Fast Synthesis of Organic Copper Halide Crystals for X-ray Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
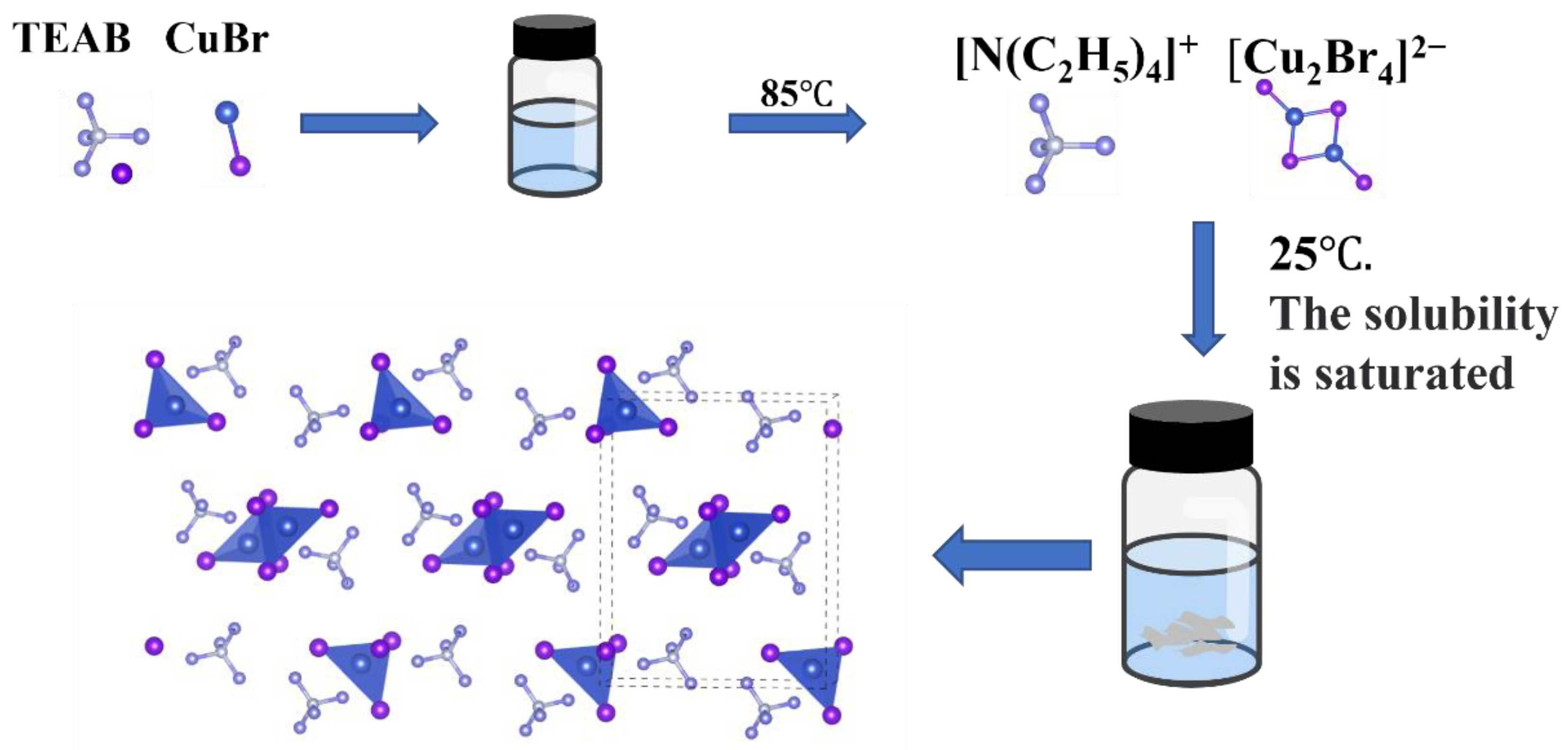
2.2. Synthesis of TEA2Cu2Br4 Crystals
2.3. Preparation of the TEA2Cu2Br4 Scintillation Screen
2.4. Characterization
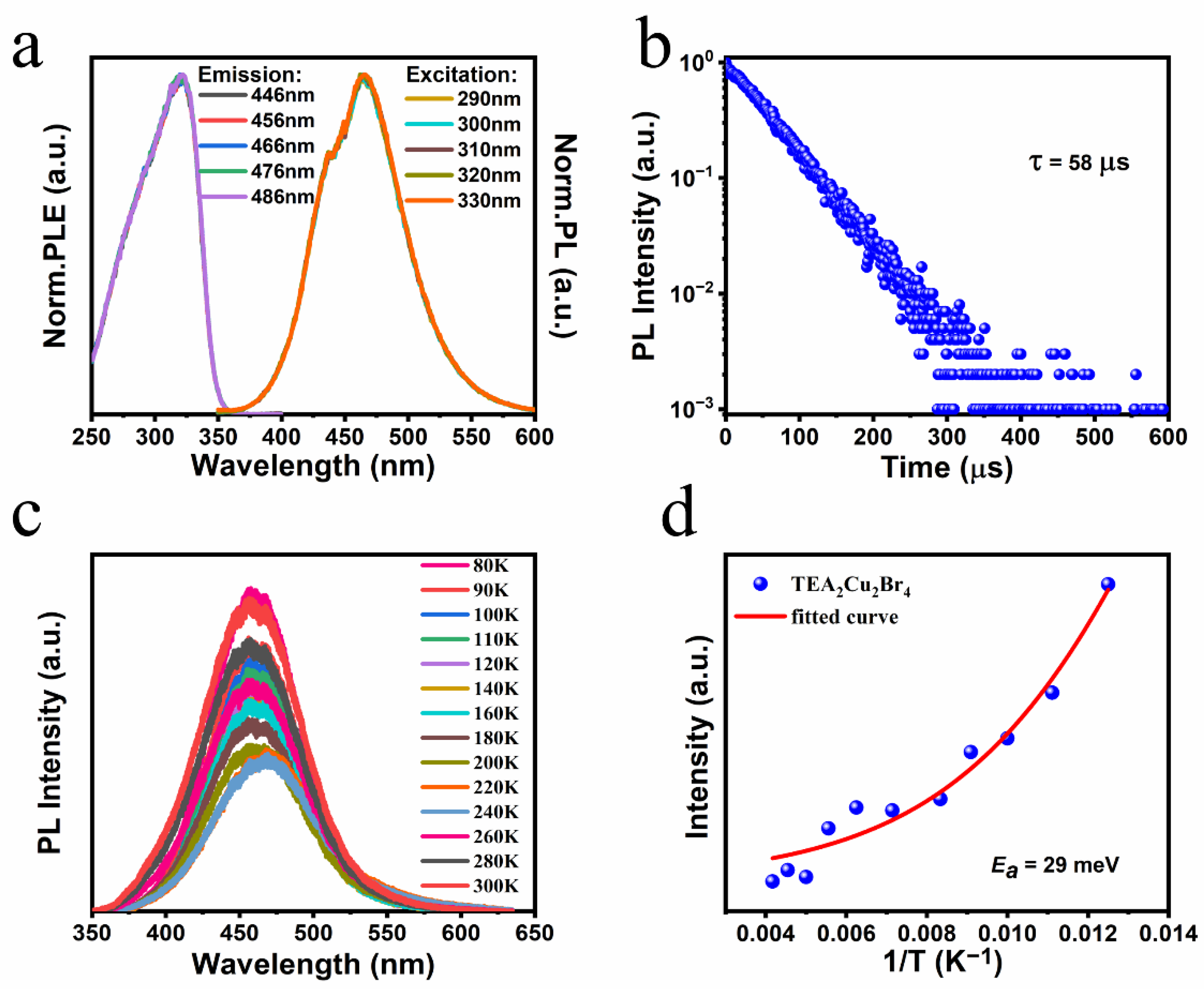
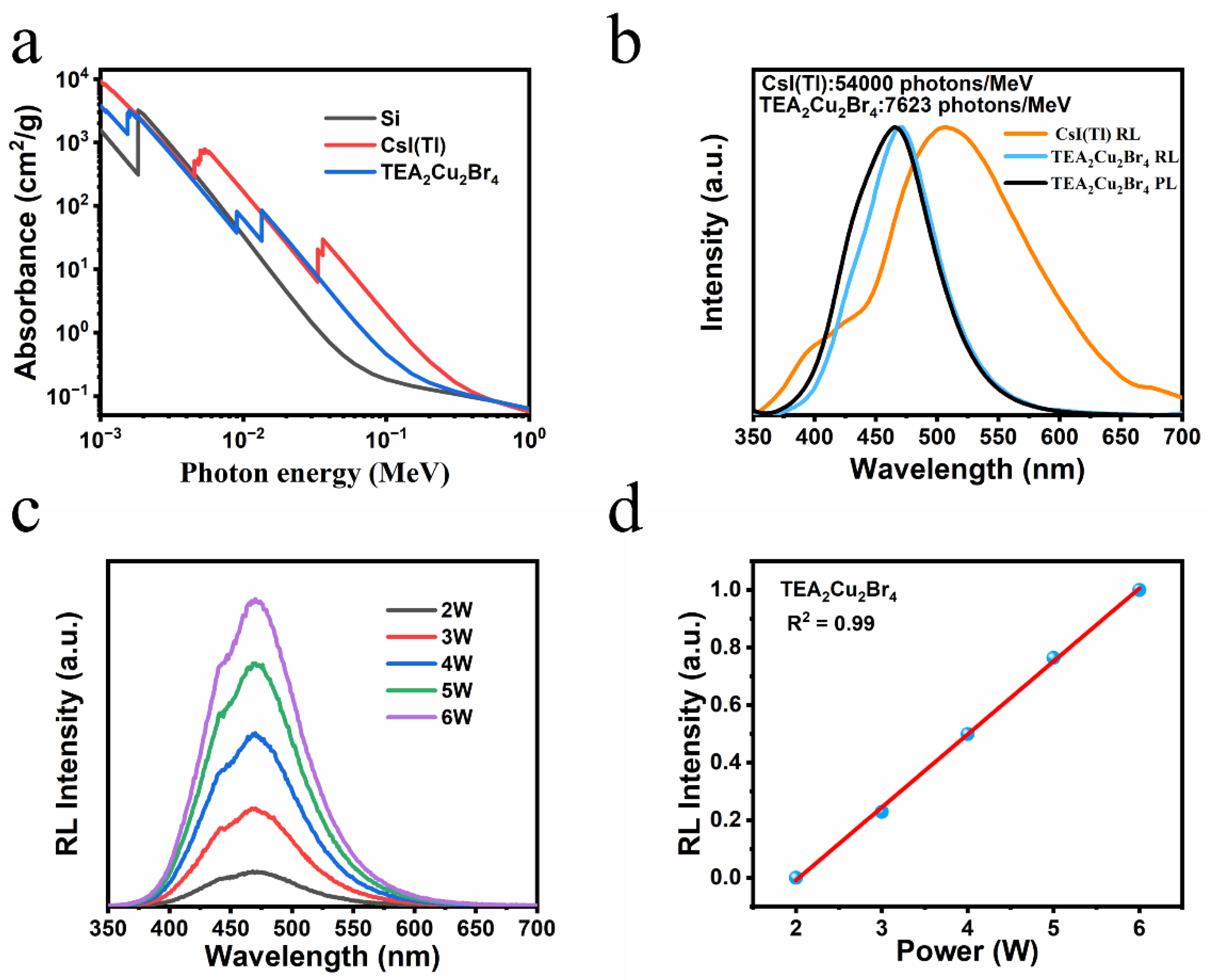
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.J.N. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef]
- Dujardin, C.; Auffray, E.; Bourret-Courchesne, E.; Dorenbos, P.; Lecoq, P.; Nikl, M.; Vasil’Ev, A.; Yoshikawa, A.; Zhu, R.-Y. Needs, trends, and advances in inorganic scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 1977–1997. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhao, L.; Deng, Y.; Xiao, X.; Ni, Z.; Xu, S.; Huang, J.J.N.P. Perovskite-filled membranes for flexible and large-area direct-conversion X-ray detector arrays. Nat. Photon. 2020, 14, 612–617. [Google Scholar] [CrossRef]
- Zhou, J.; An, K.; He, P.; Yang, J.; Zhou, C.; Luo, Y.; Kang, W.; Hu, W.; Feng, P.; Zhou, M.J.A.O.M. Solution-Processed Lead-Free Perovskite Nanocrystal Scintillators for High-Resolution X-Ray CT Imaging. Adv. Opt. Mater. 2021, 9, 2002144. [Google Scholar] [CrossRef]
