Numerical Modelling for the Experimental Improvement of Growth Uniformity in a Halide Vapor Phase Epitaxy Reactor for Manufacturing β-Ga2O3 Layers
Abstract
:1. Introduction
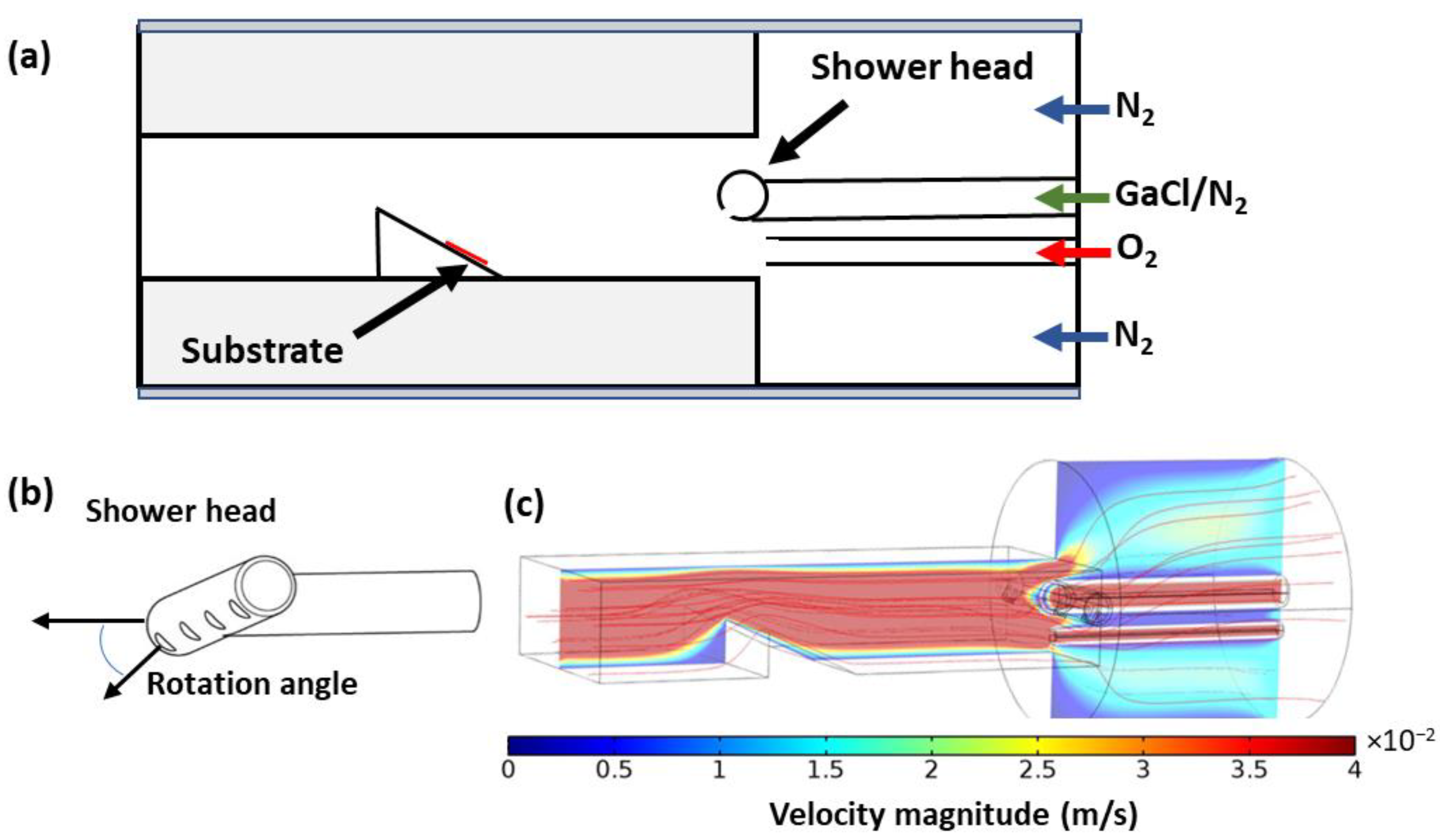
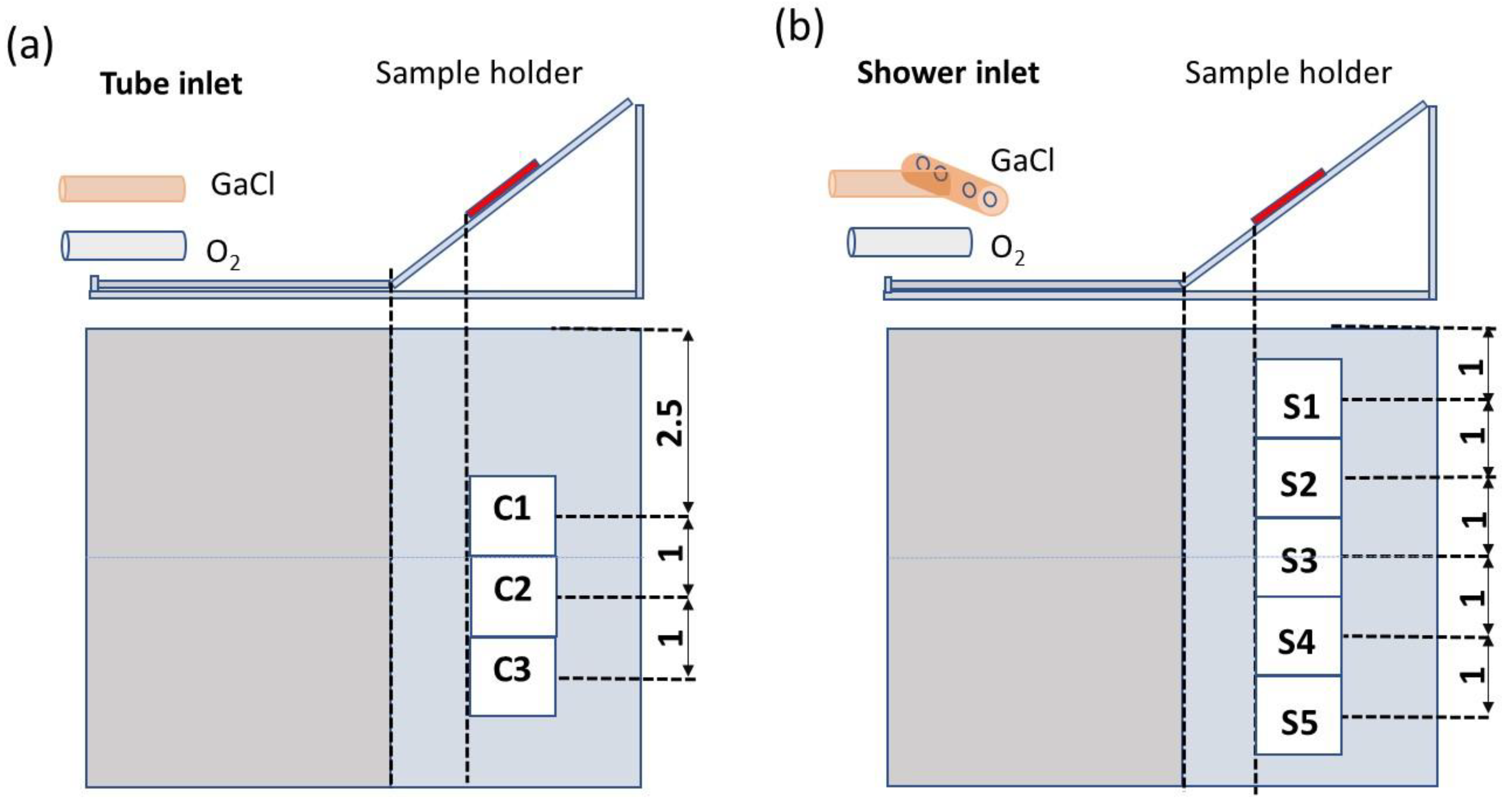
2. Materials and Methods
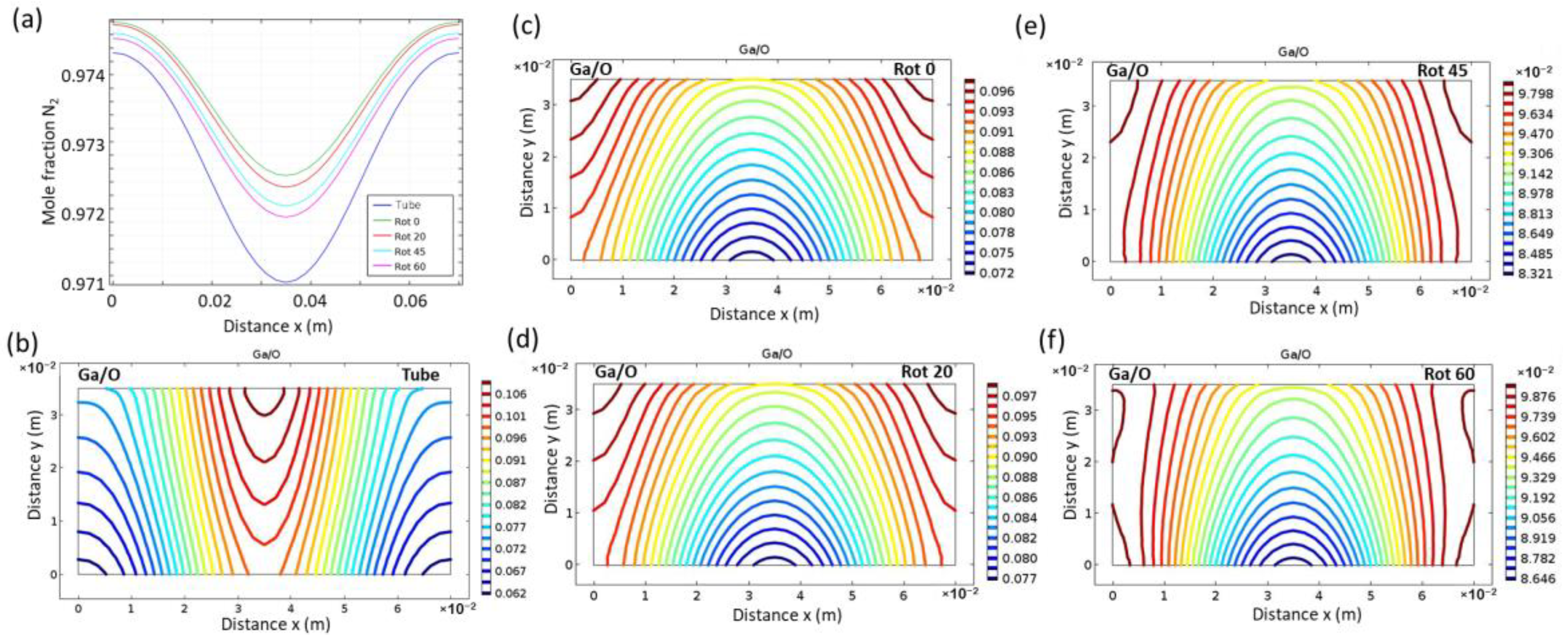
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearton, S.J.; Yang, J.; Cary, P.H., IV; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef] [Green Version]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-bandgap semiconductors: Research opportunities and challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef] [Green Version]
- Higashiwaki, M. Chapter One-Fundamental technologies for gallium oxide transistors. Semicond. Semimet. 2021, 107, 1–22. [Google Scholar]
