1. Introduction
The enamel tissue envelopes the exposed surface of human teeth, i.e., the entire and absolute anatomic crown of the tooth, and is arguably the hardest connective tissue of the human body and consists of 96% mineral, 4% organic material, and negligible water content. Structurally, enamel covers the tooth structure above the free gingiva and protects the dentin. Astoundingly, this protective layer is responsible for the teeth lasting a lifetime. In total contrast to humans, the teeth of the rodent continuously grow to compensate for the occlusal attrition. In some aquatic animals such as parrotfish and sharks, a row of a new set of teeth moves forward and replaces the old worn-out teeth. In some semiaquatic amphibious reptiles such as crocodiles of the Crocodylidae family, a lost tooth is replaced by a new one erupting in its place. Unfortunately, the same is not applicable in Homo Sapiens. Human tooth enamel is a hierarchical mineralized tissue with an intricate and baroque organization of hydroxyapatite, a biomineral of calcium and phosphate, which forms long, thin nanocrystals which are fundamental to its biomechanical performance [
1,
2].
Hydroxyapatite crystals are omnipresent in hard tissues of osteogenic and odontogenic origin. The arrangement and orientation of different dimensions of the crystals provide the much needed architectural support for the hard dental tissue’s structural integrity, form, and function. The enamel substructure exhibits itself in the mature full-fledged tissue as an assemblage of trillions of almost interchangeable, distinctly well-organized crystals of calcium and phosphate hydroxyapatite arrayed into supracrystalline structures; though they emulate a similar pattern, they have a gargantuan variation amongst different species [
3].
The hydroxyapatite crystal accounts for at least 70 to 80% weight content in dentin and enamel. The enamel is the hardest tissue in the body, predominantly composed of hydroxyapatite crystals with a range of 160 to 1000 nm length and a mean range of thickness of 25 nm, which is 1/100th the thickness of hair, and width ranging from 40 to 120 nm. Unlike bone, the enamel tissues are devoid of collagen. Amelogenins and enamelins substitute functionally for collagen by providing a framework and nidus for biomineralization. In addition, hydroxyapatite is also responsible for filtering the diffuse reflectivity of incident light and thus camouflaging the pores on the surface of the enamel, resulting in translucent opalescent appearance of enamel [
3,
4,
5].
Enamel has unique and distinct properties, including unmatched hardness, unparallel resistibility to wear, and also promises of a lifetime of subject to the dynamic oral milieu and environment [
3]. Chun KJ et al. compared the mechanical properties between enamel and dentin. It was observed on evaluation of stress and strain on the hard tissues that the enamel displayed a tendency to fracture earlier owing to its brittle nature when compared to that of dentin. However, on subjecting it to a hardness test, it was observed that that enamel was harder than dentin. Scanning electron micrographs of enamel and dentin, owing to their different internal structure and composition, indicate the enamel is more resistant to wear and thus exemplify its role in the grinding and crushing of food. Dentin displays more force resistance owing to its inherent tendency to adsorb and dissipate forces and stress [
4].
Though enamel is documented to be the strongest tissue, it still is prone to dental caries. The dynamic chemical variations which manifest during the process of carious ruination of enamel are extremely complex owing to numerous variegated factors [
5,
6]. The main component of enamel, hydroxyapatite, exhibits an intricate dissolution pattern. Analysis of the birefringent zones of the carious lesions indicate that the ruination or destruction that occurs or progresses is not simple dissolution. Substitution of the ionic constituents, coupled with selective dissolution of the soluble ionic mineral constituents, takes place and with the probability of the accompaniment of reprecipitation [
5,
6,
7,
8].
Contemporary studies with atomic and chemical force microscopy by Colin Robinson and Simon D. Connell have revealed the existence of a subunit structure of developing enamel crystals. It was observed that the crystal of mature enamel exhibited 30 to 50 nm structures which were further composed of 10–15 nm diameter subunits which were presumed to be initiation sites for mineral precipitation. The clusters of these substructures’ interfaces led to an increase in acid solubility and hence increase in enamel caries due to crystalline discontinuity. Lateral fusion of these subsurface entities can develop a discontinuity along the complete length of the crystal, primarily at its center, whereas longitudinal fusion can result in lateral discontinuities which are perpendicular to the central line. These would be the potential sites at which enamel crystals preferentially dissolve and give rise to initiation of enamel caries [
8].
Remineralization strategies have been attempted with the view to tip the ionic imbalance and overcome or circumvent the preferential enamel dissolution due to decreased acid resistance. Remineralization of subsurface lesions is a challenge. Tencate et al. inferred that to remineralize 150-micron deep subsurface lesions, there is a requirement for 5000 ppm of fluoride, and excessive doses of fluoride can lead to toxic side effects and even be fatal [
9]. The limitations of remineralizing studies is that most of the evidence-based studies are in vitro and outcomes are divergent when translated to complex dynamic oral milieu [
10].