- Liu, J.; Shabbir, B.; Wang, C.; Wan, T.; Ou, Q.; Yu, P.; Tadich, A.; Jiao, X.; Chu, D.; Qi, D.J.A.M. Flexible, printable soft-X-ray detectors based on all-inorganic perovskite quantum dots. Adv. Mater. 2019, 31, 1901644. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Shin, D.H.; Park, J.K.; Kim, D.H.; Lee, S.J.; Im, S.H.J.A.M. High-performance next-generation perovskite nanocrystal scintillator for nondestructive X-ray imaging. Adv. Mater. 2018, 30, 1801743. [Google Scholar] [CrossRef]
- Creason, T.D.; McWhorter, T.M.; Bell, Z.; Du, M.-H.; Saparov, B. K2CuX3 (X= Cl, Br): All-inorganic lead-free blue emitters with near-unity photoluminescence quantum yield. Chem. Mater. 2020, 32, 6197–6205. [Google Scholar] [CrossRef]
- Yang, B.; Yin, L.; Niu, G.; Yuan, J.H.; Xue, K.H.; Tan, Z.; Miao, X.S.; Niu, M.; Du, X.; Song, H. Lead-Free Halide Rb2CuBr3 as Sensitive X-Ray Scintillator. Adv. Mater. 2019, 31, 1904711. [Google Scholar] [CrossRef]
- Lian, L.; Zheng, M.; Zhang, W.; Yin, L.; Du, X.; Zhang, P.; Zhang, X.; Gao, J.; Zhang, D.; Gao, L. Efficient and Reabsorption-Free Radioluminescence in Cs3Cu2I5 Nanocrystals with Self-Trapped Excitons. Adv. Sci. 2020, 7, 2000195. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liu, S.; Chen, W.; Li, H.; Yan, S.; Yu, J.; Liu, C.; Zhao, L.; Zeng, T.; Han, T. Efficient and self-healing copper halide Cs3Cu2Cl5 film assisted by microwave treatment for high-resolution X-ray imaging. Ceram. Int. 2022, 48, 25086–25093. [Google Scholar] [CrossRef]