- Sasaki, K.; Kuramata, A.; Masui, T.; Villora, E.G.; Shimamura, K.; Yamakoshi, S. Device-quality β-Ga2O3 epitaxial films fabricated by ozone molecular beam epitaxy. Appl. Phys. Express 2012, 5, 035502. [Google Scholar] [CrossRef]
- Oshima, T.; Okuno, T.; Fujita, S. Ga2O3 thin film growth on c-plane sapphire substrates by molecular beam epitaxy for deep-ultraviolet photodetectors. Jpn. J. Appl. Phys. 2007, 46, 7217–7220. [Google Scholar] [CrossRef]
- Sasaki, K.; Higashiwaki, M.; Kuramata, A.; Masui, T.; Yamakoshi, S. Growth temperature dependences of structural and electrical properties of Ga2O3 epitaxial films grown on β-Ga2O3 (010) substrates by molecular beam epitaxy. J. Cryst. Growth 2014, 392, 30–33. [Google Scholar] [CrossRef]
- Park, J.H.; McClintock, R.; Jaud, A.; Dehzangi, A.; Razeghi, M. MOCVD grown β-Ga2O3 metal-oxide-semiconductor field effect transistors on sapphire. Appl. Phys. Exp. 2019, 12, 095503. [Google Scholar] [CrossRef]