Thus, an effective preventive regime would be one which has a synergistic caries-preventive effect by biomodifying the enamel crystalline structure and making it more acid resistant and also increasing the effectiveness of remineralizing pastes. Thus, a treatment strategy which can modify the tooth structure and make it more resistant to acid dissolution is the need of the hour. One such modality can be the inclusion of lasers in the caries preventive protocol. Lasers have been experimented with the aim to improve acid resistance and serve as an alternative to fluoride.
The CO
2 laser was the first laser which showed reduced dissolution of enamel in acid. The other lasers which have been investigated in the past for caries inhibition are Nd:YAG, Er:YAG, and Er Cr:YSGG. The mechanism of action has not been elucidated, but various theories have been hypothesized. In the past, preventive strategies included remineralizing paste alone or in conjunction with high-powered lasers and exhibited varying results. High-powered lasers have been discontinued for caries inhibition owing to their high cost, bulky equipment, and lack of any evidence-based therapeutic applications. Further, there was lack of any standardized protocol to bring about caries inhibition [
11,
12,
13].
Hence, the author proposes the concept that low-powered lasers, such as aluminum gallium arsenide lasers, can be used to prevent and inhibit caries. The author put forward the hypothesis that the greater selectivity of the wavelengths of 810 nm of the aluminum gallium arsenide laser can lead to removal of carbonate ions from enamel crystal, thus increasing uptake of fluoride ions and increasing the acid resistance of the tooth structure. Hence, this paper gives insight into the evidence-based approach to explore the possibility of using aluminum arsenide lasers as a caries-preventive and-inhibitory tool in caries prone populations. To understand how aluminum gallium arsenide can bring about caries inhibition, an understanding of the crystallography of enamel is imperative.
2. Crystallography of Enamel
Human teeth are cardinally subsumed by three vital components of enamel, dentin and pulp. Enamel is an acellular tissue comprised of 80–90% by volume of crystals of carbonated calcium hydroxyapatite. The apatite in enamel exhibits a number of disparities, particularly calcium and hydroxyl. Extrinsic and superfluous ions such as carbonate, magnesium, sodium, and also fluoride are more often than not observed within the hydroxy apatite crystal structure. Hydroxyl is found to be 20–30% lower in human enamel apatite compared with stoichiometric apatite. Such defects and substitutions do have a profound impact on the behavior of apatite, especially with regard to its solubility at low pH. It has been reported that the solubility product for enamel minerals, for example, is higher (ranging from 7.2 × 10
−53 to 6.4 × 10
−58) than that calculated for stoichiometric apatite, i.e., 3.04 × 10
−59. These high values are due to defects and impurities in the lattice of enamel crystals [
5,
6]. Studies on the chemistry of enamel help in understanding the clinical impact that the solubility product value has on the dissolution and redeposition of minerals in enamel crystals when continuously challenged by acids [
7,
8,
9,
14,
15,
16,
17,
18,
19,
20].
The main mineral constituent of dental enamel is Ca
10(PO
4)
6(OH)
2 [
4,
5,
6,
7,
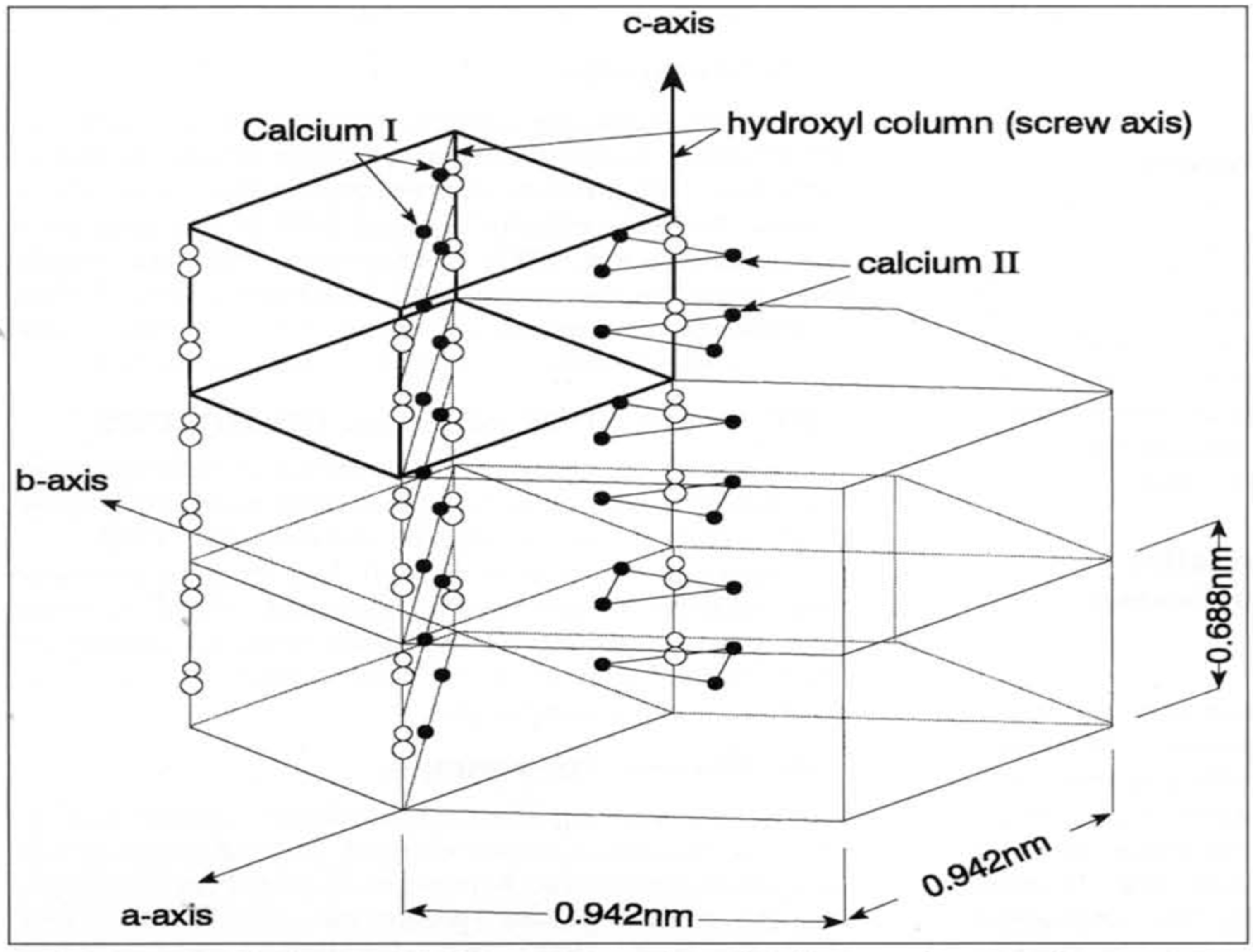