- Cheng, S.; Beitlerova, A.; Kucerkova, R.; Nikl, M.; Ren, G.; Wu, Y. Zero-Dimensional Cs3Cu2I5 Perovskite Single Crystal as Sensitive X-Ray and γ-Ray Scintillator. Phys. Status Solidi (RRL) Rapid Res. Lett. 2020, 14, 2000374. [Google Scholar] [CrossRef]
- Fang, S.; Li, H.; Xie, Y.; Li, H.; Wang, Y.; Shi, Y.J.S. Zero-Dimensional Organic–Inorganic Hybrid Copper-Based Halides with Highly Efficient Orange–Red Emission. Small 2021, 17, 2103831. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-J.; Lin, X.; He, Q.; Worku, M.; Ma, B. Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; He, T.; Yang, H.; Yang, C.; Shao, B.; Gutiérrez-Arzaluz, L.; Bakr, O.M.; Zhang, Y.; Mohammed, O. Large-Area Perovskite-Related Copper Halide Film for High-Resolution Flexible X-ray Imaging Scintillation Screens. ACS Energy Lett. 2022, 7, 844–846. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhao, F.; Gao, Y.; Chen, R.; He, T. Optical Properties of a CsMnBr3 Single Crystal. ACS omega 2022, 7, 29415–29419. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, F.; Zhu, C.; Li, J.; Lv, X.; Xing, G.; Wei, Q.; Wang, G.; Dai, J.; Dong, H. Near-unity blue luminance from lead-free copper halides for light-emitting diodes. Nano Energy 2022, 91, 106664. [Google Scholar] [CrossRef]
