Electrical and Dielectric Properties of Ca-Doped Bi-Deficient Sodium Bismuth Titanate Na0.5Bi0.49−xCaxTiO3−δ (0 ≤ x ≤ 0.08)
Abstract
:1. Introduction
2. Experimental
3. Results
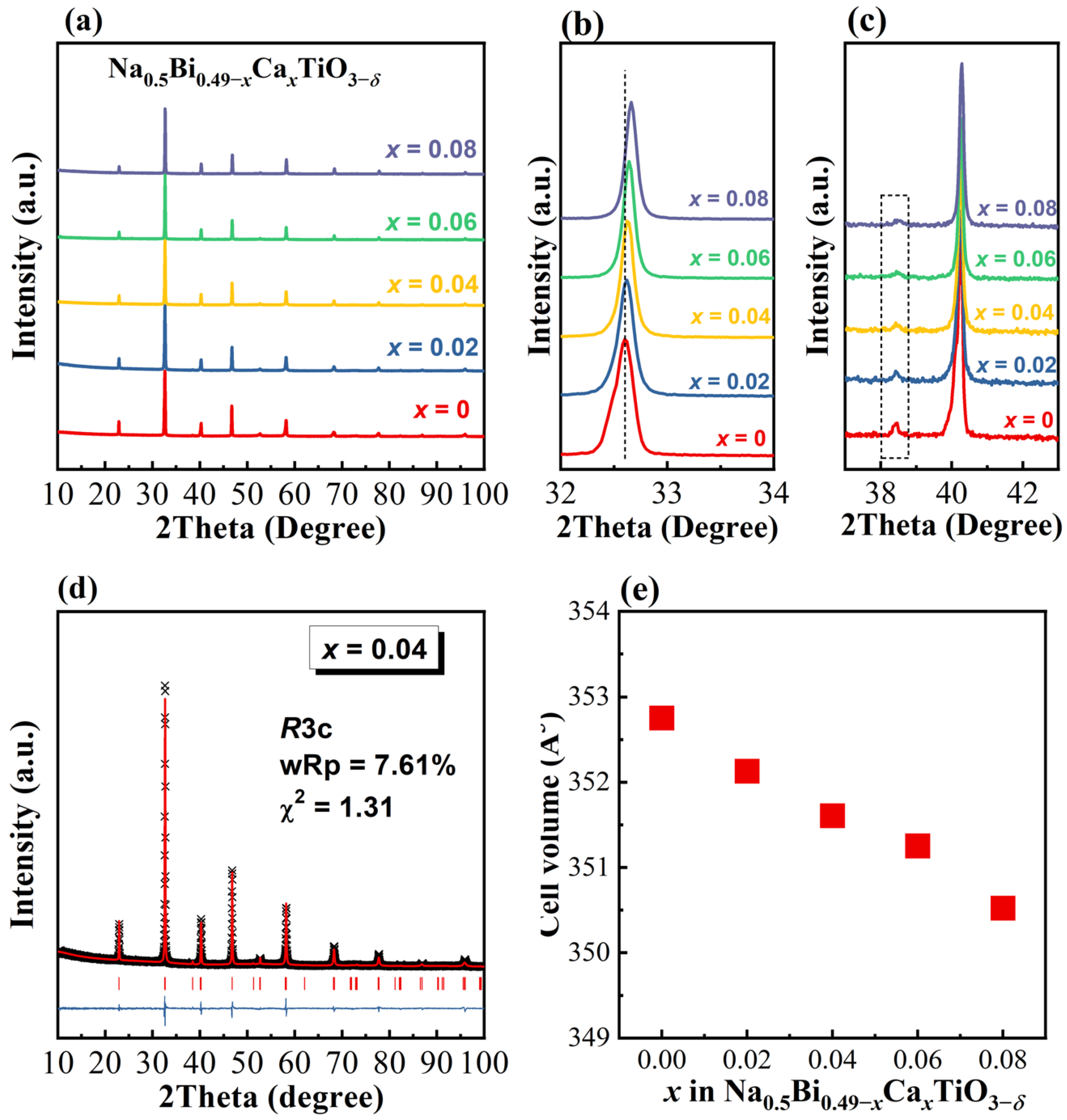
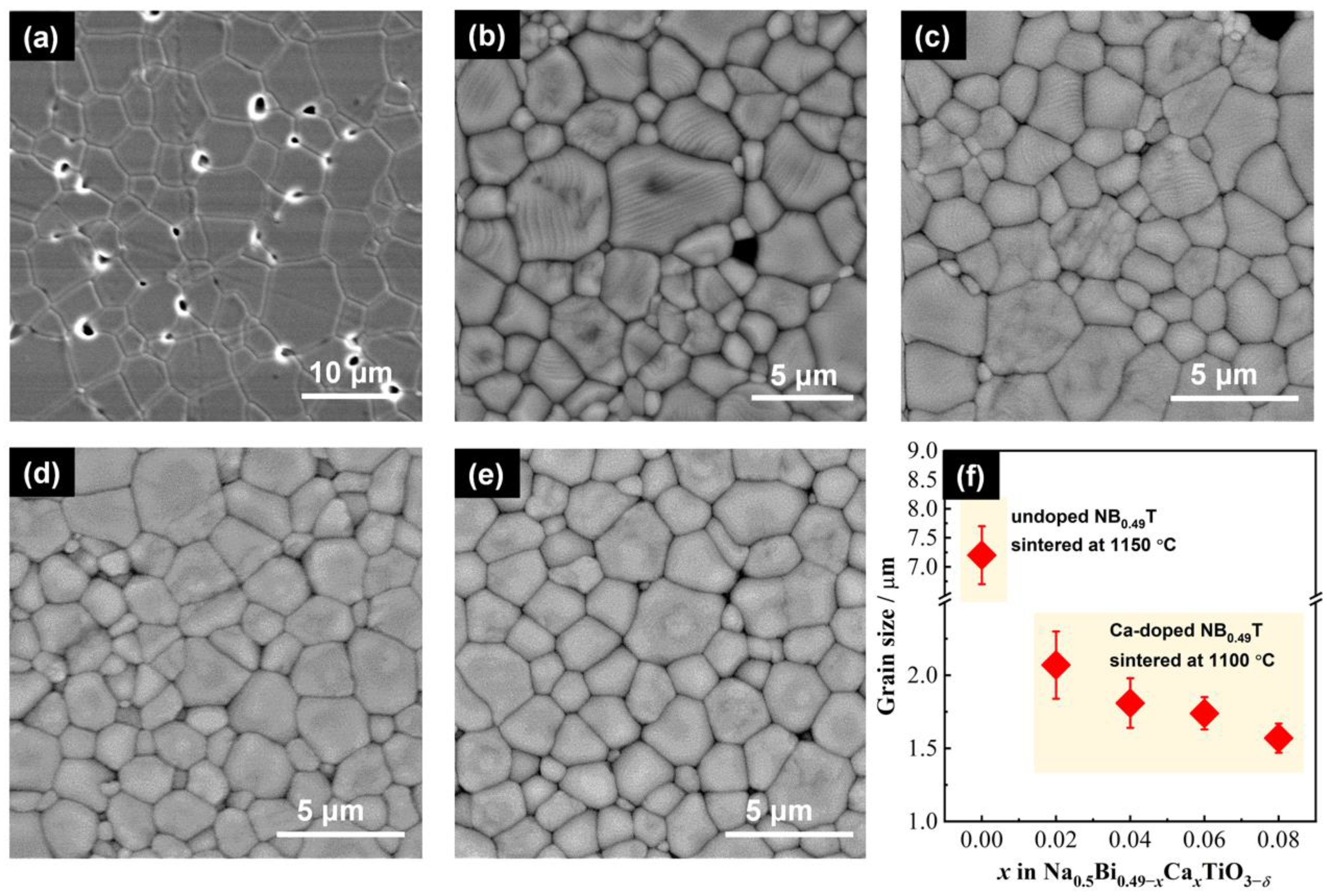
