Citrate-Capped AuNP Fabrication, Characterization and Comparison with Commercially Produced Nanoparticles
Abstract
1. Introduction
2. Experimental Work
2.1. Materials and Methods
2.2. Preparation of Gold Nanoparticles
2.3. Characterization
3. Results and Discussion
3.1. Characterization of AuNPs
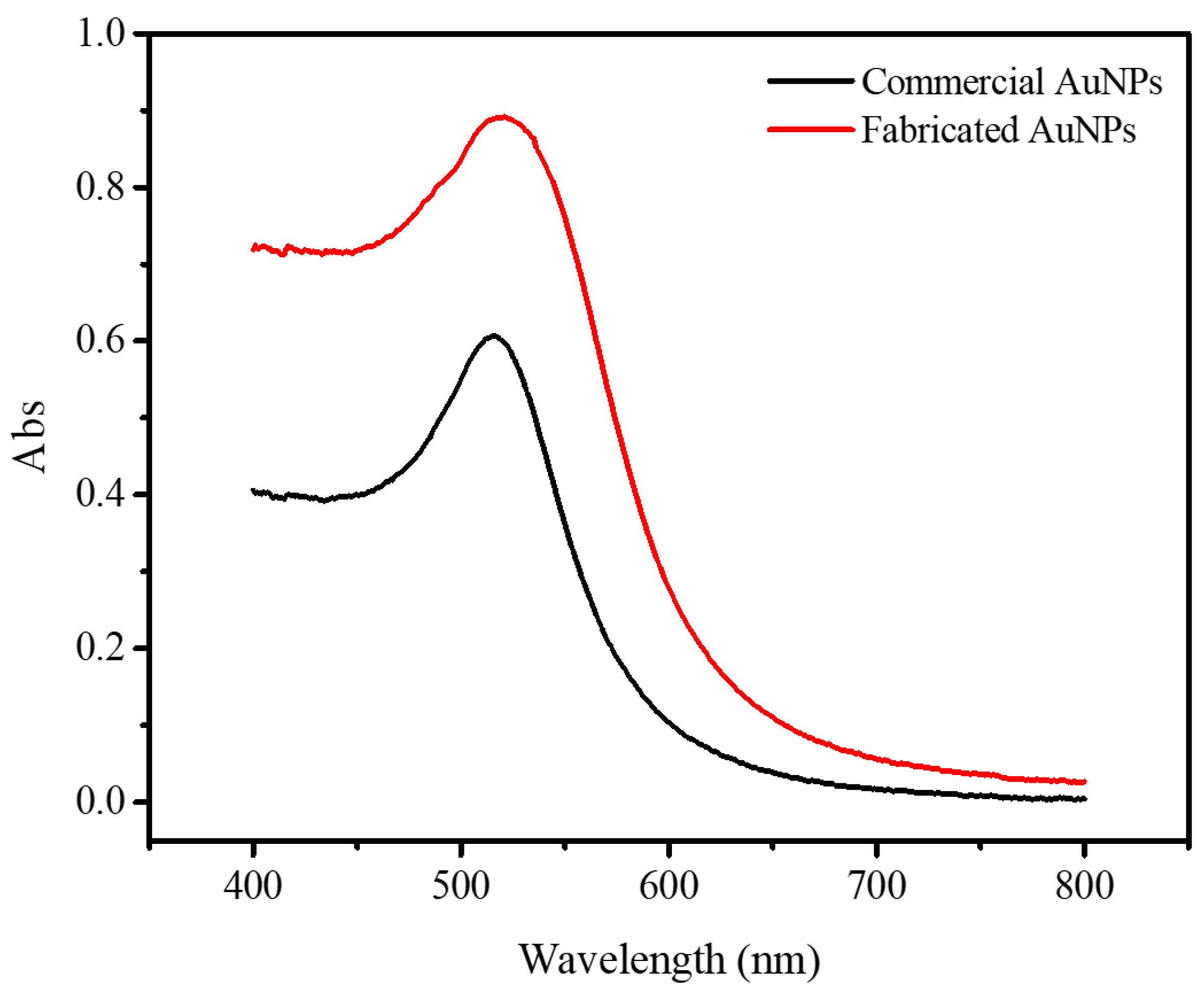
3.2. UV-Absorbance Spectra
3.3. Colorimetric Change for Dispersed vs. Aggregated AuNPs
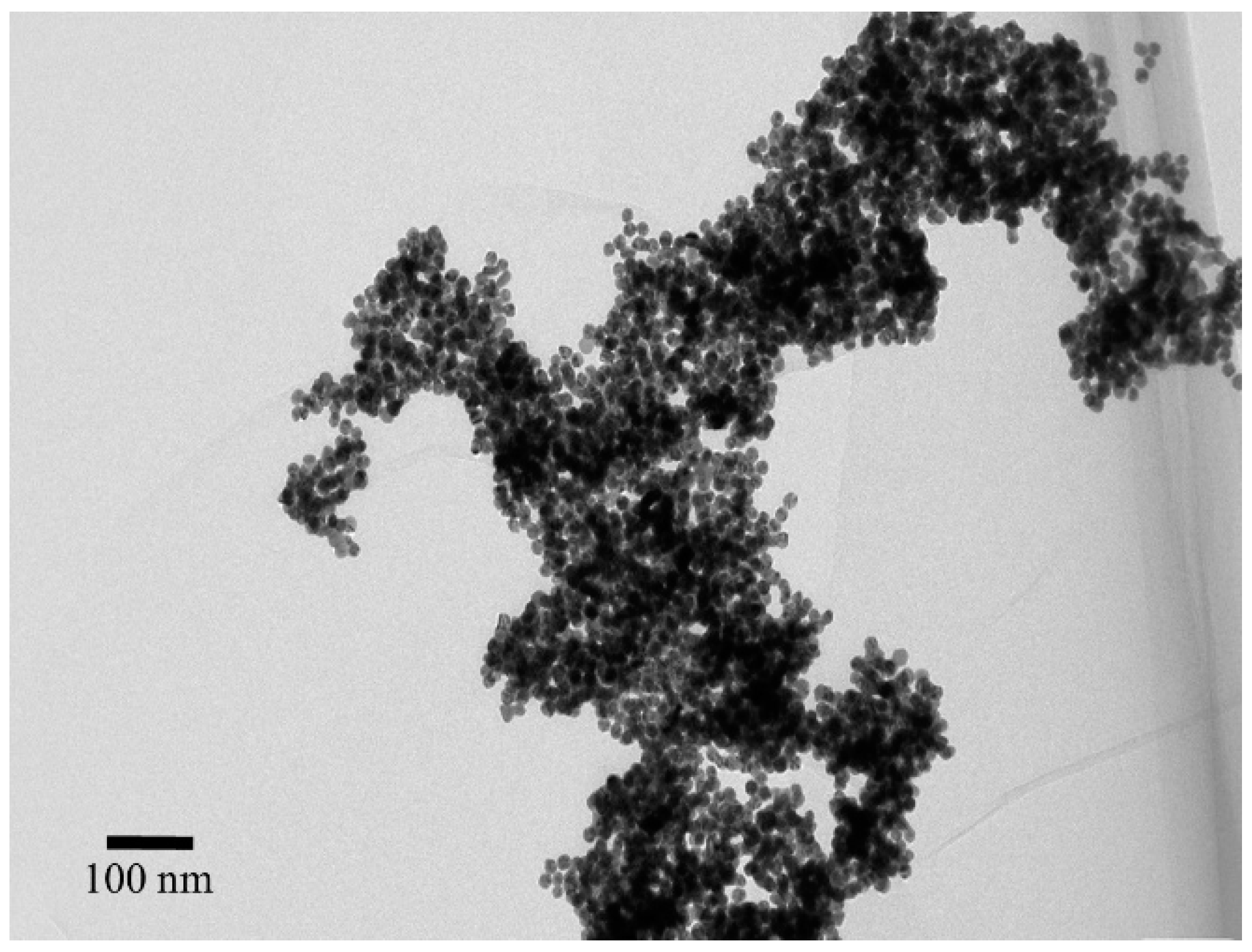
3.4. Size and Distribution Using SEM Change for Dispersed vs. Aggregated AuNPs
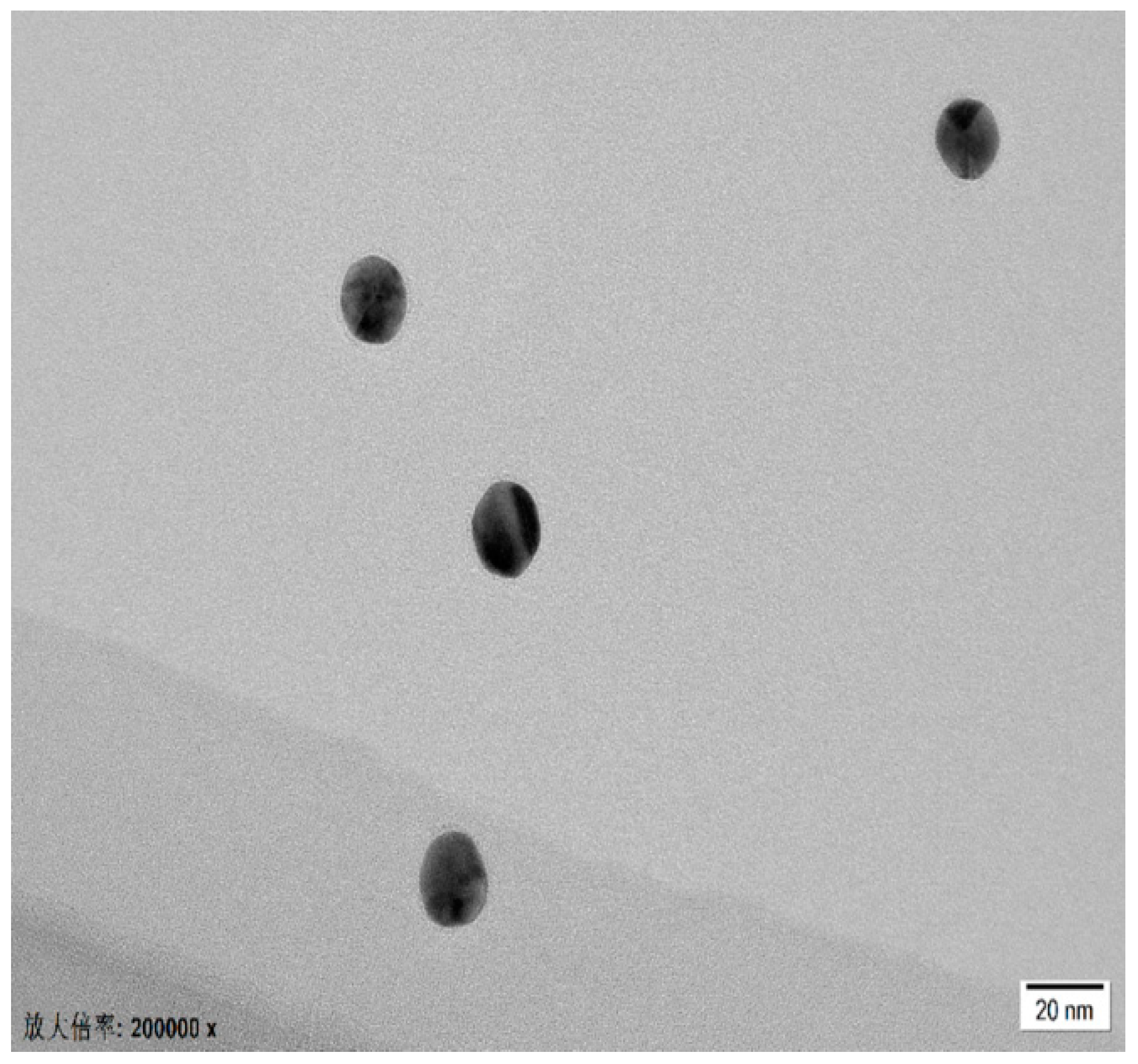
3.5. Characterization through HR-TEM
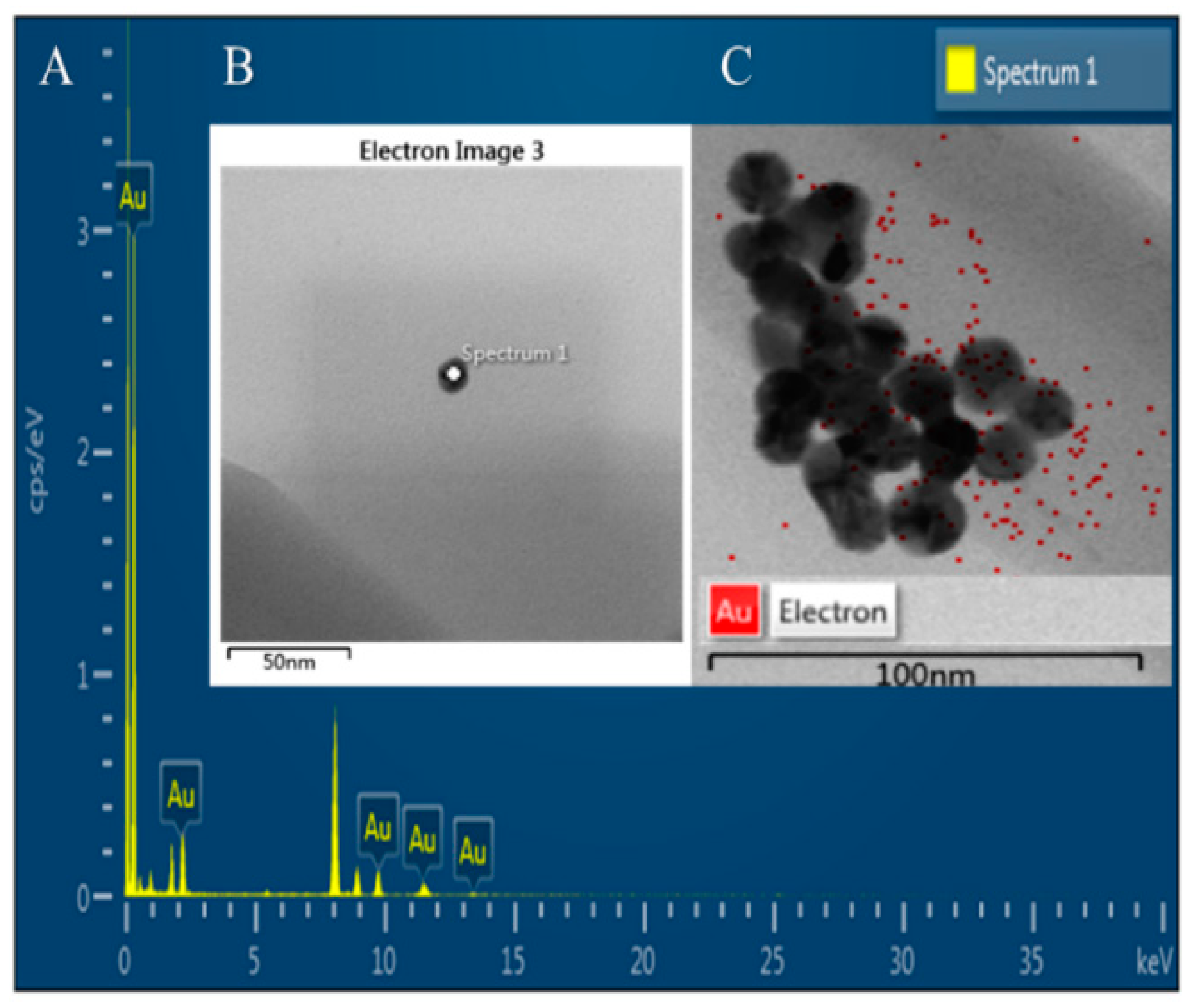
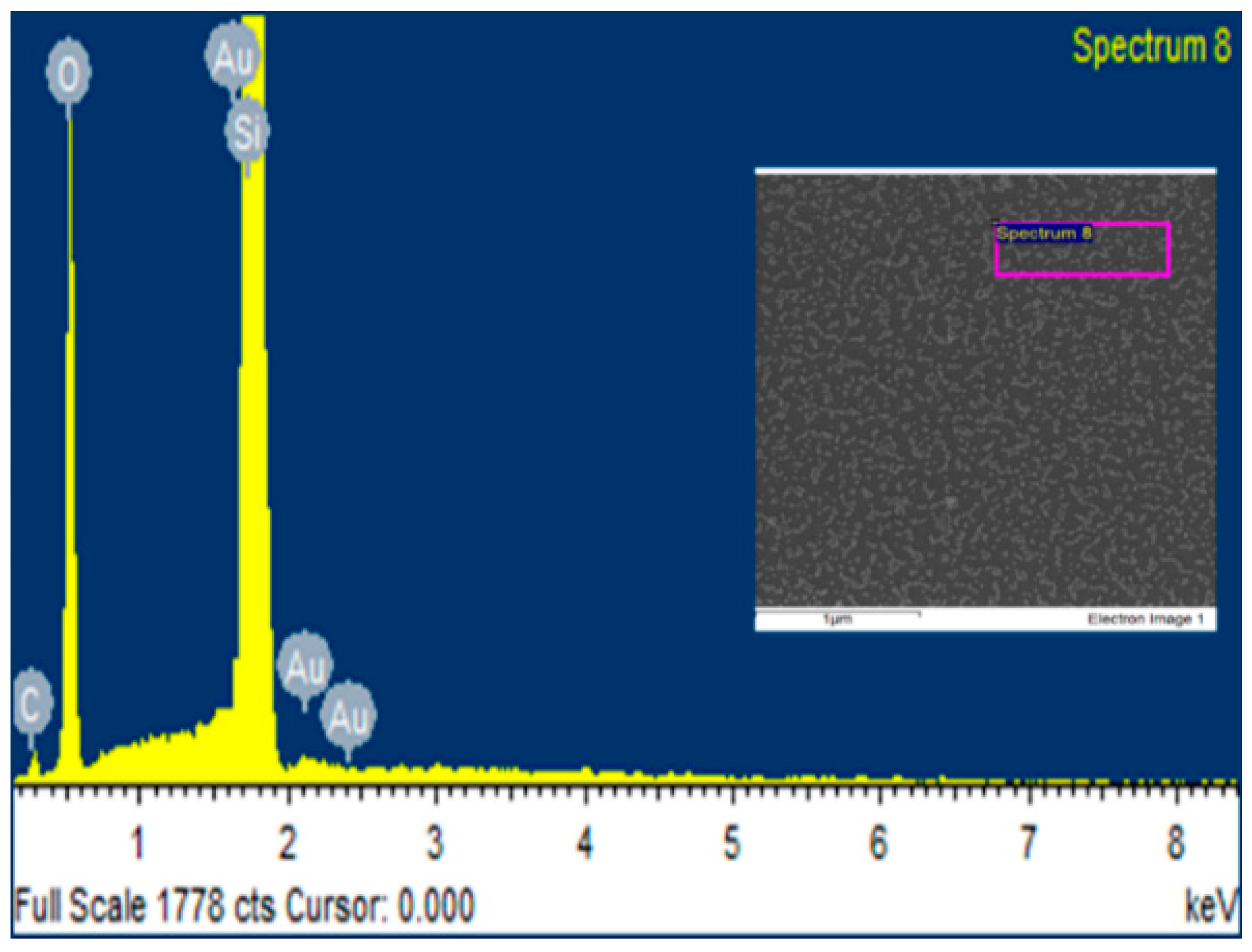
3.6. Elemental Analysis Using Energy-Dispersive X-ray Spectroscopy (EDS)
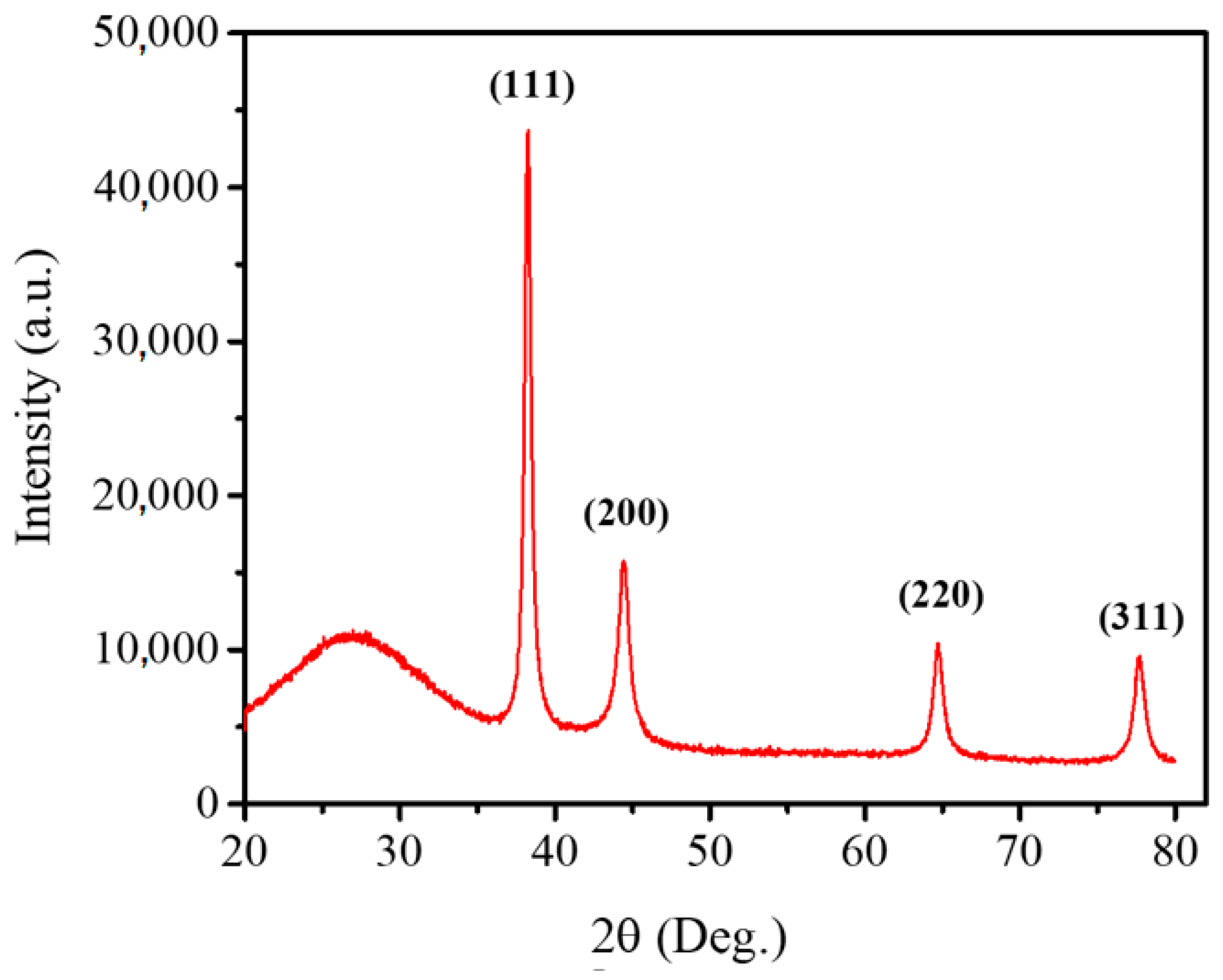
3.7. XRD Analysis
3.8. Size Distribution Using Dynamic Light Scattering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.; Dhanjal, D.S. Bio-nanotechnology for active food packaging. J. Appl. Pharm. Sci. 2016, 6, 220–226. