Crystal Structure, Raman Spectrum and Tl+ Lone-Pair Luminescence of Thallium(I) Dodecahydro-Monocarba-closo-Dodecaborate Tl[CB11H12]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single-Crystal X-ray Diffraction
2.3. Optical Spectroscopy
3. Results and Discussion
3.1. Crystal Structure
3.2. Raman Spectrum
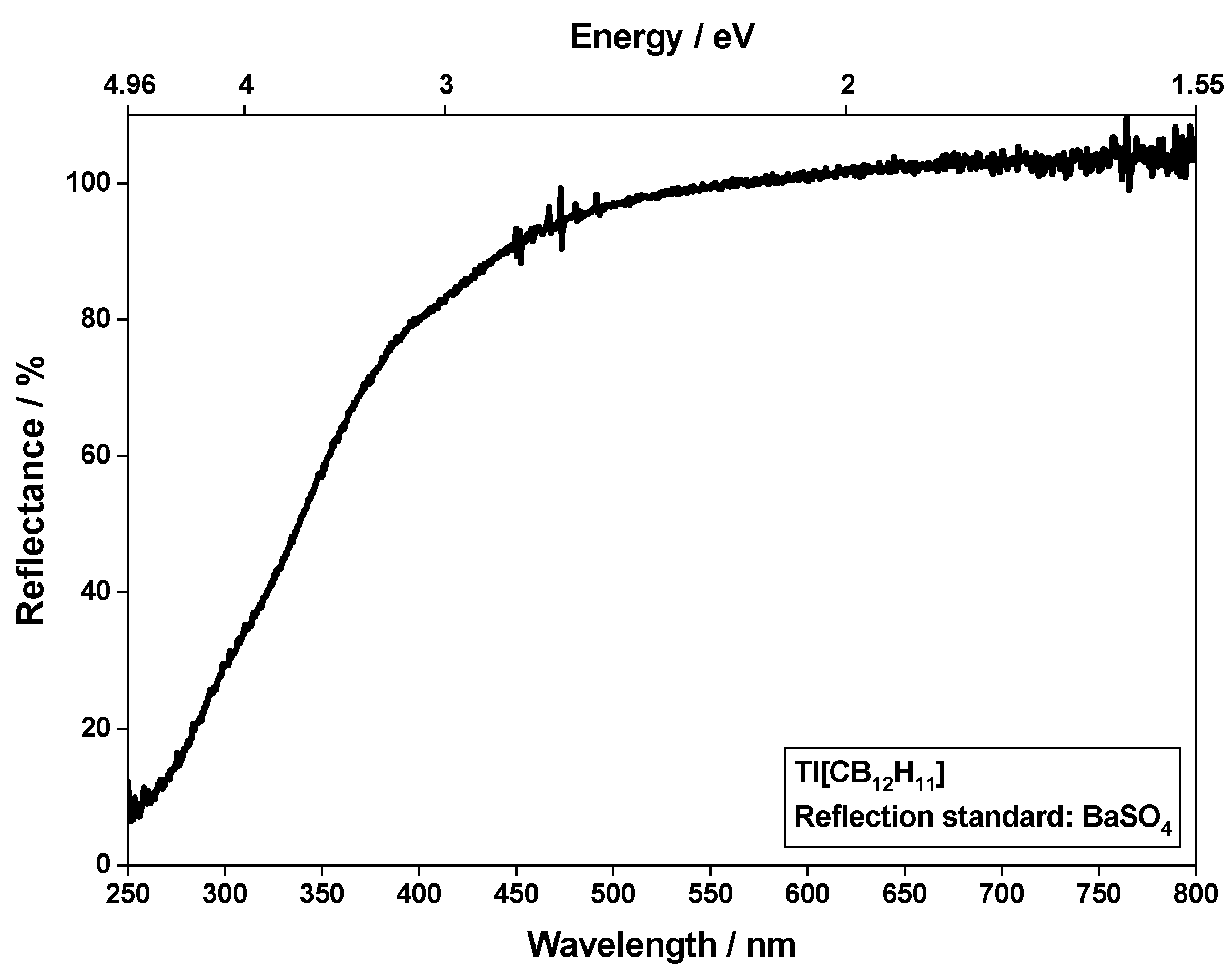
3.3. Optical Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, W.S.; Unemoto, A.; Zhou, W.; Stavila, V.; Matsuo, M.; Wu, H.; Orimo, S.-I.; Udovic, T.J. Unparalleled lithium and sodium superionic conduction in solid electrolytes with large monovalent cage-like anions. Energy Environ. Sci. 2015, 8, 3637–3645. [Google Scholar] [CrossRef] [Green Version]
- Dimitrievska, M.; Wu, H.; Stavila, V.; Babanova, O.A.; Skoryunov, R.V.; Soloninin, A.V.; Zhou, W.; Trump, B.A.; Andersson, M.S.; Skripov, A.V.; et al. Structural and Dynamical Properties of Potassium Dodecahydro-monocarba-closo-dodecaborate: K[CB11H12]. J. Phys. Chem. 2020, C 124, 17992–18002. [Google Scholar] [CrossRef]
- Bareiß, K.U.; Friedly, A.; Schleid, T. Die unerwartete Kristallstruktur des Cäsium-Dodekahydro-Monocarba-closo-Dodekaborats Cs[CB11H12]. Z. Naturforsch. 2020, 75 b, 1049–1059. [Google Scholar] [CrossRef]