8]. The calcium phosphate present in the hydroxyapatite crystal is octa calcium and tricalcium phosphate ions. The crystal of hydroxyapatite crystal can be best visualized as an array of hexagonal plates stacked one on top of another, with each plate having a 60° spatial rotation with respect to the preceding and succeeding stack to form a column (
Figure 1) [
6]. Each plate has a characteristic central hydroxyl ion, fenced by a triangle of calcium II ions, which is hemmed by a triangle of phosphate ions rotated out of phase by 60°, cordoned by a hexagon of calcium I ions. In each hydroxy apatite crystal there are atomic domain sites which are liable and prone to ionic substitutions. The calcium ion site in the hydroxy apatite structure is prone to be substituted by variable metal cations such as sodium, potassium, magnesium, manganese; the phosphate can partially be substituted by the carbonate ion, and the hydroxyl site in the c axis can accept fluoride ions, chloride ions, or be replaced by water. These ionic substitutions can lead to manifold alterations, contortions and distortions leading to complete alteration of the lattice configuration and resulting in the change in acid resistance and solubility of enamel apatite, which has a major role to play in caries initiation and progression [
5,
6,
7,
8] (
Figure 1). The unit of crystal is comprised of four columnar calcium II ions, six calcium I ions, and six phosphate ions located around the hydroxyl column in the C axis [
5,
6,
7,
8].
3. Demineralization–Remineralization
Under physiological conditions when the pH of saliva is 7.4, the oral fluids are supersaturated with respect to hydroxyapatite and fluoroapatite. The ion activity product of hydroxyapatite crystal IAP = KSP (solubility product constant of hydroxyapatite crystal) during equilibrium. When the pH in the oral fluids decreases, the solubility of the tooth mineral apatite dramatically increases. The ion activity product of hydroxyapatite crystal IAP < KSP (solubility product constant of hydroxyapatite crystal) and demineralization is noted. The solubility constant of apatite crystal is augmented by a factor of 10 with a drop noted for each unit of pH. At a critical pH, the fluids become just saturated with respect to hydroxyapatite. As fluorapatite is less soluble than hydroxyapatite, the plaque fluid remains supersaturated with respect to fluorapatite when it is undersaturated with respect to hydroxyapatite. Under these conditions, a carious lesion is initiated. This dissolution of initially enamel crystals and/or dentin crystals is known as demineralization [
21]. The net loss of mineral from tooth surfaces results in the earliest clinical manifestation, i.e., a white spot. The complete depth of the enamel layer may be involved by caries, but still the lesion may not be cavitated. This is because enamel is not homogenous throughout in its thickness and composition [
21,
22,
23]. At the ultrastructural level, enamel is formed by closely appositioned hydroxyl apatite crystals. The outer 50–100 microns of the enamel layer is heavily impregnated with fluoride. Below this zone, there is more concentration of carbonate ions and less of concentration of fluoride ions [
9,
10,
21,
22,
23]. Thus the sui
generis of enamel crystallographic structure is that caries are always subsurface to begin with. This can be understood if one understands the structure and mineral content and orientation of the enamel crystal. The most conservative and desirable management of such non-cavitated carious lesions is to reverse the carious lesion by remineralization [
24,
25,
26,
27]. However, is the reversal of subsurface lesions a clinical reality or is remineralization a predictable outcome?