- Asplund, M.; Jagner, S.; Hamalainen, R.; Kohl, F.; Seip, R. Crystal Structure of bis-(tetraethylammonium) di-mu-bromo-dibromodicuprate (I),[N-(C2H5) 4] 2 [Cu2Br4]. Acta Chem. Scand. A 1984, 38, 135–139. [Google Scholar] [CrossRef]
- Lian, L.; Wang, X.; Zhang, P.; Zhu, J.; Zhang, X.; Gao, J.; Wang, S.; Liang, G.; Zhang, D.; Gao, L. Highly luminescent zero-dimensional organic copper halides for X-ray scintillation. J. Phys. Chem. Lett. 2021, 12, 6919–6926. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Ning, Y.; Chen, H.; Guo, C.; Cui, J.; Peng, G.; Ci, Z.; Jin, Z. Environmentally stable one-dimensional copper halide based ultra-flexible composite film for low-cost X-ray imaging screens. Chem. Eng. J. 2022, 430, 132826. [Google Scholar] [CrossRef]
- Wang, B.; Peng, J.; Yang, X.; Cai, W.; Xiao, H.; Zhao, S.; Lin, Q.; Zang, Z.J.L.; Reviews, P. Template Assembled Large-Size CsPbBr3 Nanocomposite Films toward Flexible, Stable, and High-Performance X-Ray Scintillators. Laser Photonics Rev. 2022, 16, 2100736. [Google Scholar] [CrossRef]
- Li, N.; Xu, Z.; Xiao, Y.; Liu, Y.; Yang, Z.; Liu, S. Flexible, High Scintillation Yield Cu3Cu2I5 Film Made of Ball-Milled Powder for High Spatial Resolution X-Ray Imaging. Adv. Opt. Mater. 2022, 10, 2102232. [Google Scholar] [CrossRef]