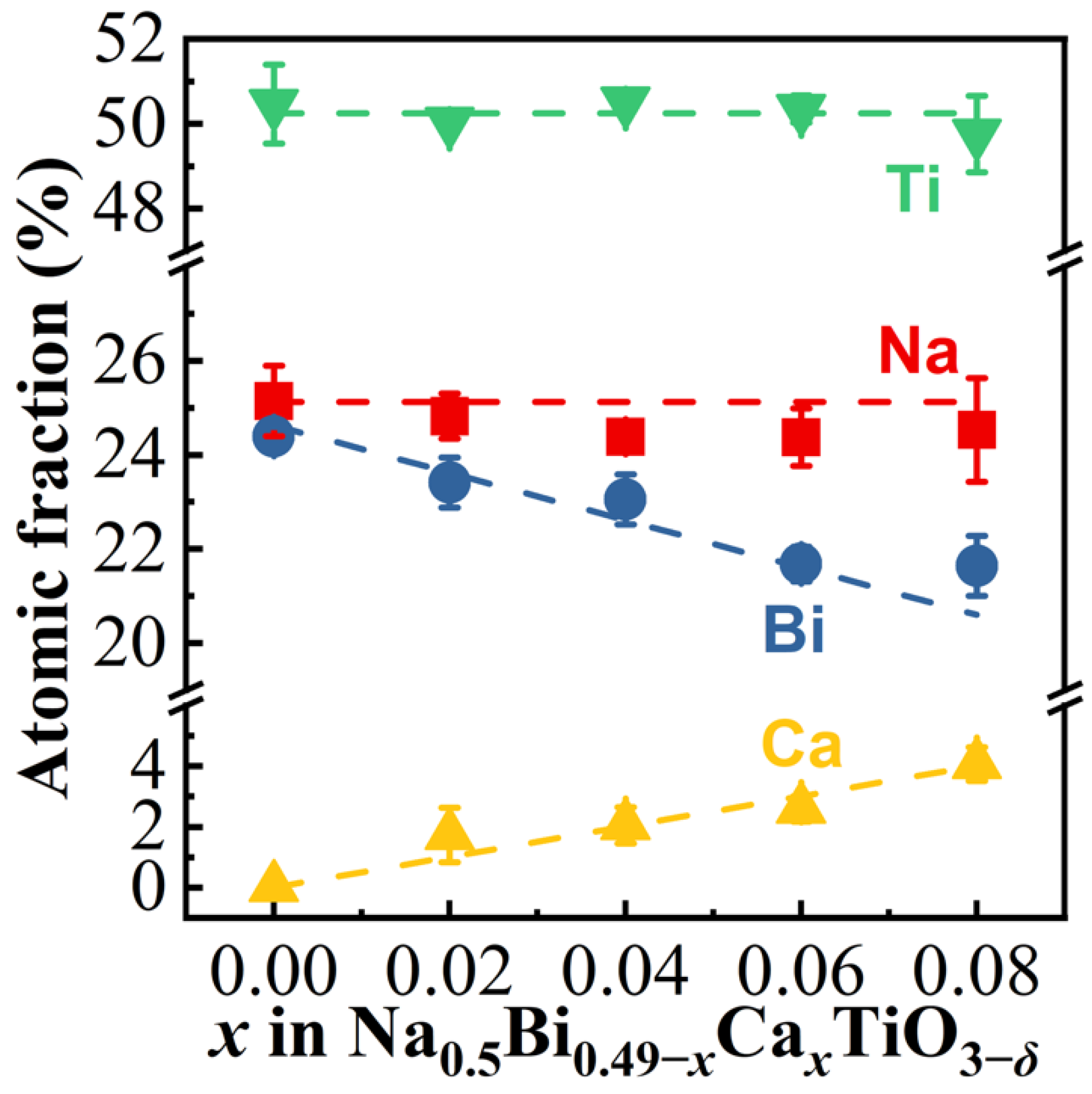
3.1. Crystal Structure, Microstructure, and Composition
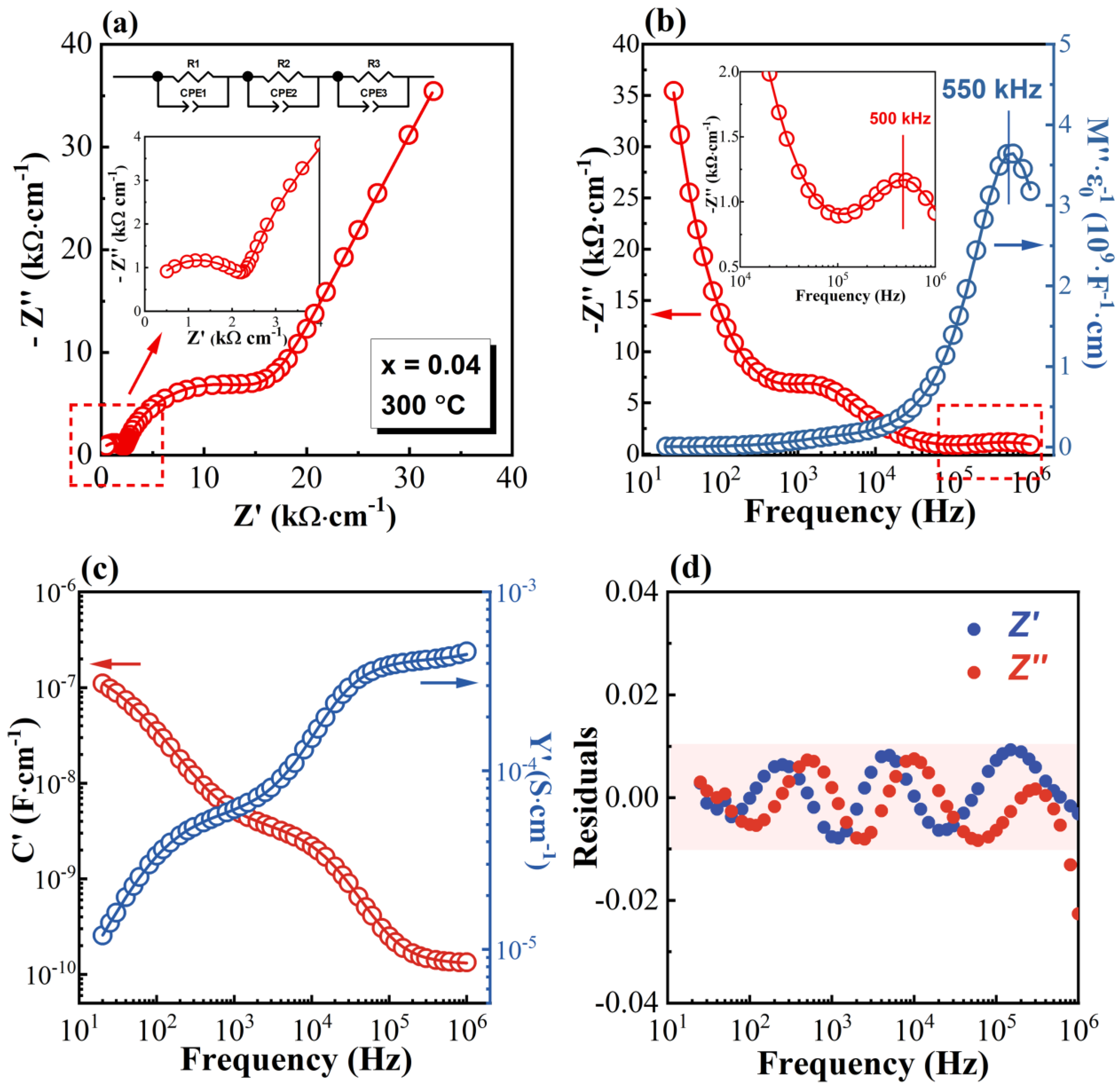
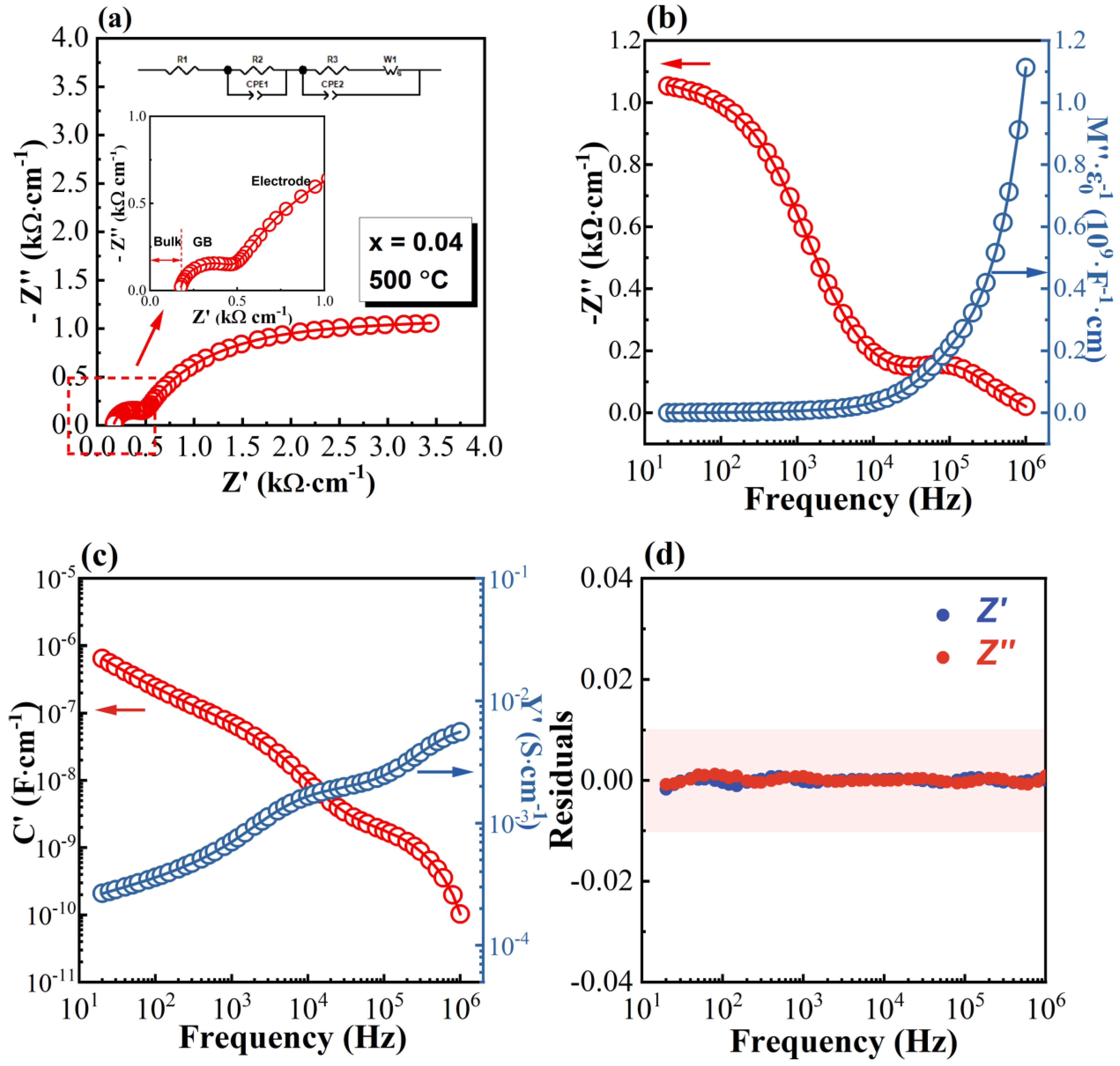
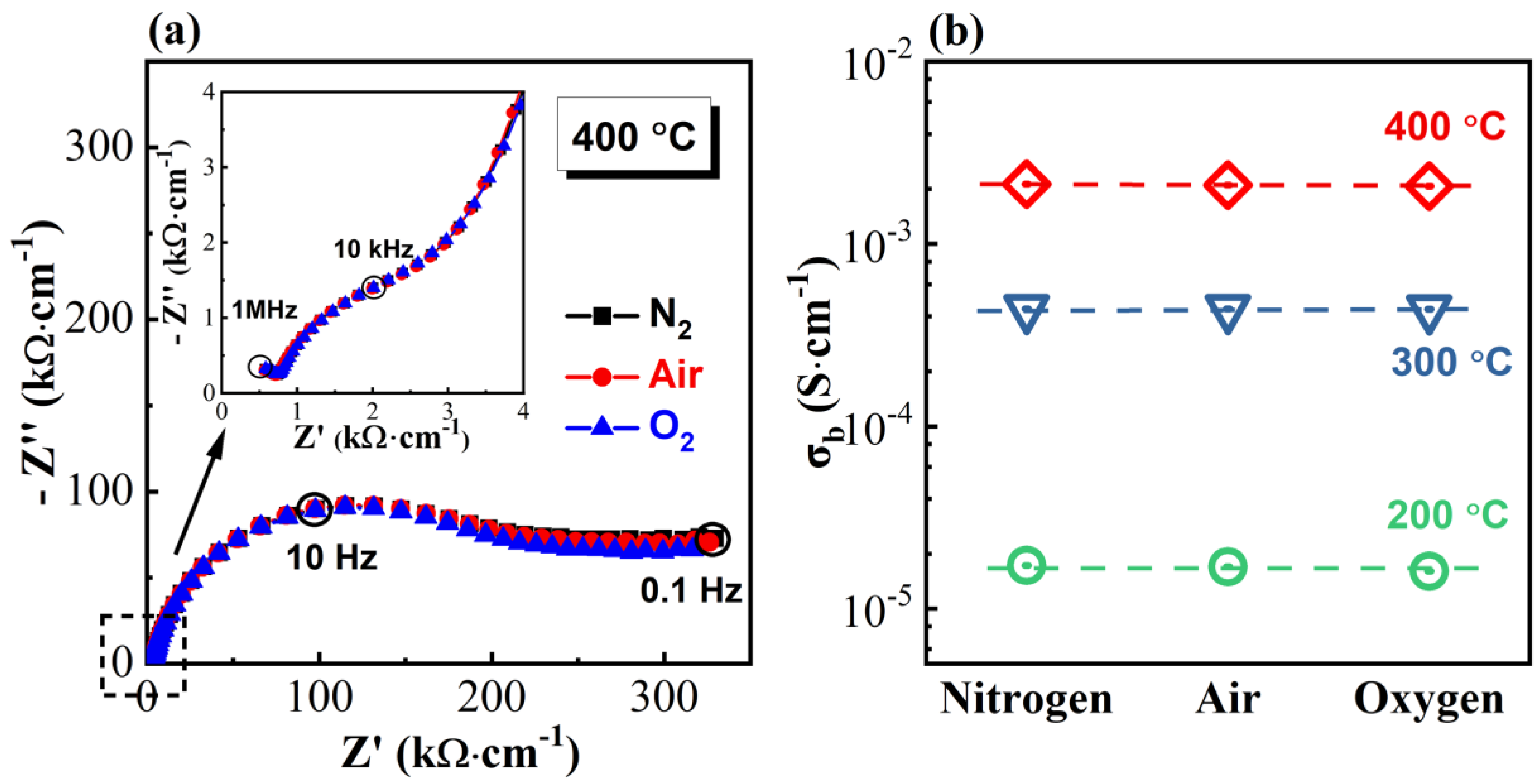