- Du, X.; Mi, W.; Luan, C.; Li, Z.; Xia, C.; Ma, J. Characterization of homoepitaxial β-Ga2O3 films prepared by metal–organic chemical vapor deposition. J. Cryst. Growth 2014, 404, 75–79. [Google Scholar] [CrossRef]
- Baldini, M.; Albrecht, M.; Fiedler, A.; Irmscher, K.; Klimm, D.; Schewski, R.; Wagner, G. Semiconducting Sn-doped β-Ga2O3 homoepitaxial layers grown by metal organic vapour-phase epitaxy. J. Mater. Sci. 2016, 51, 3650–3656. [Google Scholar] [CrossRef]
- Oshima, Y.; Villora, E.G.; Shimamura, K. Quasi-heteroepitaxial growth of β-Ga2O3 on off-angled sapphire (0001) substrates by halide vapor phase epitaxy. J. Cryst. Growth 2015, 410, 53–58. [Google Scholar] [CrossRef]
- Xiong, Z.N.; Xiu, X.Q.; Li, Y.W.; Hua, X.M.; Xie, Z.L.; Chen, P.; Liu, B.; Han, P.; Zhang, R.; Zheng, Y.D. Growth of β-Ga2O3 films on sapphire by hydride vapor phase epitaxy. Chin. Phys. Lett. 2018, 35, 058101. [Google Scholar] [CrossRef]