- Romerosa, A.M. Thermal, structural and possible ionic-conductor behaviour of CsB10CH13 and CsB11CH12. Thermochim. Acta 1993, 217, 123–128. [Google Scholar] [CrossRef]
- Černý, R.; Brighi, M.; Murgia, F. The crystal chemistry of inorganic hydroborates. Chemistry 2020, 2, 805–826. [Google Scholar] [CrossRef]
- Bareiß, K.U.; Kleeberg, F.M.; Enseling, D.; Jüstel, T.; Schleid, T. Tl2[B10H10] und Tl2[B12H12]: Kristallstrukturen, Raman-Spektren und Tl+-Lone-Pair-Lumineszenz im Vergleich. Z. Naturforsch. 2022, 77 b, 179–187. [Google Scholar] [CrossRef]
- Van, N.-D.; Tiritiris, I.; Schleid, T. Tl2[B12H12]: Thallium(I) Dodecahydro-closo-dodecaborate with Cs2[B12H12]-Type Crystal Structure. Z. Anorg. Allg. Chem. 2004, 630, 1764. [Google Scholar] [CrossRef]
- Bareiß, K.U.; Bette, S.; Enseling, D.; Jüstel, T.; Schleid, T. Extraordinary intense blue Tl+ lone-pair photoluminescence from thallium(I) chloride hydroborate Tl3Cl[B12H12]. Dalton Trans. 2022, 51, 13331–13341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS/L-2013, Programs for Crystal Structure Determination; University of Göttingen: Göttingen, Germany, 2013. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C 71, 3–8. [Google Scholar]