4. Challenges in Remineralization of Subsurface Non-Cavitated Carious Lesions
The limitations of remineralizing studies are that most of the evidence-based studies are in vitro and are very different when translated to the complex dynamic oral milieu. The other limitations are remineralization occurs within the body of a subsurface lesion, and calcium and phosphate ions must penetrate the surface layer of the enamel. The surface layer is highly mineralized and has a charged surface nature and hence poses an unsurmountable challenge for the ion to penetrate to the subsurface layer. Fluoride ions require a high concentration of 5000 ppm to penetrate to the subsurface layer [
9]. CPP ACP products, if ingested in significant quantities, cause side effects. The potential risk increases with patients who have allergic diathesis, especially IgE immunglobulinopathies. The effectiveness of CPP ACP in remineralizing subsurface lesions is contentious and debatable. Another limitation is that repeated application of remineralizing paste is needed to maintain and replenish a constant supply of the lost remineralizing ions [
14,
15]. Further repeated topical application of fluoride may give rise to fluoride-resistant strains developing [
23,
24,
25]. The injudicious topical application of fluoride leads to the evolution of generations of fluoride-resistant strains of opportunistic cariogenic microbiota, of which the most detrimental is the rise of fluoride-resistant
Streptococcus mutans (
S. mutans). These mutated
S. mutans, owing to their fluoride-resistant status, now display robust cariogenic virulence. The acidogenicity and extracelluar polysaccharide (EPS) formation are both exponentially enhanced, and this may further detrimentally impact the anti-cariogenic effect of fluorides [
25,
26,
27].
Thus, an effective remineralizing protocol is one which brings about a change in enamel crystal and makes it more resistant to the acidic challenges of the oral cavity. Enamel does not contain any growth-inducing constituent to bring about cellular regeneration after structural loss solely and wholly relies upon physico-chemical-centered repair mechanisms to restore its aboriginal ionic inorganic composition [
25,
26,
27].
Sonali et al. have worked on the concept of utilizing a crystal irradiation to bring about remineralization of altered crystals with an increase in acid resistance [
28,
29,
30,
31,
32,
33,
34,
35,
36]. The crystal utilized was aluminum gallium arsenide laser irradiation.
5. Crystals of Aluminum Gallium Arsenide
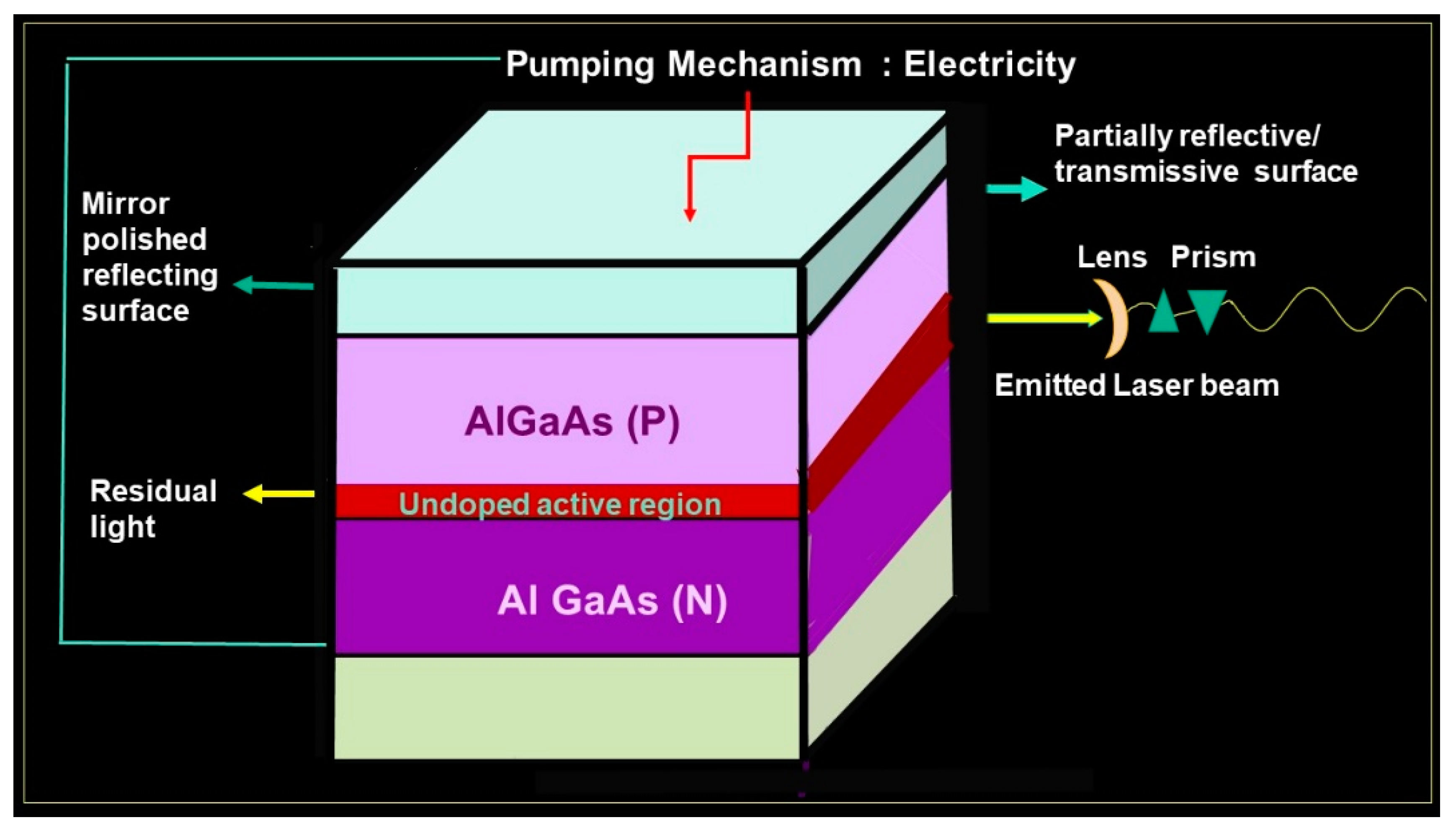
