Growth Mechanism of Ice Lens in Saturated Clay Considering Surface Charge
Abstract
:1. Introduction
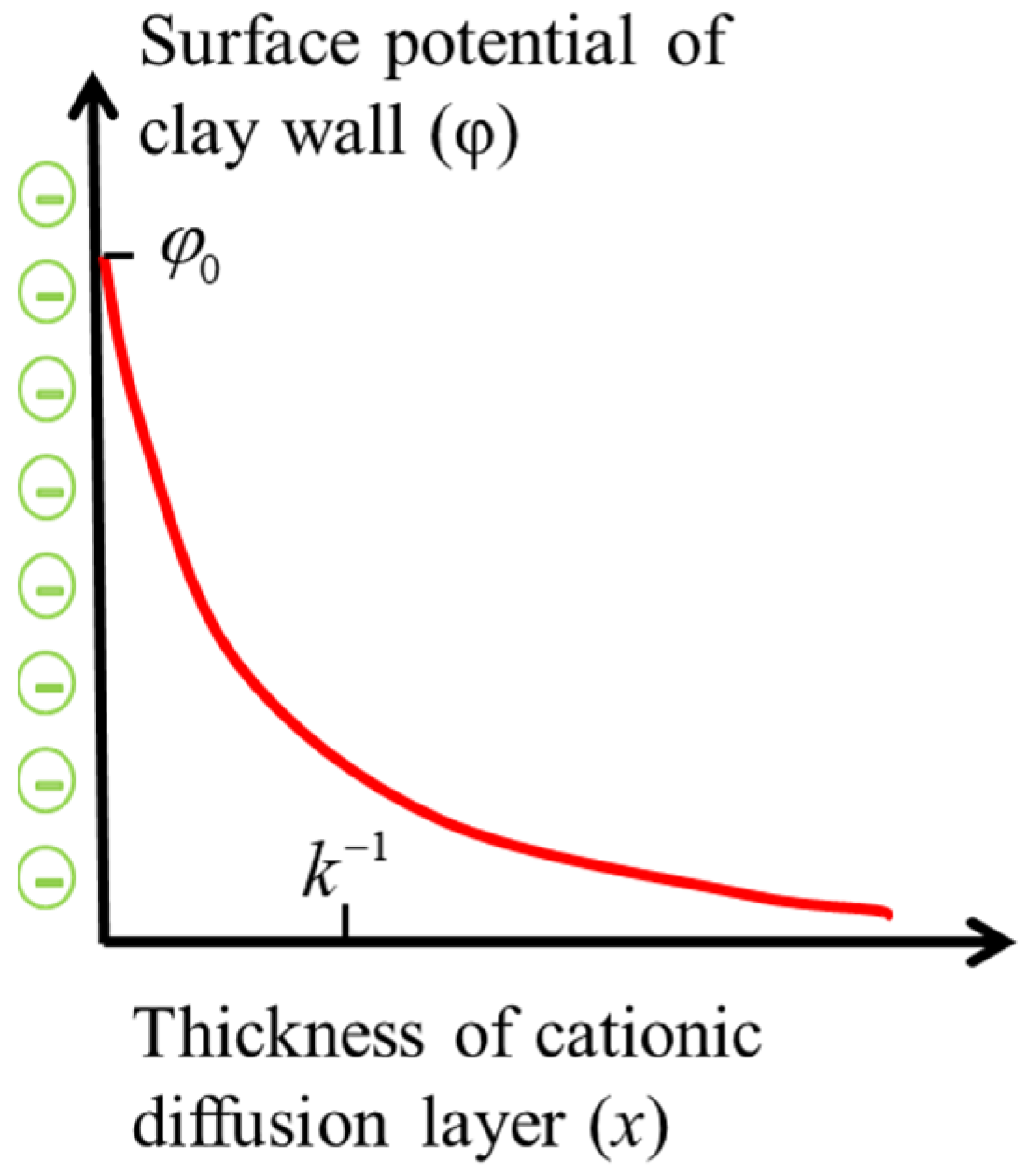
2. Electrical Properties of Clay Surface
3. Basic Assumptions and Physical Models
3.1. Basic Hypotheses
- Saturated clay is regarded as an ideal elastoplastic porous medium without impurities;
- The surface charge of clay particles is uniformly distributed and fixed, and the distribution law of the diffusion layer accords with the Boltzmann distribution;
- There is only one symmetrical electrolyte in the solution, and the charge number of positive and negative ions is equal.