- Hanson, H.P.; Herman, F.; Lea, J.E.; Skillman, S. HFS atomic scattering factors. Acta Crystallogr. 1964, 17, 1040–1044. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Siegbahn, P.E.; Eisenstein, O.; Rheingold, A.L.; Koetzle, T.F. A New Intermolecular Interaction: Unconventional Hydrogen Bonds with Element–Hydride Bonds as Proton Acceptor. Acc. Chem. Res. 1996, 29, 348–354. [Google Scholar] [CrossRef]
- Klooster, W.T.; Koetzle, T.F.; Siegbahn, P.E.M.; Richardson, T.B.; Crabtree, R.H. Study of the N−H···H−B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction. J. Am. Chem. Soc. 1999, 121, 6337–6343. [Google Scholar] [CrossRef]
- Custelcean, R.; Jackson, J.E. Dihydrogen bonding: Structures, energetics, and dynamics. Chem. Rev. 2001, 101, 1963–1980. [Google Scholar] [CrossRef]
- Batsanov, S.S. Bis(but-2-enoato-κO)triphenylbismuth(V). Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Brighi, M.; Murgia, F.; Łodziana, Z.; Černý, R. Structural Phase Transitions in closo-Dicarbadodecaboranes C2B10H12. Inorg. Chem. 2022, 61, 5813–5823. [Google Scholar] [CrossRef]
- Kononova, E.G.; Bukalov, S.S.; Leites, L.A.; Lyssenko, K.A.; Ol’shevskaya, V.A. Vibrational spectra and structures of cesium salts with the icosahedral monocarba-closo-dodecaborate anion, [CB11H12]−, and its nido-derivative, [CB10H13]− Russ. Chem. Bull. Int. Ed. 2003, 85–92. [Google Scholar] [CrossRef]
