Phys. Condens. Matter 2011, 23, 155901. [Google Scholar] [CrossRef]
- Levin, I.; Krayzman, V.; Woicik, J.C.; Karapetrova, J.; Proffen, T.; Tucker, M.G.; Reaney, I.M. Structural changes underlying the diffuse dielectric response in AgNbO3. Phys. Rev. B 2009, 79, 104113. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hu, T.F.; Nie, H.C.; Fu, Z.Q.; Xu, C.H.; Xu, F.F.; Wang, G.S.; Dong, X.L. Giant energy density and high efficiency achieved in silver niobate-based lead-free antiferroelectric ceramic capacitors via domain engineering. Energy Storage Mater. 2021, 34, 417–426. [Google Scholar] [CrossRef]
- Han, K.; Lu, N.N.; Mao, S.F.; Zhuo, F.P.; Chen, X.Y.; Liu, L.J.; Hu, C.Z.; Wang, X.P.; Wei, Y.Z. Realizing high low-electric-field energy storage performance in AgNbO3 ceramics by introducing relaxor behaviour. J. Mater. 2019, 5, 597–605. [Google Scholar] [CrossRef]
- Liu, Z.L.; Bao, W.C.; Wang, G.; Sun, S.K.; Li, L.H.; Li, J.L.; Jang, H.J.; Ji, H.F.; Feteira, A.; Li, D.J.; et al. Mechanism of enhanced energy storage density in AgNbO3-based lead-free antiferroelectrics. Nano Energy 2021, 79, 105423. [Google Scholar] [CrossRef]
- Valant, M.; Axelsson, A.K.; Alford, N. Review of Ag(Nb, Ta)O3 as a functional material. J. Eur. Ceram. Soc. 2007, 27, 2549–2560. [Google Scholar] [CrossRef]
- Fu, D.; Endo, M.; Taniguchi, H.; Taniyama, T.; Koshihara, S.; Itoh, M. Piezoelectric properties of lithium modified silver niobate perovskite single crystals. Appl. Phys. Lett. 2008, 92, 172905. [Google Scholar] [CrossRef]
- Kania, A.; Miga, S. Preparation and dielectric properties of Ag1−xLixNbO3 (ALN) solid solutions ceramics. Mat. Sci. Eng. B-Adv. 2001, 86, 128. [Google Scholar] [CrossRef]
- Niewiadomski, A.; Kania, A.; Kugel, G.E.; Hafid, M.; Ditko, D. Raman spectroscopy, dielectric properties and phase transitions of Ag0.96Li0.04NbO3 ceramics. Mater. Res. Bull. 2015, 65, 123. [Google Scholar] [CrossRef]
- Fu, D.; Endo, M.; Taniguchi, H.; Taniyama, T.; Itoh, M.; Koshihara, S. Ferroelectricity of Li-doped silver niobate (Ag, Li)NbO3. J. Phys. Condens. Matter 2011, 23, 075901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newiadomski, A.; Kajewski, D.; Kania, A.; Balin, K.; Miga, S.; Pawlik, M.; Koperski, S. Microstructure and characterization of Ag1−xLixNbO3 ceramics. Ceram. Int. 2016, 42, 4445. [Google Scholar] [CrossRef]
- Khan, H.U.; Sterianou, I.; Miao, J.; Pokorny, J.; Reaney, I.M. The effect of Li-substitution on the M-phases of AgNbO3. J. Appl. Phys. 2012, 111, 024107. [Google Scholar] [CrossRef]
- Sakabe, Y.; Takeda, T.; Ogiso, Y.; Wada, N. Ferroelectric properties of (Ag, Li)(Nb, Ta)O-3 ceramics. Japan. J. Appl. Phys. 2001, 42, 5675. [Google Scholar] [CrossRef]
- Farid, U.; Gibbs, A.S.; Kennedy, B.J. Impact of Li doping on the struture and phase stability in AgNbO3. Inorg. Chem. 2020, 59, 12595–12607. [Google Scholar] [CrossRef]
- Porokhonskyy, V.; Bovtun, V.; Kamba, S.; Buixaderas, E.; Petzelt, J.; Kania, A.; Miga, S.; Yakimenko, Y. Microwave dielectric properties of the Ag1−xLixNbO3 (x=0 divided by 0.06) ceramics. Ferroelectrics 2000, 238, 137. [Google Scholar] [CrossRef]
- Palaimiene, E.; Macutkevic, J.; Kezionis, A.; Banys, J.; Gruszka, I.; Koperski, J.; Kania, A. Dielectric properties and infrared spectra of Ag0.92Li0.08NbO3 ceramics. Solid State Commun. 2021, 32, 114338. [Google Scholar] [CrossRef]
- Palaimiene, E.; Macutkevic, J.; Banys, J.; Gruszka, I.; Kania, A. Broadband and infrared spectroscopy of Ag0.98Li0.02NbO3 ceramics. Lith. J. Phys. 2020, 60, 247–252. [Google Scholar] [CrossRef]
- Kania, A.; Roleder, K.; Kugel, G.E.; Fontana, M.D. Raman scattering, central peak and phase transitions in AgNbO3. J. Phys. C Solid State Phys. 1986, 19, 9. [Google Scholar] [CrossRef]