3.2. Impedance Spectra
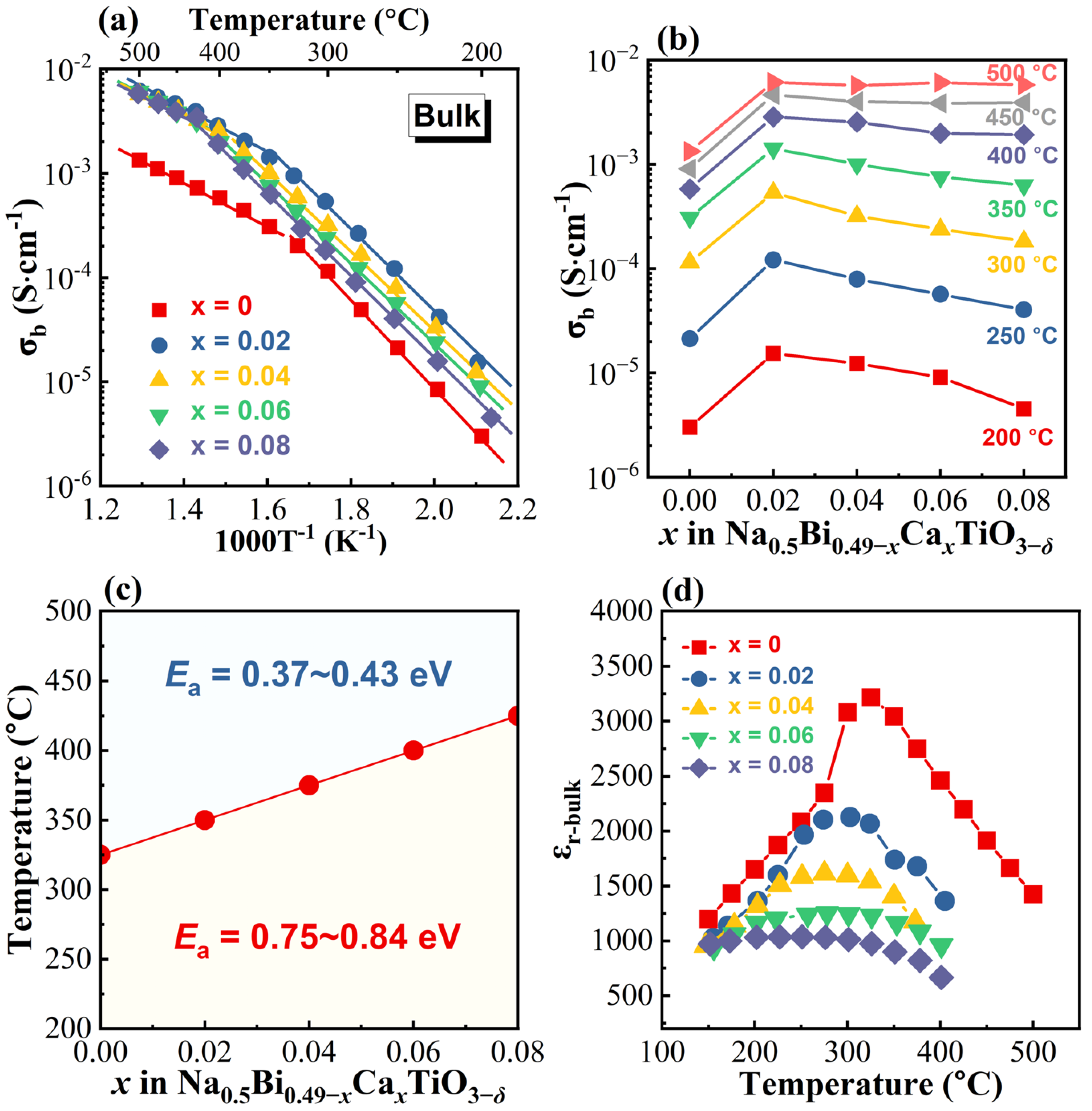
3.3. Dielectric Properties
4. Discussion
- (1)
- The average structure. Oxide ion conductivity in a perovskite lattice can usually be predicted empirically by the tolerance factor, t [37]; the lattice free volume, Vsf [27]; and the critical radius, rC [38]. Equations for calculating the above three parameters can be found in our previous publications [7,39,40], and the values for Ca-doped NB0.49T are listed in Table 2. With increasing doping level, t decreases towards the optimum value of 0.96 [27]; furthermore, Vsf and rC both increase, which are beneficial to the oxygen ion conduction in perovskites. This fails to explain the σb–x relationship in Figure 6b.