- Weidlein, J.; Müller, U.; Dehnicke, K. Schwingungsspektroskopie, 2nd ed.; Thieme: Stuttgart, Germany, 1988; pp. 142–144. ISBN 978-3-136-25101-0. [Google Scholar]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324–1326. [Google Scholar] [CrossRef]
- Seitz, F. Interpretation of the Properties of Alkali Halide - Thallium Phosphors. J. Chem. Phys. 1938, 6, 150–162. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmeier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1994; ISBN 978-3-540-58019-5. [Google Scholar]
- Ronda, C.R. Luminescence; Wiley-VCH: Weinheim, Germany, 2006; ISBN 978-3-527-31402-7. [Google Scholar]
- Fonger, W.H.; Struck, C.W. Eu3+ 5D Resonance Quenching to the Charge-Transfer States in Y2O2S, La2O2S, and LaOCl. J. Chem. Phys. 1970, 52, 6364–6372. [Google Scholar] [CrossRef]
- Struck, C.W.; Fonger, W.H. Role of the charge-transfer states in feeding and thermally emptying the 5D states of Eu3+ in yttrium and lanthanum oxysulfides. J. Lumin. 1970, 1-2, 456–469. [Google Scholar] [CrossRef]
- Struck, C.W.; Fonger, W.H. Thermal Quenching of Tb3+, Tm3+, Pr3+, and Dy3+ 4fn Emitting States in La2O2S. J. Appl. Phys. 1971, 42, 4515–4516. [Google Scholar] [CrossRef]