- Volkov, A.A.; Gorshunov, B.P.; Komandin, G.; Fortin, W.; Kugel, G.E.; Kania, A.; Grigas, J. High-frequency dielectric spectra of AgTaO3-AgNbO3 mixed ceramics. J. Phys. Condens. Matter 1995, 7, 785. [Google Scholar] [CrossRef]
- Fortin, W.; Kugel, G.E.; Grigas, J.; Kania, A. Manifestation of Nb dynamics in Raman, microwave, and infrared spectra of the AgTaO3-AgNbO3 mixed system. J. Appl. Phys. 1996, 79, 4273. [Google Scholar] [CrossRef]
- Ratuszna, A.; Pawluk, J.; Kania, A. Temperature Evolution of the Crystal Structure of AgNbO3. Phase Transit. 2003, 76, 611. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Hu, Q.; Jin, L.; Yu, K.; Li, J.; Politova, E.D.; Yu, S.; Stefanovich, S.Y.; Xu, Z.; et al. Ferroelectric transitions in silver niobate ceramics. J. Mater. Chem. C 2019, 7, 1028. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, L.; Zhang, H.F.; Xu, Z.; Wei, X.Y.; Politova, E.D.; Stefanovich, S.Y.; Tarakina, N.V.; Abrahams, I.; Yan, H.X. High energy density in silver niobate ceramics. J. Mater. Chem. A 2016, 4, 17279. [Google Scholar] [CrossRef]
- He, X.; Chen, C.; Li, C.B.; Zhen, H.R.; Yi, Z.G. Ferroelectric, photoelectric, and photovolataic performance of silver niobate ceramics. Adv. Funct. Mater. 2019, 29, 1900918. [Google Scholar] [CrossRef]
- Yan, Z.N.; Zhang, D.; Zhou, X.F.; Qi, H.; Luo, H.; Zhou, K.C. Silver niobate based lead-free ceramics with high energy storage density. J. Mater. Chem. A 2019, 7, 10702. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Ye, J.M.; Wang, G.S.; Dang, X.L.; Whithers, E.; Liu, Y. Antiferroelectrics for Energy Storage Applications: A Review. Adv. Mater. Technol. 2018, 3, 10800111. [Google Scholar] [CrossRef]
- Kania, A. Flux growth of AgTaxNb1−xO3 (ATN) solid-solutions single-crystals. J. Cryst. Growth 1989, 96, 703–704. [Google Scholar] [CrossRef]
- Grigas, J. Microwave Dielectric Spectroscopy of Ferroelectrics. Ferroelectrics 2009, 380, 37–41. [Google Scholar] [CrossRef]
- Macutkevic, J.; Banys, J.; Vysochanskii, Y. Broadband dielectric spectroscopy of CuInP2Se6 crystals. Phys. Stat. Sol. A 2009, 206, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Macutkevic, J.; Banys, J.; Grigalaitis, R.; Vysochanskii, Y. Asymmetric phase diagram of mixed CuInP2(SxSe1-x)6 crystals. Phys. Rev. B 2008, 78, 064101. [Google Scholar] [CrossRef] [Green Version]
- Jonscher, A.K. The “universal” dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Dziaugys, A.; Banys, J.; Macutkevic, J.; Vysochanskii, Y.; Pritz, I.; Gurzan, M. Phase transitions in CuBiP2Se6 crystals. Phase Transit. 2011, 84, 147–156. [Google Scholar] [CrossRef]
- Raymond, M.V.; Smith, D.M. Defects and charge transport in perovskite ferroelectrics. J. Phys. Chem. Solid. 1996, 57, 1507. [Google Scholar] [CrossRef]
- Levstik, A.; Kutnjak, Z.; Filipic, C.; Pirc, R. Glassy freezing in relaxor ferroelectric lead magnesium niobate. Phys. Rev. B 1998, 57, 11204–11211. [Google Scholar] [CrossRef]







| Ceramics | ν0 | EA/k, K | T0, K |
|---|---|---|---|
| Ag0.99Li0.01NbO3 | 10.7 GHz | 221 | 313 |
| Ag0.98Li0.02NbO3 | 154 THz | 193 | 313 |
| Ag0.97Li0.03NbO3 | 2.1 THz | 299 | 303 |
| Ag0.95Li0.05NbO3 | 2.2 GHz | 240 | 293 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macutkevic, J.; Banys, J.; Kania, A. Electrical Conductivity and Dielectric Relaxation in Ag1−xLixNbO3. Crystals 2022, 12, 158. https://doi.org/10.3390/cryst12020158
Macutkevic J, Banys J, Kania A. Electrical Conductivity and Dielectric Relaxation in Ag1−xLixNbO3. Crystals. 2022; 12(2):158. https://doi.org/10.3390/cryst12020158
Chicago/Turabian StyleMacutkevic, Jan, Juras Banys, and Antoni Kania. 2022. "Electrical Conductivity and Dielectric Relaxation in Ag1−xLixNbO3" Crystals 12, no. 2: 158. https://doi.org/10.3390/cryst12020158
APA StyleMacutkevic, J., Banys, J., & Kania, A. (2022). Electrical Conductivity and Dielectric Relaxation in Ag1−xLixNbO3. Crystals, 12(2), 158. https://doi.org/10.3390/cryst12020158