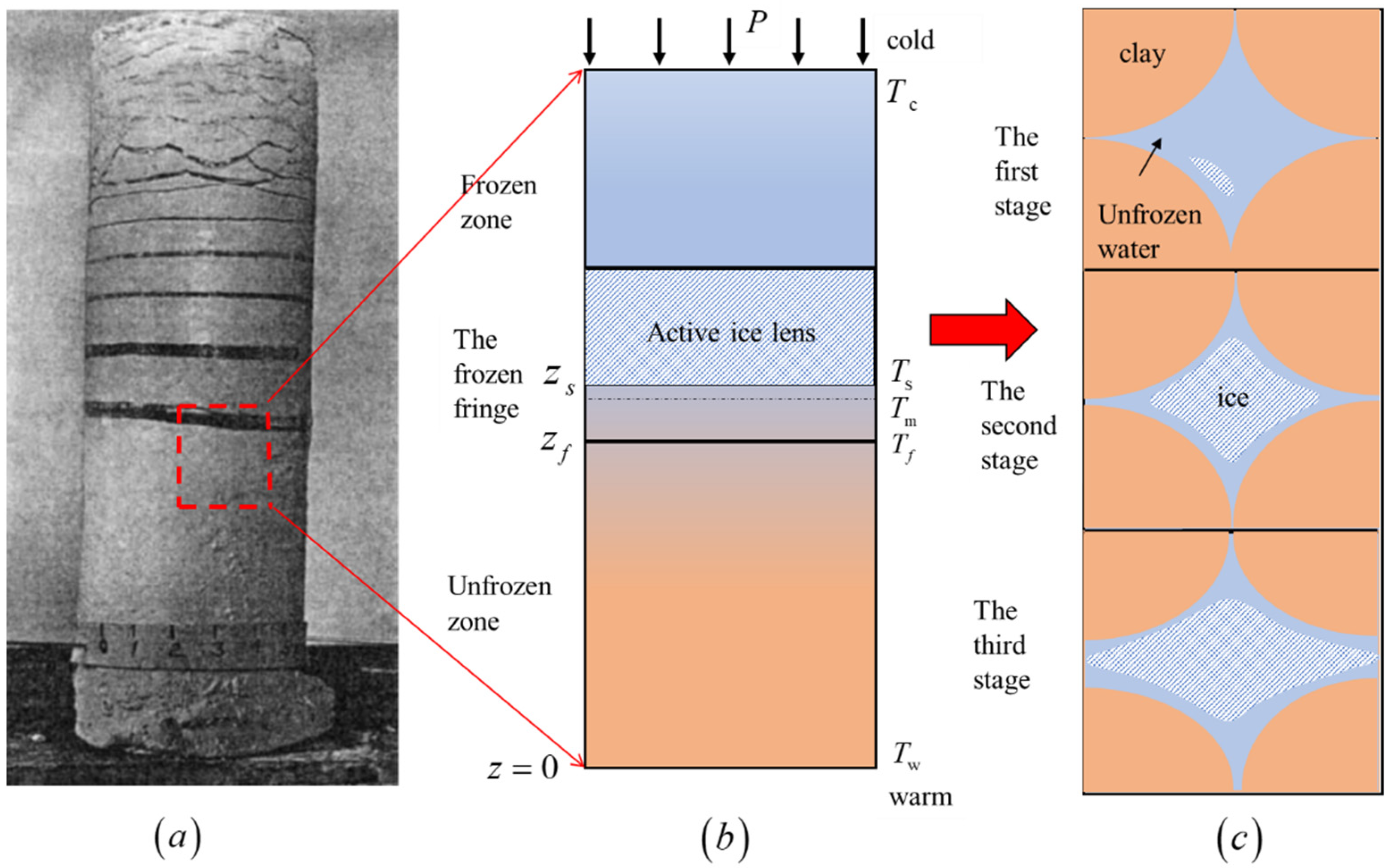
3.2. Growth Process Model of Ice Lens in Saturated Clay
4. Mechanism of Ice Lens Growth in Saturated Clay
4.1. Pore Structure of Frozen Clay
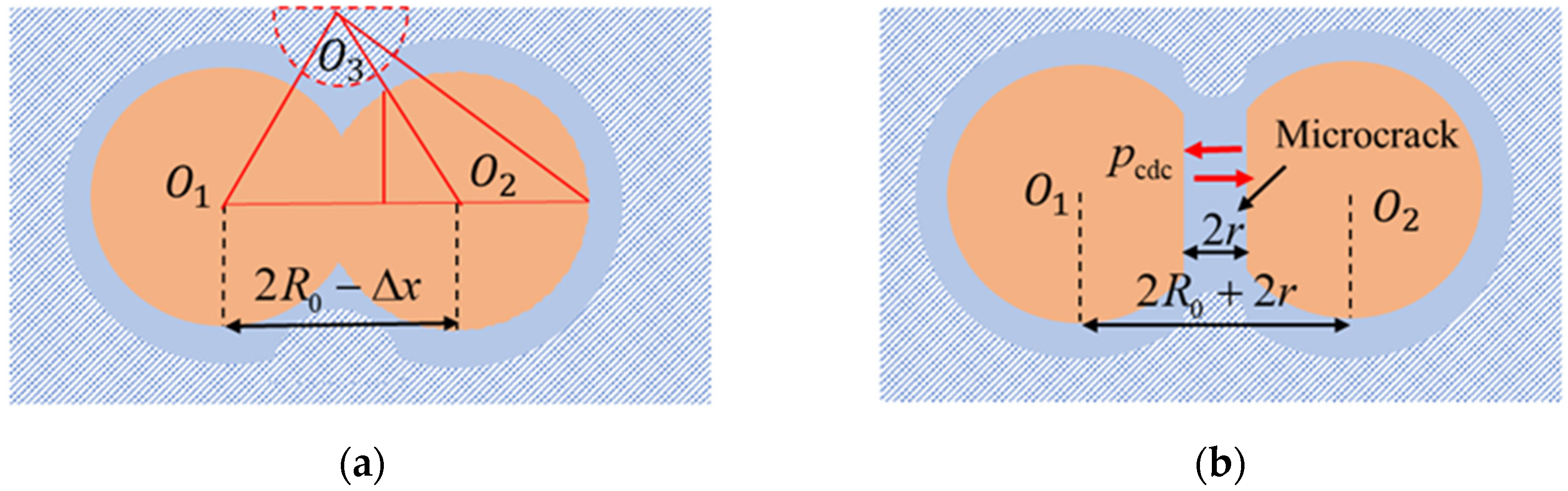
4.2. Micromechanical Analysis of Frozen Clay Pores
4.3. The Formation of Initial Cracks
4.4. Crack Propagation
4.5. Mechanism of Ice Lens Formation
5. Model Verification and Discussion
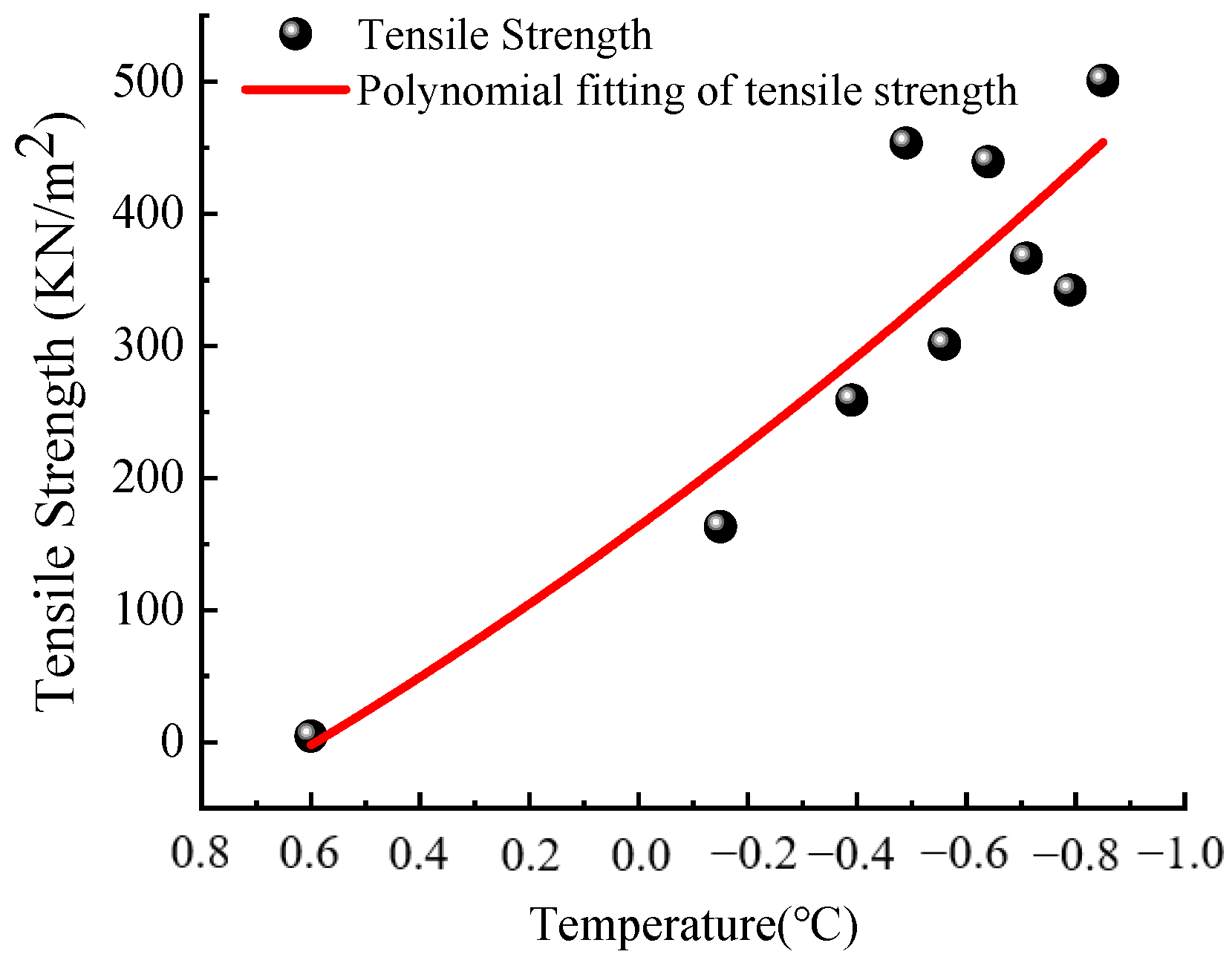
5.1. Model Validation
5.2. Discussion
6. Conclusions
- An ice lens growth model of saturated clay considering the effect of the surface charge is proposed. That is, the ice lens growth process of frozen saturated clay is as follows: the ice nucleus on the clay surface is born, the ice crystals grow in the pores and the ice crystal pressure causes the cracks to extend to the adjacent pores, resulting in a new ice lens. The formulas for calculating the pressure on the clay wall under the action of ice crystals in clay pores when ice crystals are formed and filled with cracks were given.