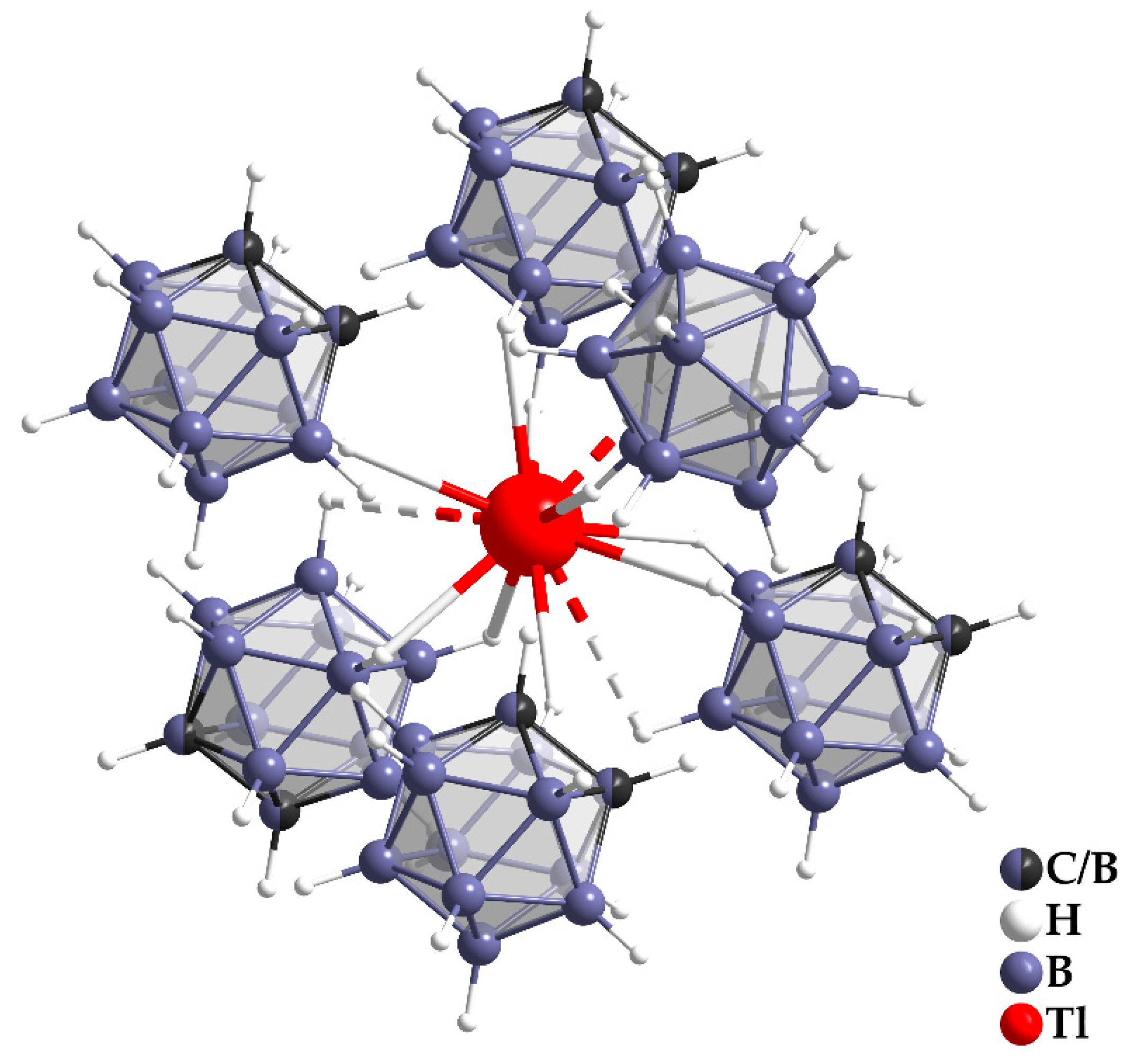
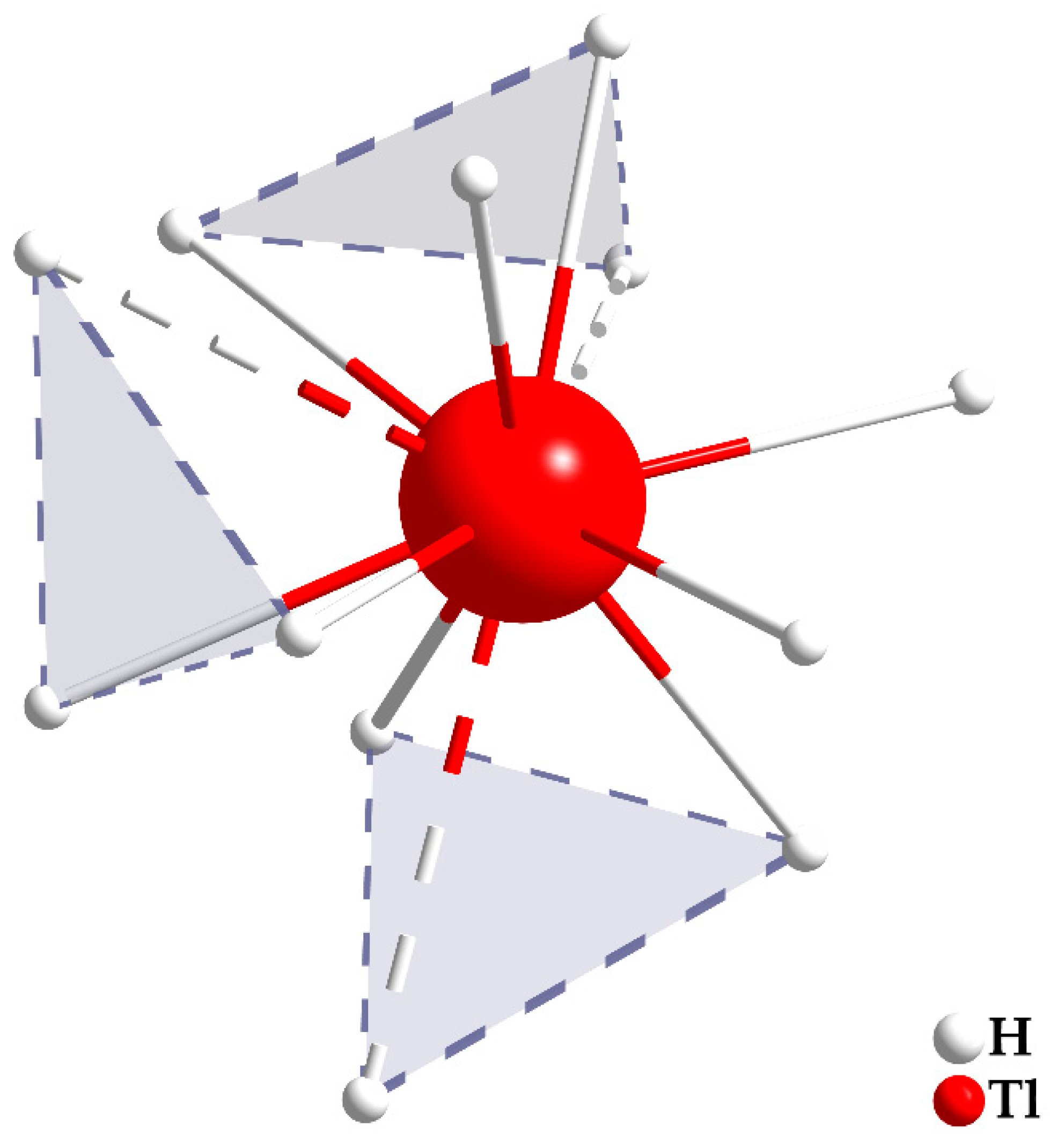
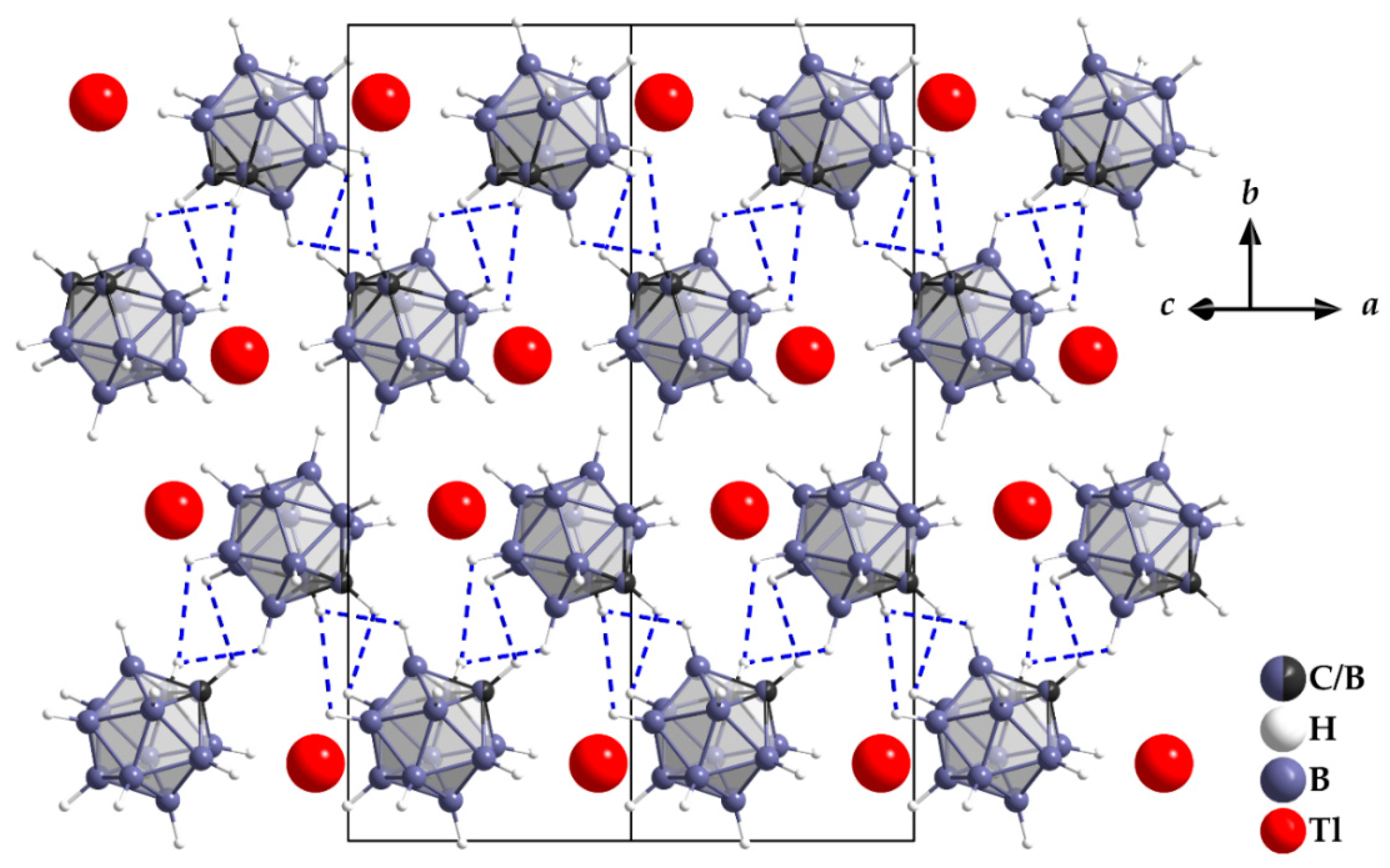



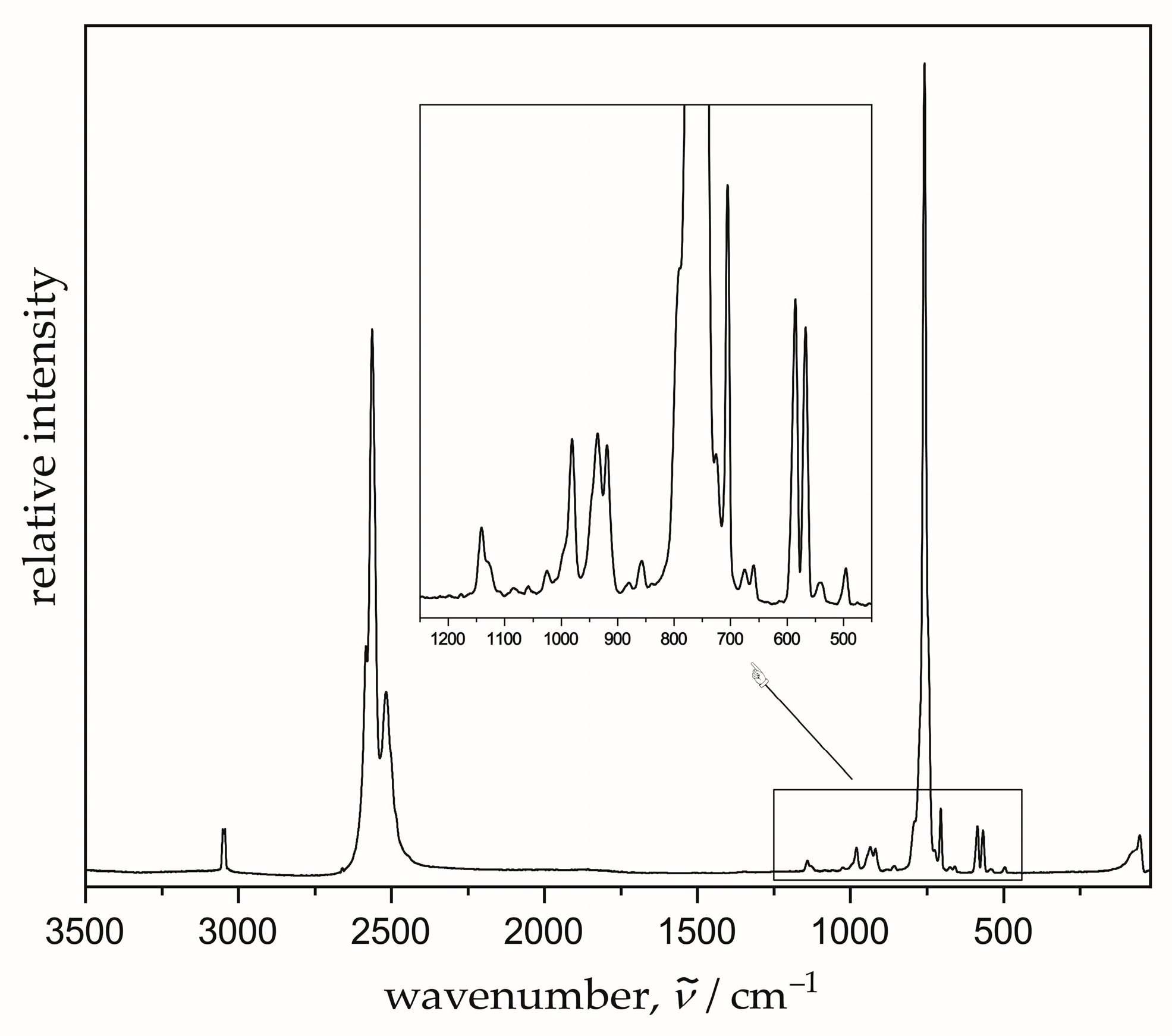



| empirical formula | Tl[CB11H12] |
| crystal system | monoclinic |
| space group | (no. 14) |
| lattice parameters | |
| /pm | 685.64(3) |
| /pm | 1978.21(9) |
| /pm | 1006.89(5) |
| /° | 132.918(3) |
| number of formula units, | 4 |
| calculated density, /g | 2.307 |
| molar volume, / | 150.57 |
| diffractometer | -CCD (Bruker-Nonius) |
| radiation wavelength | Mo-: pm |
| diffraction limit, 2/° | 54.92 |
| hkl range, max, max, max | 8, 25, 12 |
| (000)/ | 616 |
| absorption coefficient, / | 16.07 |
| extinction coefficient, ε | 0.0114(5) |
| measured reflections | 17,476 |
| unique reflections | 2283 |
| , | 0.064, 0.038 |
| GooF | 0.034, 0.081, 1.028 |
| Residual electron density(max., min. / ) | 1.31, –1.14 |
| CCDC number | 2221677 |
| Atom | / | / | / | |
|---|---|---|---|---|
| Tl | 0.62389(6) | 0.094958(15) | 0.73978(4) | 627(2) |
| C1|B11 * | 0.4456(14) | 0.1820(3) | 0.0867(9) | 501(2) |
| H11 | 0.380 | 0.218 | −0.021 | 601 |
| C2|B12 * | 0.7656(15) | 0.1829(4) | 0.2906(10) | 484(2) |