A diode is a device which has wafers or crystals of semiconductor material that primarily and essentially act as a unidirectional switch for the flow of current. It facilitates the flow of the current in a single direction but completely inhibits the flow of current in the opposite direction. The wavelength emitted by these laser diodes falls into the infrared region and ranges between 600 and 1064 nm; 800 nm is the wavelength for the active medium containing aluminum and 980 is the wavelength for the diode with active media composed of indium. Lasers are named or christened generically based on the active medium composition, state, or characteristic. The core of the optical cavity of the laser is comprised of chemical elements, molecules, or compounds and is called the active medium, which can be a container of gas, a crystal, or a solid-state semiconductor The aluminum gallium arsenide laser essentially is a diode laser with crystalline wafers of the active media of aluminum. Aluminum gallium arsenide (AlGaAs) wafers are crystalline wafers which are used as a semiconductor in photo-optics and biophotonic applications especially. The structure of AlGaAs is more or less similar to that of gallium arsenide, but the AlGaAs laser has a larger band gap. These crystal wafers are accommodated in the core of the optical cavity of the laser [
11,
12,
13].
There are two mirrors, one at each end of the optical cavity, placed parallel to each other. Surrounding this core is an excitation source, either a flash lamp strobe device or an electrical coil which provides the energy to the active medium. The housing includes a focusing lens, cooling systems, and controls. Due to the activation of the active media and the initiation of the cascade of stimulated emission, the result is population inversion, which signifies that a major proportion of the atoms of the active medium are in an elevated state or orbit rather than in the lower energy level or resting state. There must be a constant supply of energy, called a pumping mechanism, to maintain this excitation or stimulated emission. The method to enhance the optical pumping is by electric discharge [
11,
12,
13].
The mirrors positioned at each end of the active medium are intended to reflect the generated or stimulated photons to and fro so as to facilitate further stimulated emission, and each time it passes, it successively penetrates or contacts the active medium to amplify the power of the laser beam exponentially, and this protocol is labeled amplification [
11,
12,
13].
The active region is aluminum, and when pumped sufficiently by electric discharge, it leads to stimulated emission of photons, along with population inversion which further leads to exponential photon activation by stimulated emission, and the end results are that the device with active media of crystals emits lased light [
11,
12,
13].