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Christy, A.N. Green synthesis of platinum nanoparticles using Azadirachta indica—An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Scharfe, B.; Feroze, S.; Forberich, K.; Lipovšek, B.; Brabec, C.J.; Egelhaaf, H.-J. Solution prcessed oxygen and moisture barrier based on glass flakes for encapsulation of organic (opto) electronic devices. Flex. Print. Electron. 2021, 6, 025006. [Google Scholar] [CrossRef]
- Ahmed, F.; Zain-ul-abdein, M.; Channa, I.A.; Yaseen, M.K.; Gilani, S.J.; Makhdoom, M.A.; Mansoor, M.; Shahzad, U.; Jumah, M.N.B. Effect of Ultrasonic Surface Mechanical Attrition Treatment-Induced Nanograins on the Mechanical Properties and Biocompatibility of Pure Titanium. Materials 2022, 15, 5097. [Google Scholar] [CrossRef]
- Masood, F.; Makhdoom, M.A.; Channa, I.A.; Gilani, S.J.; Khan, A.; Hussain, R.; Batool, S.A.; Konain, K.; Rahman, S.U.; Wadood, A.; et al. Development and Characterization of Chitosan and Chondroitin Sulfate Based Hydrogels Enriched with Garlic Extract for Potential Wound Healing/Skin Regeneration Applications. Gels 2022, 8, 676. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Ahmad, J.; Memon, A.G.; Shaikh, A.A.; Ismail, T.; Giwa, A.S.; Mahmood, A. Insight into single-element nobel metal anisotropic silver nanoparticle shape-dependent selective ROS generation and quantification. RSC Adv. 2021, 11, 8314–8322. [Google Scholar] [CrossRef]