| H12 | 0.916 | 0.218 | 0.322 | 581 |
| B1 | 0.5088(16) | 0.2123(4) | 0.2735(10) | 493(2) |
| H1 | 0.489 | 0.266 | 0.293 | 592 |
| B2 | 0.2288(14) | 0.1606(4) | 0.1112(10) | 447(2) |
| H2 | 0.025 | 0.180 | 0.024 | 536 |
| B3 | 0.3194(14) | 0.1016(4) | 0.0286(10) | 459(2) |
| H3 | 0.175 | 0.083 | −0.112 | 551 |
| B4 | 0.6534(15) | 0.1166(4) | 0.1404(10) | 468(2) |
| H4 | 0.727 | 0.108 | 0.073 | 562 |
| B5 | 0.8532(15) | 0.1012(4) | 0.3737(10) | 489(2) |
| H5 | 1.057 | 0.082 | 0.459 | 587 |
| B6 | 0.7650(15) | 0.1614(4) | 0.4569(9) | 489(2) |
| H6 | 0.912 | 0.181 | 0.596 | 587 |
| B7 | 0.4234(14) | 0.1461(3) | 0.3429(10) | 414(2) |
| H7 | 0.347 | 0.156 | 0.408 | 497 |
| B8 | 0.3074(15) | 0.0776(4) | 0.1926(10) | 443(2) |
| H8 | 0.156 | 0.042 | 0.160 | 665 |
| B9 | 0.5716(13) | 0.0500(3) | 0.2102(9) | 417(2) |
| H9 | 0.592 | −0.003 | 0.190 | 500 |
| B10 | 0.6406(15) | 0.0775(4) | 0.4084(10) | 437(2) |
| H10 | 0.707 | 0.043 | 0.517 | 524 |
| C1|B11 | C2|B12 | 170.1(10) | C2|B12 | C1|B11 | 170.1(10) |
| B3 | 171.1(9) | B5 | 172.8(11) | ||
| B4 | 171.7(10) | B6 | 173.0(10) | ||
| B2 | 171.7(9) | B4 | 173.7(10) | ||
| B1 | 172.4(9) | B1 | 174.7(10) | ||
| H11 | 110 | H12 | 110 | ||