An arsenide is an anion with the charge of −3. Arsenide is a rare mineral group composed of one or more compound metals with arsenic. These salts of arsenide are postulated to exhibit very high lattice energies and, as a result, have multiple applications as semiconductors, lasers, and light-emitting diodes. A crystalline semiconductor alloy with wafers of aluminum gallium arsenide can be employed in single- and double-hetero-structure diode lasers as the light confinement layer. The aluminum gallium arsenide diode laser thus consists of a double heterojunction which is essentially formed by an undoped or lightly p-doped active region surrounded by higher bandgaps of p region and n regions. A piece of P-type junction of the gallium arsenide layer and the N-type gallium arsenide layer is interspersed with a layer of the active region of aluminum. The reflecting polished mirror at both ends keeps amplifying the electrons by cross reflection, and one of the mirrors is partially transmissive. The aluminum gallium arsenide laser also has layers of cladding which furnish the much needed energy barrier and hence restrict and limit the carriers to the active zone. The actual real time operational wavelengths range
from 600 to 880 nm and varies due to multifactorial variations such as the actual dimension of the active region, the characteristics and effect of the dopants, and the compositional variation of the cladding and active layers. In studies by Sonali et al. [
20,
21,
22,
23,
24,
25,
26,
27,
28], the wavelength of 810 nm was employed. When an effective voltage is applied in the forward direction, electrons and holes are interjected into the active layer. Since the band gap energy is of greater dimension in the cladding layers than effectively in the active layer, the injected electrons and holes are prevented and barricaded from permeating across the junction by the potential barriers milled between the cladding layers and the active layer. The electrons and holes which are limited and confined to the active layer constitute a state of population inversion, thus potentiating the amplification of light by stimulated emission. Thus, emission of LASER of aluminum gallium arsenide crystals takes place from one of the partially transmissive mirrors (
Figure 2) [
11,
12,
13].
The cladding layers serve two functions; one is the injection of the charge carriers and the other is confinement of the light. As the active region has an effective smaller bandgap compared to the cladding layers and vice versa, its refractive index is slightly greater than that of the adjacent layers. The gallium arsenide effective refractive index at these wavelengths is larger when compared to the refractive index of the aluminum gallium arsenide cladding layers [
11,
12,
13,
37,
38].
6. Anticariogenic Sanative Effect of Crystals on Crystals: Mechanism of Action
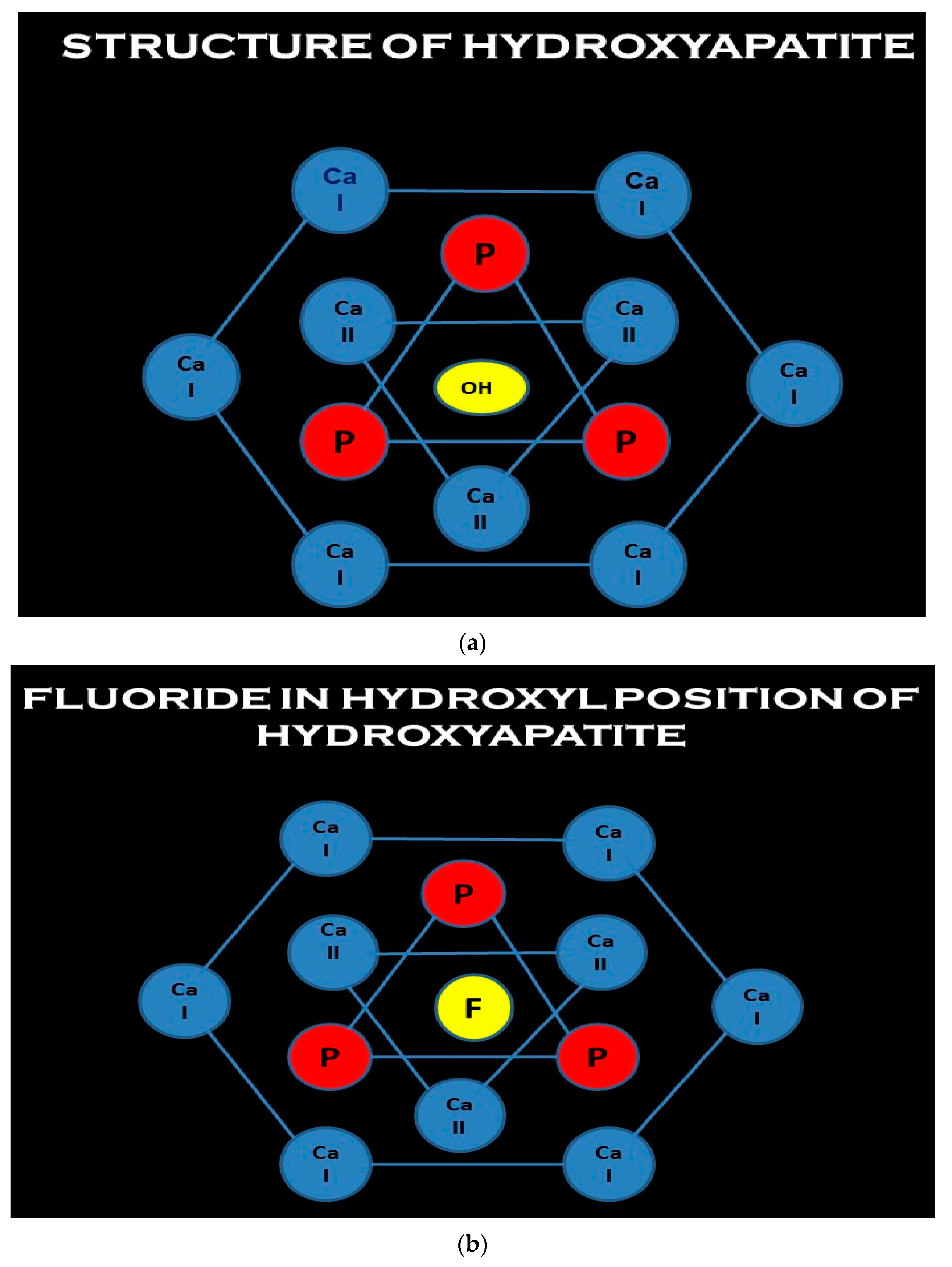