- (2)
- The local structure. Previous studies by first principles calculations have revealed the significant impact of the local structure on oxygen ion diffusion in NBT [22,41]. In perovskites, oxygen ion migration occurs through a saddle point, which is a triangle formed by two A-site cations and one B-site cation [42]. In NBT, the energy barriers for oxygen ion migration through the Na-Na-Ti, Na-Bi-Ti, and Bi-Bi-Ti saddle points are 1.0–1.3, 0.6–0.85 and 0.22 eV, respectively [22]. As high polarizability of Bi3+ and weak Bi–O bonds are critical to the high mobility of oxygen ions, replacement of Bi3+ by Ca2+ with a lower polarizability and a stronger bond with oxygen increases the energy barrier for oxygen migration, and therefore reduces the oxygen ion mobility.
- (3)
- Trapping of oxygen vacancies by . Trapping of the positively charged oxygen vacancies by the negatively charged acceptor-type dopants has been widely observed in oxide ion conductors. Evidence comes from the “volcano”-shaped relationship between conductivity and doping level, and a decrease in the activation energy at higher temperatures when the defect associates are released [43]. In NBT, trapping between the B-site acceptor dopants and oxygen vacancies has been supported by first-principles calculations. Although the calculations suggest A-site acceptor dopants are beneficial to oxygen ion diffusion, experimental results on Li- and K-doped NBT still show a drop in the σb when the doping level exceeds 4% [18]. Trapping of oxygen vacancies by can be another possible reason for the reduced oxygen ion mobility.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, M.; Pietrowski, M.J.; de Souza, R.A.; Zhang, H.; Reaney, I.M.; Cook, S.N.; Kilner, J.A.; Sinclair, D.C. A family of oxide-ion conductors based on the ferroelectric perovskite Na0.5Bi0.5TiO3. Nat. Mater. 2014, 13, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H.; Cook, S.N.; Li, L.; Kilner, J.A.; Reaney, I.M.; Sinclair, D.C. Dramatic influence of A-site nonstoichiometry on the electrical conductivity and conduction mechanisms in the perovskite oxide Na0.5Bi0.5TiO3. Chem. Mater. 2015, 27, 629–634. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, J.; Yan, X.; Zhou, M.; Tang, P.; Liang, T.; Li, W. Effects of Bi deficiency on the microstructural and conductive properties of Na0.5Bi0.5TiO3 (NBT) perovskite. Solid State Ion. 2017, 209, 152–162. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, M.; Shi, J.; Liang, T.; Zeng, J.; Yan, X.; Luo, N.; Li, W.; Wei, Y. Microstructure and electrical conductivity of A-site fully stoichiometric Na0.5+xBi0.5−xTiO3−δ with different Na/Bi ratios. Ceram. Int. 2019, 45, 11438–11447. [Google Scholar] [CrossRef]
- Shi, J.; Liu, X.; Zhu, F.; Tian, W.; Xia, Y.; Li, T.; Rao, R.; Zhang, T.; Liu, L. Oxygen vacancy migration and its lattice structural origin in A-site non-stoichiometric bismuth sodium titanate perovskites. J. Mater. 2022, 8, 719–729. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Li, L.; Reaney, I.M.; Sinclair, D.C. High ionic conductivity with low degradation in A-site strontium-doped nonstoichiometric sodium bismuth titanate perovskite. Chem. Mater. 2016, 28, 5269–5273. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wu, P.; Sinclair, D.C. Enhanced bulk conductivity of A-site divalent acceptor-doped non-stoichiometric sodium bismuth titanate. Solid State Ion. 2017, 299, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Li, M.; Li, L.; Wu, P.; Pradal-Velázquez, E.; Sinclair, D.C. Optimisation of oxide-ion conductivity in acceptor-doped Na0.5Bi0.5TiO3 perovskite: Approaching the limit? J. Mater. Chem. A 2017, 5, 21658–21662. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, R.; Omar, S. Electrical conductivity study of B-site Ga doped non-stoichiometric sodium bismuth titanate ceramics. J. Alloy. Compd. 2018, 746, 54–61. [Google Scholar] [CrossRef]