- Murakami, H.; Nomura, K.; Goto, K.; Sasaki, K.; Kawara, K.; Thieu, Q.T.; Togashi, R.; Kumagai, Y.; Higashiwaki, M.; Kuramata, A.; et al. Homoepitaxial growth of β-Ga2O3 layers by halide vapor phase epitaxy. Appl. Phys. Exp. 2015, 8, 015503. [Google Scholar] [CrossRef]
- Konishi, K.; Goto, K.; Togashi, R.; Murakami, H.; Higashiwaki, M.; Kuramata, A.; Yamakoshi, S.; Monemar, B.; Kumagai, Y. Comparison of O2 and H2O as oxygen source for homoepitaxial growth of β-Ga2O3 layers by halide vapor phase epitaxy. J. Cryst. Growth 2018, 492, 39–44. [Google Scholar] [CrossRef]
- Nikolaev, V.I.; Pechnikov, A.I.; Stepanov, S.I.; Nikitina, I.P.; Smirnov, A.N.; Chikiryaka, A.V.; Sharofidinov, S.S.; Bougrov, V.E.; Romanov, A.E. Epitaxial growth of (−201) β-Ga2O3 on (0001) sapphire substrates by halide vapour phase epitaxy. Mater. Sci. Semicon. Process. 2016, 47, 16–19. [Google Scholar] [CrossRef]