Sonali Sharma has extensively worked with crystals and proposed the concept of Aluminum Gallium Arsenide Laser Caries Inhibition. The hypothesis of the anticariogenic sanative effect of crystals was based on the inference that the selectivity of these wavelengths of aluminum gallium arsenide crystals is greater in the targeting and removal of the carbonate group from the enamel mineral molecule. Additionally, the altered mineral has greater uptake of topically applied fluoride and thus greater acid resistance and leads to remineralization of non-cavitated lesions. (
Figure 3a–e) This can be understood by derivation of the structure of hydroxyapatite [
5]. The hydroxyl ion is located in the central c axis, and it is surrounded by triangles of calcium and phosphates and a hexagonal of calcium (
Figure 3a). During remineralization, fluoride is classically incorporated by filling in existing hydroxyl vacancies or eventually displacing hydroxyl ions; thus, fluoride decreases the solubility product of hydroxyapatite crystal (
Figure 3b). The fluoride ion which replaces the hydroxyl has a tighter fit in the c axis and thus reduces the lattice energy [
5]. Fluoride-resistant
S. mutans demonstrated a robust cariogenic virulence, with increased potential of acidogenicity and extracellular polysaccharide matrix production, which might have a detrimental effect on the preventive action of fluoride in the formation of biofilm [
25,
26].
However, during demineralization in the initial stages, there is ionic substitution. Carbonate can replace either hydroxyl Type A or phosphate Type B conditions, and it may do so in the presence of sodium, which may be needed for calcium substitution. As the carbonate has a poorer fit, the hydroxyapatitite crystal lattice’s end resultant phase is unstable, weaker, and less acid resistant, and hence the solubility product is increased and demineralization initiates and progresses (
Figure 3c). Calcium can also be replaced by magnesium to a limited, miniscule extent. Magnesium, due to charge density, has a similar increase in solubility product as carbonate also exhibits a very similar trend of destabilization of the apatite crystals. On incorporation into the apatite crystal lattice, the synergistic effect of carbonate and magnesium concomitantly increases the precipitation or leaching out of calcium phosphate ions by increasing the solubility product constant [
5]. This synergistic effect sets the ball rolling for vicious cycle of prolonged demineralization (
Figure 3d).
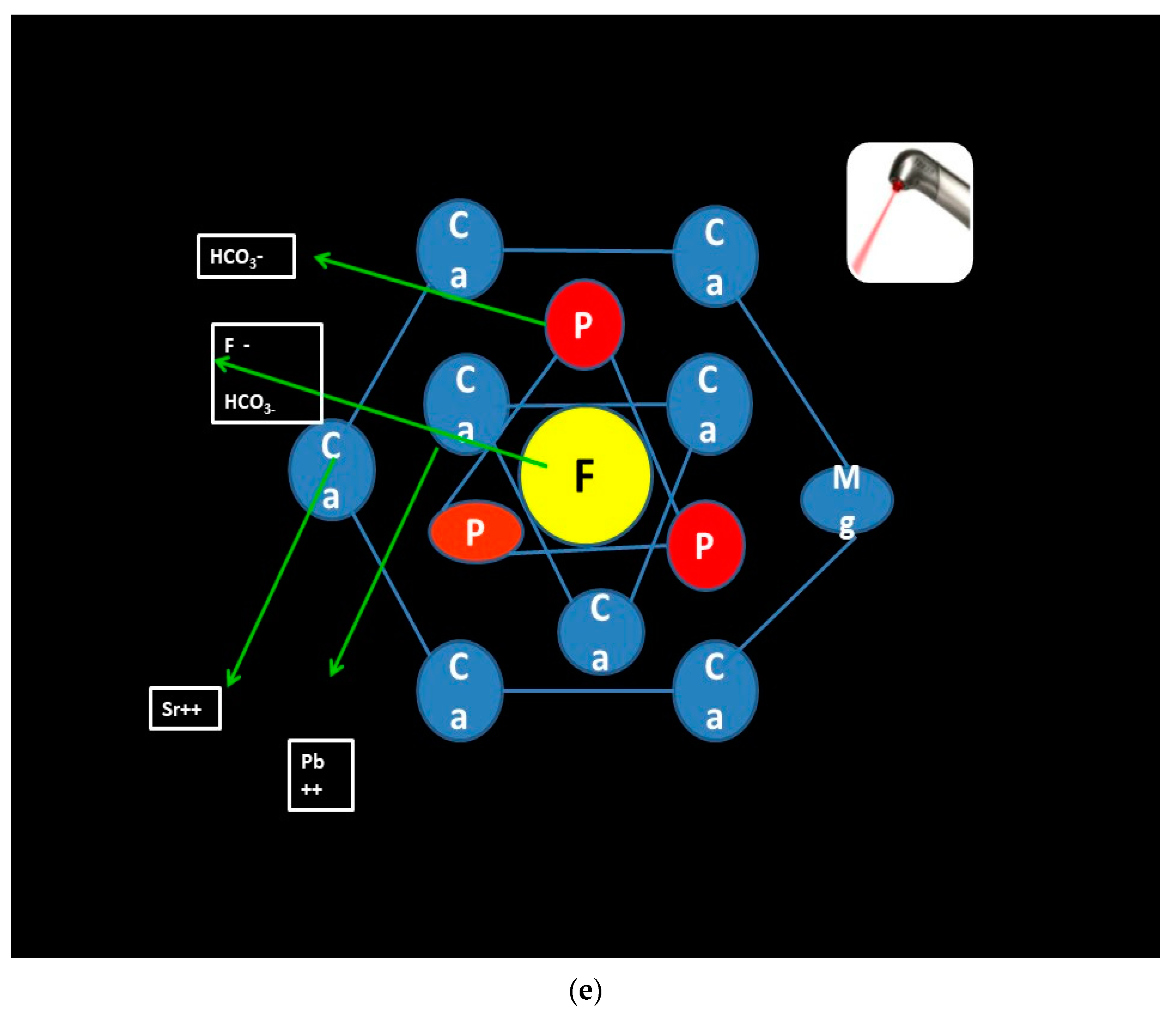
According to studies conducted by Sonali Sharma et al., if the subsurface lesions prior to cavitation are irradiated by 3.5 watts of 810 aluminum gallium laser for 30 s and thereafter the casein-phospho-peptide amorphous calcium phosphate with fluoride (CPP ACP F) remineralizing paste is applied, then the acid resistance is observed to exponentially increase [
28,
29,
30,
31,
32,
33,
34,
35,