- Li, M.Y.; He, C.; Wang, W.G.; Hao, G.L.; Li, X.Y.; Liu, T.; Wang, X.F.; Wang, D. Investigation of Ga doping for non-stoichiometric sodium bismuth titanate ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 16104–16112. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, R.; Singh, P. Polyol-mediated synthesis of Bi-deficient Mg2+-doped sodium bismuth titanate and study of oxide ion migration behavior with functional properties. J. Alloy. Compd. 2021, 860, 158492. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Das, S.; Omar, S. High ionic conductivity of Mg2+-doped non-stoichiometric sodium bismuth titanate. Acta Mater. 2018, 159, 8–15. [Google Scholar] [CrossRef]
- Lu, Y.; López, C.A.; Wang, J.; Alonso, J.A.; Sun, C. Insight into the structure and functional application of Mg-doped Na0.5Bi0.5TiO3 electrolyte for solid oxide fuel cells. J. Alloy. Compd. 2018, 752, 213–219. [Google Scholar] [CrossRef]
- Koch, L.; Steiner, S.; Meyer, K.; Seo, I.; Albe, K.; Frömling, T. Ionic conductivity of acceptor doped sodium bismuth titanate: Influence of dopants, phase transitions and defect associates. J. Mater. Chem. C 2017, 5, 8958–8965. [Google Scholar] [CrossRef]
- Steiner, S.; Seo, I.; Ren, P.; Li, M.; Keeble, D.J.; Frömling, T. The effect of Fe-acceptor doping on the electrical properties of Na1/2Bi1/2TiO3 and 0.94(Na1/2Bi1/2)TiO3-0.06BaTiO3. J. Am. Ceram. Soc. 2019, 102, 5295–5304. [Google Scholar] [CrossRef] [Green Version]
- Groszewicz, P.B.; Koch, L.; Steiner, S.; Ayrikyan, A.; Webber, K.G.; Frömling, T.; Albe, K.; Buntkowsky, G. The fate of aluminium in (Na,Bi)TiO3-based ionic conductors. J. Mater. Chem. A 2020, 8, 18188–18197. [Google Scholar] [CrossRef]
- Shih, D.P.C.; Aguadero, A.; Skinner, S.J. Improvement of ionic conductivity in A-site lithium doped sodium bismuth titanate. Solid State Ion. 2018, 317, 32–38. [Google Scholar] [CrossRef]
- Shih, D.P.C.; Aguadero, A.; Skinner, S.J. A-site acceptor-doping strategy to enhance oxygen transport in sodium-bismuth-titanate perovskite. J. Am. Ceram. Soc. 2023, 106, 100–108. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Hu, H.; Du, H.; Shi, J. Ionic conductive and dielectric properties of samarium isovalent doping in non-stoichiometric bismuth sodium titanate perovskite. Ionics 2019, 25, 2729–2734. [Google Scholar] [CrossRef]
- Shi, J.; Rao, R.; Tian, W.; Xu, X.; Liu, X. Anomalous electrical performance of A-site double-bivalent-doped Bi0.49Na0.5TiO3-δ ceramics from nominal oxygen deficiency to excess. Ceram. Int. 2022, 48, 5210–5216. [Google Scholar] [CrossRef]
- Liu, X.; Du, H.; Shi, J.; Hu, H.; Hao, X. Ionic conduction and anomalous diffusion in Sr and Ga acceptor co-doped bismuth sodium titanate solid solutions. ECS J. Solid State Sci. Technol. 2018, 7, N96–N100. [Google Scholar] [CrossRef]
- He, X.; Mo, Y. Accelerated materials design of Na0.5Bi0.5TiO3 oxygen ionic conductors based on first principles calculations. Phys. Chem. Chem. Phys. 2015, 17, 18035–18044. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Albe, K. Influence of phase transitions and defect associates on the oxygen migration in the ion conductor Na1/2Bi1/2TiO3. J. Mater. Chem. A 2017, 5, 4368–4375. [Google Scholar] [CrossRef]
- Koch, L.; Steiner, S.; Hoang, A.; Klomp, A.J.; Albe, K.; Frömling, T. Revealing the impact of acceptor dopant type on the electrical conductivity of sodium bismuth titanate. Acta Mater. 2022, 229, 117808. [Google Scholar] [CrossRef]
- Ranjan, R.; Garg, R.; Kothai, V.; Agrawal, A.; Senyshyn, A.; Boysen, H. Phases in the (1−x)Na0.5Bi0.5TiO3-(x)CaTiO3 system. J. Phys. Condens. Matter 2010, 22, 075901. [Google Scholar] [CrossRef]
- Roukos, R.; Zaiter, N.; Chaumont, D. Relaxor behaviour and phase transition of perovskite ferroelectrics-type complex oxides (1−x)Na0.5Bi0.5TiO3−xCaTiO3 system. J. Adv. Ceram. 2018, 7, 124–142. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, H.; Inaba, H.; Matsuyama, M.; Lan, N.G.; Mokiya, M.; Tagawa, H. Structural consideration on the ionic conductivity of perovskite-type oxides. Solid State Ion. 1999, 122, 1–15. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Drelle, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR 86-748 (2004); Los Alamos National Laboratory: Los Alamos, NM, USA, 2004. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 31, 751–767. [Google Scholar] [CrossRef]