- Pozina, G.; Hsu, C.W.; Abrikossova, N.; Kaliteevski, M.A.; Hemmingsson, C. Development of β-Ga2O3 layers growth on sapphire substrates employing modeling of precursors ratio in halide vapor phase epitaxy reactor. Sci. Rep. 2020, 10, 22261. [Google Scholar] [CrossRef]
- Nakagomi, S.; Kokubun, Y. Crystal orientation of β-Ga2O3 thin films formed on c-plane and a-plane sapphire substrate. J. Cryst. Growth 2012, 349, 12–18. [Google Scholar] [CrossRef]
- Harwig, T.; Kellendonk, F.; Slappendel, S. The ultraviolet luminescence of β-galliumsesquioxide. J. Phys. Chem. Solids 1978, 39, 675–680. [Google Scholar] [CrossRef]
- Harwig, T.; Kellendonk, F. Some observations on the photoluminescence of doped β-galliumsesquioxide. J. Solid State Chem. 1978, 24, 255–263. [Google Scholar] [CrossRef]
- Villora, E.G.; Atou, T.; Sekiguchi, T.; Sugawara, T.; Kikuchi, M.; Fukuda, T. Cathodoluminescence of undoped β-Ga2O3 single crystals. Solid State Commun. 2001, 120, 455–458. [Google Scholar] [CrossRef]