- Chandio, A.D.; Channa, I.A.; Rizwan, M.; Akram, S.; Javed, M.S.; Siyal, S.H.; Saleem, M.; Makhdoom, M.A.; Ashfaq, T.; Khan, S.; et al. Polyvinyl alcohol and nano-clay based solution processed packaging coatings. Coatings 2021, 11, 942. [Google Scholar] [CrossRef]
- Adam, T.; Hashim, U.; Mohan, M.; Uda, M.N.A.; Uda, M.N.A. Integrated ZnO-Al2O3 nano particles as absorbent for heavy metal ions and organic impurities. AIP Conf. Proc. 2021, 2339, 020128. [Google Scholar] [CrossRef]
- Rehman, H.; Ali, Z.; Hussain, M.; Gilani, S.R.; Shahzady, T.G.; Zahra, A.; Hussain, S.; Hussain, H.; Hussain, I.; Farooq, M.U. Synthesis and characterization of zno nanoparticles and their use as an adsorbent for the arsenic removal from drinking water. Dig. J. Nanomater. Biostruct. 2019, 14, 1033–1040. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85082714155&partnerID=40&md5=95e3f5e384791fe58093c55d8c0aa8e9 (accessed on 25 October 2022).
- Tang, Y.-C.; Huang, X.-H.; Wu, C.-N. Removal of arsenic(III) from drinking water by adsorption with titanium and ferrous oxide nanoparticles. Asian J. Chem. 2013, 25, 2491–2496. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and ‘green’ method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Faraday, M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kim, F.; Connor, S.; Song, H.; Kuykendall, T.; Yang, P. Platonic gold nanocrystals. Angew. Chem.-Int. Ed. 2004, 43, 3673–3677. [Google Scholar] [CrossRef]
- Busbee, B.D.; Obare, S.O.; Murphy, C.J. An improved synthesis of high-aspect-ratio gold nanorods. Adv. Mater. 2003, 15, 414–416. [Google Scholar] [CrossRef]
- Aguirre, C.M.; Kaspar, T.R.; Radloff, C.; Halas, N.J. CTAB Mediated Reshaping of Metallodielectric Nanoparticles. Nano Lett. 2003, 3, 1707–1711. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodríguez-González, B.; de Abajo, F.J.G.; Liz-Marzán, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Millstone, J.E.; Park, S.; Shuford, K.L.; Qin, L.; Schatz, G.C.; Mirkin, C.A. Observation of a quadrupole plasmon mode for a colloidal solution of gold nanoprisms. J. Am. Chem. Soc. 2005, 127, 5312–5313. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-S.; Lee, C.