| B1 | C1|B11 | 172.5(9) | B2 | C1|B11 | 171.7(9) |
| C2|B12 | 174.7(10) | B8 | 174.9(10) | ||
| B6 | 175.9(11) | B7 | 175.1(10) | ||
| B7 | 176.3(9) | B1 | 176.9(10) | ||
| B2 | 176.9(10) | B3 | 177.3(10) | ||
| H1 | 110 | H2 | 110 | ||
| B3 | C1|B11 | 171.0(9) | B4 | C1|B11 | 171.7(10) |
| B9 | 175.2(10) | C2|B12 | 173.8(10) | ||
| B4 | 175.7(10) | B9 | 175.4(10) | ||
| B2 | 177.3(10) | B3 | 175.7(10) | ||
| B8 | 177.4(10) | B5 | 176.1(10) | ||
| H3 | 110 | H4 | 110 | ||
| B5 | C2|B12 | 172.9(11) | B6 | C2|B12 | 173.0(10) |
| B4 | 176.1(10) | B1 | 175.9(11) | ||
| B9 | 177.1(10) | B10 | 177.6(10) | ||
| B10 | 177.8(10) | B5 | 178.2(10) | ||
| B6 | 178.2(10) | B7 | 179.8(10) | ||
| H5 | 110 | H6 | 110 | ||
| B7 | B2 | 175.1(10) | B8 | B2 | 174.9(10) |
| B1 | 176.3(10) | B7 | 176.6(10) | ||
| B8 | 176.7(10) | B3 | 177.3(10) | ||
| B10 | 177.6(10) | B9 | 178.0(9) | ||
| B6 | 179.8(10) | B10 | 178.4(10) | ||
| H7 | 110 | H8 | 110 | ||
| B9 | B3 | 175.2(10) | B10 | B6 | 177.5(10) |
| B4 | 175.5(10) | B7 | 177.6(10) | ||
| B5 | 177.1(10) | B5 | 177.8(10) | ||
| B8 | 178.0(9) | B8 | 178.4(10) | ||
| B10 | 179.3(9) | B9 | 179.3(9) | ||
| H9 | 110 | H10 | 110 |
| Tl | H9 | 272.5 |
| H7 | 275.0 | |
| H2 | 280.7 | |
| H10 | 285.4 | |
| H5 | 286.3 | |