- Onuma, T.; Fujioka, S.; Yamaguchi, T.; Higashiwaki, M.; Sasaki, K.; Masui, T.; Honda, T. Correlation between blue luminescence intensity and resistivity in β-Ga2O3 single crystals. Appl. Phys. Lett. 2013, 103, 041910. [Google Scholar] [CrossRef] [Green Version]
- Binet, L.; Gourier, D. Origin of the blue luminescence of β-Ga2O3. J. Phys. Chem. Solids 1998, 59, 1241–1249. [Google Scholar] [CrossRef]
- Ho, Q.D.; Frauenheim, T.; Deak, P. Origin of photoluminescence in β-Ga2O3. Phys. Rev. B 2018, 97, 115163. [Google Scholar] [CrossRef]

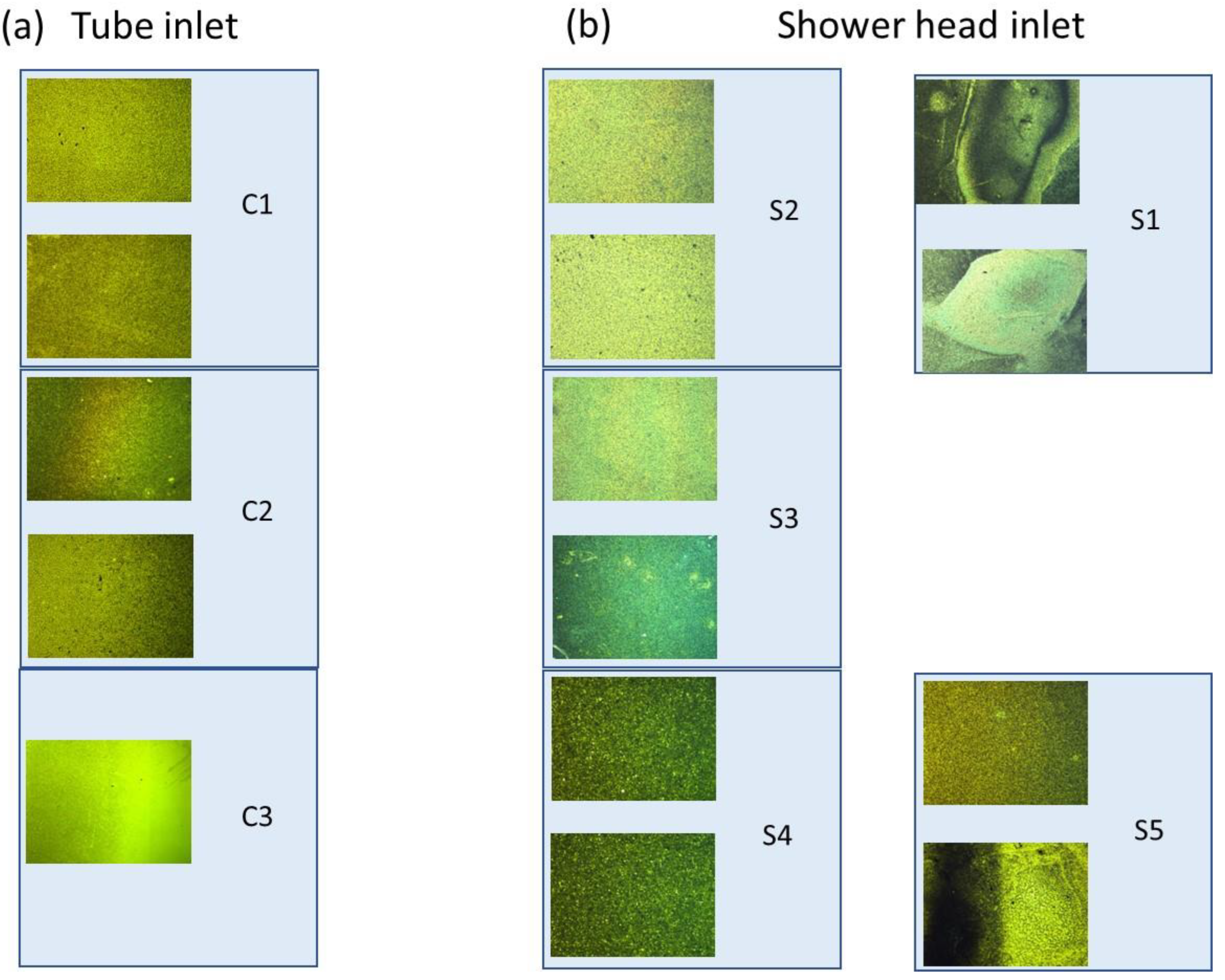
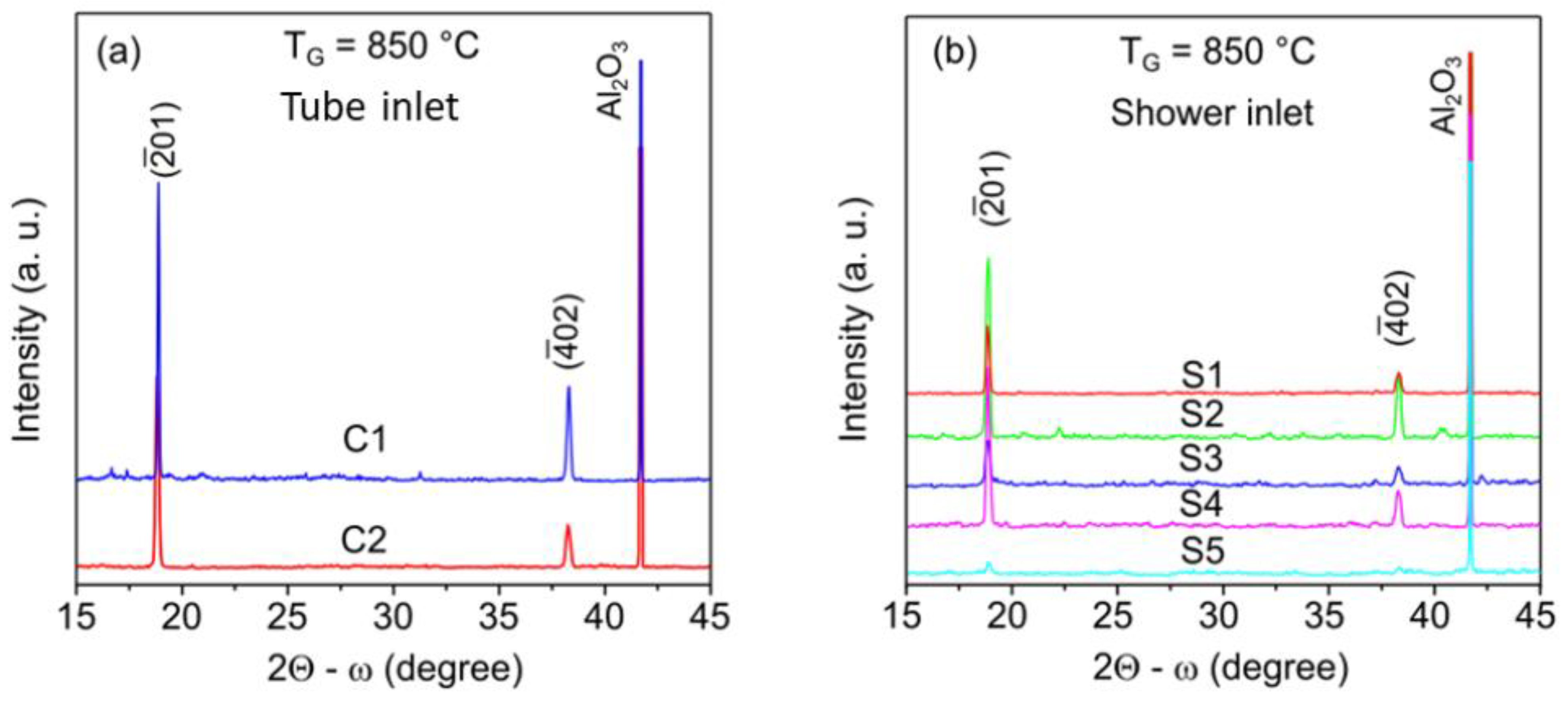
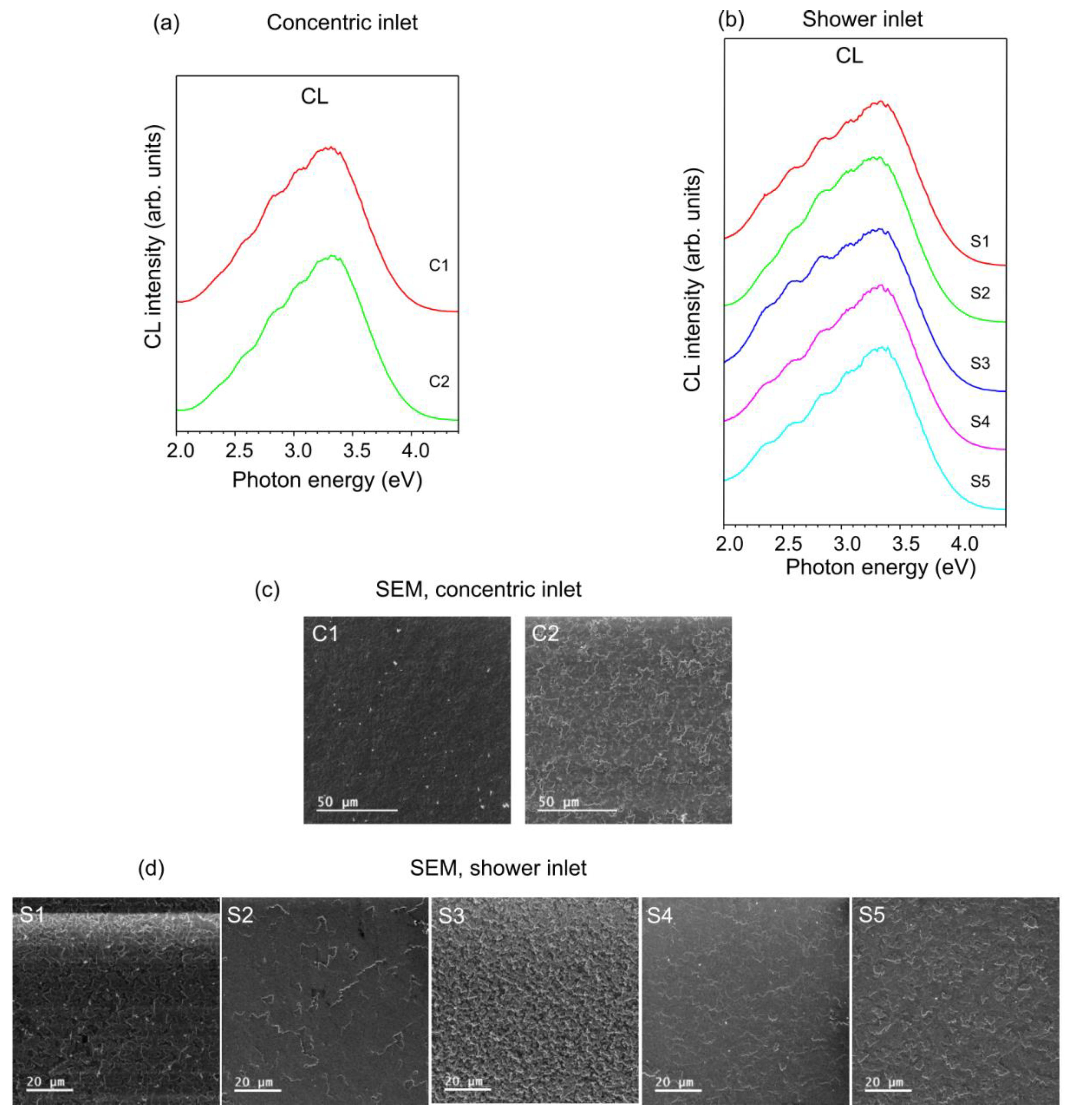
| Geometry | Sample | Thickness of Grown Layer, µm |
|---|---|---|
| Tube | C1 | 4.26 |
| C2 | 0.91 | |
| C3 | ~no growth | |
| Shower head | S1 | 1.06 |
| S2 | 1.62 | |
| S3 | 1.56 | |
| S4 | 1.47 | |
| S5 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozina, G.; Hsu, C.-W.; Abrikossova, N.; Hemmingsson, C. Numerical Modelling for the Experimental Improvement of Growth Uniformity in a Halide Vapor Phase Epitaxy Reactor for Manufacturing β-Ga2O3 Layers. Crystals 2022, 12, 1790. https://doi.org/10.3390/cryst12121790
Pozina G, Hsu C-W, Abrikossova N, Hemmingsson C. Numerical Modelling for the Experimental Improvement of Growth Uniformity in a Halide Vapor Phase Epitaxy Reactor for Manufacturing β-Ga2O3 Layers. Crystals. 2022; 12(12):1790. https://doi.org/10.3390/cryst12121790
Chicago/Turabian StylePozina, Galia, Chih-Wei Hsu, Natalia Abrikossova, and Carl Hemmingsson. 2022. "Numerical Modelling for the Experimental Improvement of Growth Uniformity in a Halide Vapor Phase Epitaxy Reactor for Manufacturing β-Ga2O3 Layers" Crystals 12, no. 12: 1790. https://doi.org/10.3390/cryst12121790