- Birowosuto, M.; Cortecchia, D.; Drozdowski, W.; Brylew, K.; Lachmanski, W.; Bruno, A.; Soci, C. X-ray scintillation in lead halide perovskite crystals. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, L.; Liu, B.; Jin, P.; Gao, R.; Zhou, L.; Wan, P.; Xu, Q.; Ouyang, X. Scintillation performance of two-dimensional perovskite (BA) 2 PbBr 4 microcrystals. J. Mater. Chem. C 2021, 9, 17124–17128. [Google Scholar] [CrossRef]
- Hu, X.; Yan, P.; Ran, P.; Lu, L.; Leng, J.; Yang, Y.M.; Li, X. In Situ Fabrication of Cs3Cu2I5: Tl Nanocrystal Films for High-Resolution and Ultrastable X-ray Imaging. J. Phys. Chem. Lett. 2022, 13, 2862–2870. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, X.; Wu, L.; Liu, J.; Lin, T.; Zeng, R. Fast Synthesis of Organic Copper Halide Crystals for X-ray Imaging. Crystals 2022, 12, 1799. https://doi.org/10.3390/cryst12121799
Bin X, Wu L, Liu J, Lin T, Zeng R. Fast Synthesis of Organic Copper Halide Crystals for X-ray Imaging. Crystals. 2022; 12(12):1799. https://doi.org/10.3390/cryst12121799
Chicago/Turabian StyleBin, Xiangshi, Lingshi Wu, Jiaxing Liu, Tao Lin, and Ruosheng Zeng. 2022. "Fast Synthesis of Organic Copper Halide Crystals for X-ray Imaging" Crystals 12, no. 12: 1799. https://doi.org/10.3390/cryst12121799
APA StyleBin, X., Wu, L., Liu, J., Lin, T., & Zeng, R. (2022). Fast Synthesis of Organic Copper Halide Crystals for X-ray Imaging. Crystals, 12(12), 1799. https://doi.org/10.3390/cryst12121799