-L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Chang, S.-S.; Shih, C.-W.; Chen, C.-D.; Lai, W.-C.; Wang, C.R.C. The Shape Transition of Gold Nanorods. Langmuir 1999, 15, 701–709. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Jung, H.L.; Wang, Z.; Liu, J.; Lu, Y. Highly sensitive and selective colorimetric sensors for uranyl (UO 22+): Development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.H.; Lu, Y. Label-Free Colorimetric Detection of Lead Ions with a Nanomolar Detection Limit and Tunable Dynamic Range by using Gold Nanoparticles and DNAzyme. Adv. Mater. 2008, 20, 3263–3267. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Yang, X.; Liu, P.; Wang, K.; Huang, J.; Liu, J.; Song, C.; Wang, J. Colorimetric detection of mercury ion based on unmodified gold nanoparticles and target-triggered hybridization chain reaction amplification. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 283–287. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, X.; Shi, H.; Luo, Y. T-T mismatch-driven biosensor using triple functional DNA-protein conjugates for facile detection of Hg2+. Biosens. Bioelectron. 2016, 78, 418–422. [Google Scholar] [CrossRef]
- Li, H.; Rothberg, L.J. Label-Free Colorimetric Detection of Specific Sequences in Genomic DNA Amplified by the Polymerase Chain Reaction. J. Am. Chem. Soc. 2004, 126, 10958–10961. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zuo, X.; Yang, R.; Xiao, Y.; Kang, D.; Vallée-Bélisle, A.; Gong, X.; Yuen, J.D.; Hsu, B.B.Y.; Heeger, A.J.; et al. Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Natl. Acad. Sci. USA 2010, 107, 10837–10841. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.G.; Zhou, X.; Liu, J.; Wang, R.; Liu, L.; Yu, B.; He, M.; Shi, H. Utilization of unmodified gold nanoparticles for label-free detection of mercury (II): Insight into rational design of mercury-specific oligonucleotides. J. Hazard. Mater. 2017, 321, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Xing, Y.P.; Liu, L.H.; Zhou, X.H.; Shi, H.C. Fenton reaction-triggered colorimetric detection of phenols in water samples using unmodified gold nanoparticles. Sens. Actuators B Chem. 2016, 225, 593–599. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Memon, A.G.; Xing, Y.; Zhou, X.; Wang, R.; Liu, L.; Zeng, S.; He, M.; Ma, M.; Ma, M. Ultrasensitive colorimetric aptasensor for Hg2+ detection using Exo-III assisted target recycling amplification and unmodified AuNPs as indicators. J. Hazard. Mater. 