| H4 | 293.3 | |
| H8 | 293.4 | |
| H3 | 298.1 | |
| H1 | 306.5 | |
| ∙∙∙ H10 | 335.4 | |
| ∙∙∙ H8 | 335.9 | |
| ∙∙∙ H6 | 357.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bareiß, K.U.; Enseling, D.; Jüstel, T.; Schleid, T. Crystal Structure, Raman Spectrum and Tl+ Lone-Pair Luminescence of Thallium(I) Dodecahydro-Monocarba-closo-Dodecaborate Tl[CB11H12]. Crystals 2022, 12, 1840. https://doi.org/10.3390/cryst12121840
Bareiß KU, Enseling D, Jüstel T, Schleid T. Crystal Structure, Raman Spectrum and Tl+ Lone-Pair Luminescence of Thallium(I) Dodecahydro-Monocarba-closo-Dodecaborate Tl[CB11H12]. Crystals. 2022; 12(12):1840. https://doi.org/10.3390/cryst12121840
Chicago/Turabian StyleBareiß, Kevin U., David Enseling, Thomas Jüstel, and Thomas Schleid. 2022. "Crystal Structure, Raman Spectrum and Tl+ Lone-Pair Luminescence of Thallium(I) Dodecahydro-Monocarba-closo-Dodecaborate Tl[CB11H12]" Crystals 12, no. 12: 1840. https://doi.org/10.3390/cryst12121840
APA StyleBareiß, K. U., Enseling, D., Jüstel, T., & Schleid, T. (2022). Crystal Structure, Raman Spectrum and Tl+ Lone-Pair Luminescence of Thallium(I) Dodecahydro-Monocarba-closo-Dodecaborate Tl[CB11H12]. Crystals, 12(12), 1840. https://doi.org/10.3390/cryst12121840