- Jones, G.O.; Thomas, P.A. Investigation of the structure and phase transitions in the novel A-site substituted distorted perovskite compound Na0.5Bi0.5TiO3. Acta Crystallogr. Sect. B 2002, 58, 168–178. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Masó, N.; West, A.R. Electronic conductivity in yttria-stabilized zirconia under a small dc bias. Chem. Mater. 2015, 27, 1552–1558. [Google Scholar] [CrossRef]
- MacDonald, J.R. Impedance Spectroscopy—Emphasizing Solid Materials and Systems; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Fleig, J. The grain boundary impedance of random microstructures: Numerical simulations and implications for the analysis of experimental data. Solid State Ion. 2002, 150, 181–193. [Google Scholar] [CrossRef]
- Grimes, N.W.; Grimes, R.W. Dielectric polarizability of ions and the corresponding effective number of electrons. J. Phys. Condens. Matter 1998, 10, 3029–3034. [Google Scholar] [CrossRef]
- Beskow, G. V. M. Goldschmidt: Geochemische Verteilungsgesetze der Elemente. Geol. Fören. I Stockh. Förh. 1924, 46, 738–743. [Google Scholar] [CrossRef]
- Kilner, J.A.; Brook, R.J. A study of oxygen ion conductivity in doped non-stoichiometric oxides. Solid State Ion. 1982, 6, 237–252. [Google Scholar] [CrossRef]
- Yang, F.; Wu, P.; Sinclair, D.C. Suppression of electrical conductivity and switching of conduction mechanisms in ‘stoichiometric’ (Na0.5Bi0.5TiO3)1−x(BiAlO3)x (0 ≤ x ≤ 0.08) solid solutions. J. Mater. Chem. C 2017, 5, 7243–7252. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wu, P.; Sinclair, D.C. Electrical conductivity and conduction mechanism in (Na0.5Bi0.5TiO3)1−x(BiScO3)x (0.00 ≤ x ≤ 0.35) solid solutions. J. Mater. Chem. C 2018, 6, 11598–11607. [Google Scholar] [CrossRef]
- Dawson, J.A.; Chen, H.; Tanaka, I. Crystal structure, defect chemistry and oxygen ion transport of the ferroelectric perovskite, Na0.5Bi0.5TiO3: Insights from the first principles calculations. J. Mater. Chem. A 2015, 3, 16574–16582. [Google Scholar] [CrossRef]
- Islam, M.S. Ionic transport in ABO3 perovskite oxides: A computer modelling tour. J. Mater. Chem. 2000, 10, 1027–1038. [Google Scholar] [CrossRef]
- Arachi, Y.; Sakai, H.; Yamamoto, O.; Takeda, Y.; Imanishai, N. Electrical conductivity of the ZrO2-Ln2O3 (Ln = lanthanides) system. Solid State Ionics 1999, 121, 133–139. [Google Scholar] [CrossRef]

| x | Space Group | Lattice Parameters | Fitting Qualities | |||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | V (Å3) | wRp (%) | χ2 | ||
| 0 | R3c | 5.4901 | 13.5137 | 352.75 | 9.83 | 2.14 |
| 0.02 | R3c | 5.4903 | 13.4890 | 352.13 | 6.15 | 1.59 |
| 0.04 | R3c | 5.4957 | 13.4425 | 351.61 | 7.61 | 1.31 |
| 0.06 | R3c | 5.4931 | 13.4416 | 351.25 | 9.01 | 1.59 |
| 0.08 | R3c | 5.4902 | 13.4280 | 350.52 | 9.78 | 2.17 |
| x | δ | (%) | t | Vsf | rC (Å) |
|---|---|---|---|---|---|
| 0 | 0.015 | 0.5 | 0.9792 | 0.1949 | 0.9071 |
| 0.02 | 0.025 | 0.83 | 0.9789 | 0.1969 | 0.9074 |
| 0.04 | 0.035 | 1.17 | 0.9785 | 0.1988 | 0.9076 |
| 0.06 | 0.045 | 1.5 | 0.9781 | 0.2008 | 0.9079 |
| 0.08 | 0.055 | 1.83 | 0.9778 | 0.2027 | 0.9082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Hu, Y.; Hu, Q.; Wu, P.; Sinclair, D.C. Electrical and Dielectric Properties of Ca-Doped Bi-Deficient Sodium Bismuth Titanate Na0.5Bi0.49−xCaxTiO3−δ (0 ≤ x ≤ 0.08). Crystals 2022, 12, 1800. https://doi.org/10.3390/cryst12121800
Yang F, Hu Y, Hu Q, Wu P, Sinclair DC. Electrical and Dielectric Properties of Ca-Doped Bi-Deficient Sodium Bismuth Titanate Na0.5Bi0.49−xCaxTiO3−δ (0 ≤ x ≤ 0.08). Crystals. 2022; 12(12):1800. https://doi.org/10.3390/cryst12121800
Chicago/Turabian StyleYang, Fan, Yidong Hu, Qiaodan Hu, Patrick Wu, and Derek C. Sinclair. 2022. "Electrical and Dielectric Properties of Ca-Doped Bi-Deficient Sodium Bismuth Titanate Na0.5Bi0.49−xCaxTiO3−δ (0 ≤ x ≤ 0.08)" Crystals 12, no. 12: 1800. https://doi.org/10.3390/cryst12121800
APA StyleYang, F., Hu, Y., Hu, Q., Wu, P., & Sinclair, D. C. (2022). Electrical and Dielectric Properties of Ca-Doped Bi-Deficient Sodium Bismuth Titanate Na0.5Bi0.49−xCaxTiO3−δ (0 ≤ x ≤ 0.08). Crystals, 12(12), 1800. https://doi.org/10.3390/cryst12121800