2019, 384, 120948. [Google Scholar] [CrossRef]
- Memon, A.G.; Zhou, X.; Xing, Y.; Wang, R.; Liu, L.; Khan, M.; He, M. Label-free colorimetric nanosensor with improved sensitivity for Pb2+ in water by using a truncated 8–17 DNAzyme. Front. Environ. Sci. Eng. 2019, 13, 12. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J. Am. Chem. Soc. 2004, 126, 12298–12305. [Google Scholar] [CrossRef]
- Jin, R.; Wu, G.; Li, Z.; Mirkin, C.A.; Schatz, G.C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2003, 125, 1643–1654. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; bin Jumah, M.N. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]





| AuNPs | Z-Average (r. nm) | D(i,10) | PdI | Conductivity (mS/cm) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 2 1 | 20.31 | 13.5 | 0.273 | 0.953 | −17.4 |
| 2 2 | 20.29 | 14 | 0.271 | 1.09 | −19.4 |
| 2 3 | 20.18 | 13.8 | 0.266 | 1.13 | −16.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, A.G.; Channa, I.A.; Shaikh, A.A.; Ahmad, J.; Soomro, A.F.; Giwa, A.S.; Baig, Z.T.; Mahdi, W.A.; Alshehri, S. Citrate-Capped AuNP Fabrication, Characterization and Comparison with Commercially Produced Nanoparticles. Crystals 2022, 12, 1747. https://doi.org/10.3390/cryst12121747
Memon AG, Channa IA, Shaikh AA, Ahmad J, Soomro AF, Giwa AS, Baig ZT, Mahdi WA, Alshehri S. Citrate-Capped AuNP Fabrication, Characterization and Comparison with Commercially Produced Nanoparticles. Crystals. 2022; 12(12):1747. https://doi.org/10.3390/cryst12121747
Chicago/Turabian StyleMemon, Abdul Ghaffar, Iftikhar Ahmed Channa, Asif Ahmed Shaikh, Jabran Ahmad, Abdul Fatah Soomro, Abdulmoseen Segun Giwa, Zenab Tariq Baig, Wael A. Mahdi, and Sultan Alshehri. 2022. "Citrate-Capped AuNP Fabrication, Characterization and Comparison with Commercially Produced Nanoparticles" Crystals 12, no. 12: 1747. https://doi.org/10.3390/cryst12121747
APA StyleMemon, A. G., Channa, I. A., Shaikh, A. A., Ahmad, J., Soomro, A. F., Giwa, A. S., Baig, Z. T., Mahdi, W. A., & Alshehri, S. (2022). Citrate-Capped AuNP Fabrication, Characterization and Comparison with Commercially Produced Nanoparticles. Crystals, 12(12), 1747. https://doi.org/10.3390/cryst12121747