36]. The inference drawn is that fluoride uptake is increased and the ion which replaces carbonate in the c-axis is larger in size. The high charge density on the fluoride ion, together with its symmetry, leads to a much adaptive and hermetic fit within the calcium II triangle. The cumulative effect is lowering of the lattice energy and effectively stabilizing the enamel crystal structure, the end result being increase in acid resistance (
Figure 3e) [
28,
29,
30,
31,
32,
33,
34,
35,
36].
7. Evidence Based Studies
To establish the caries inhibitory role of aluminum gallium arsenide, a series of studies were conducted and thus validated the proposed hypothesis by Sharma et al. [
28,
29,
30,
31,
32,
33,
34,
35,
36].
For standardization of protocol, in vitro studies were conducted by Sharma et al. on enamel samples prior to clinical trial to ascertain the optimum wattage and time to be employed in the study. Results of the in vitro studies reiterated that the optimum wattage and time which brought about the caries inhibitory potentiality of the 810 nm aluminum gallium arsenide laser were 3.5 watts and 30 s, respectively [
28,
31].
Sonali Sharma conducted in vitro studies for standardization of a caries-inhibitory surface treatment protocol of enamel subsurface caries. It was observed that laser irradiation followed by application of CPP ACP F is a more effective protocol than different remineralizing pastes when used alone [
32].
Standardization of devices for monitoring the effect of aluminum gallium arsenide (Al Ga As) lasers on non-cavitated enamel lesions was also carried out by in vitro studies of sectioned enamel samples by Sonali Sharma et al. It was observed that laser fluorescence devices, i.e., DIAGNOdent, could detect demineralization and subsequent remineralization of artificially created demineralized lesions [
33].
To corroborate the result of the trials, multiple in vitro studies were carried out by Sonali Sharma et al. The microhardness of different surface treatments was ascertained, and it was observed that laser 3.5-watt irradiation for 30 s followed by remineralizing paste application of CPP ACP F gave the best result [
34].
The compositional change of different surface treatments by FTIR analysis also proved that laser irradiation of 3.5 watts followed by application of CPP ACP F brought about the change conducive for remineralization. This study was conducted by Sonali Sharma et al. [
35].
Sharma et al. observed that adherence of
streptococci was reduced when the enamel carious lesions were first irradiated with the aluminum gallium arsenide laser [
36].
Aluminum gallium arsenide lasers have not been evaluated as a clinical caries-inhibitory tool in the past, and further, in vivo studies are far and few between. Hence, more clinical studies need to be conducted. However, based on the results of these documented studies, it can be inferred that aluminum gallium arsenide lasers seem a viable adjunct in the caries prevention protocol.