- The appearance and growth of pore ice in frozen soil reduces its moisture content. When there is only adsorbed water in the soil, the frozen edge soil can be regarded as a linear elastic solid, and the ultimate tensile strength of the soil is taken as the criterion for soil skeleton fracturing. It is proved that there is a good consistency between the soil tensile strength of the macroscopic dimension and the intergranular separation pressure of the molecular dimension in judging the production conditions of the new lens.
- The conditions for the initial crack and propagation of frozen clay are as follows: when KIC = 0, = 0, there are no cracks of soil skeleton; when KIC > 0, , there are cracks in the soil structure at the freezing fringe, which expand with the infiltration of ice crystals.
- The expansion of soil cracks at the frozen fringe is the main cause of frost heaving. Only when the initial cracks of the soil under the action of ice pressure make the clay skeleton form a permeable path can the pores with more pore sizes be frozen. The lateral extension of cracks promotes the formation of zonal lenses, and the formation of new lenses should be the result of the growth of old lenses along vertical cracks rather than secondary nucleation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, C.R.; Fernandes, M.C.; Creyts, T.T.; Rice, J.R. Effects of ice deformation on Röthlisberger channels and implications for transitions in subglacial hydrology. J. Glaciol. 2016, 62, 750–762. [Google Scholar] [CrossRef]
- Taber, S. The mechanics of frost heaving. J. Geol. 1930, 38, 303–317. [Google Scholar] [CrossRef]
- O’Neill, K.; Miller, R.D. Exploration of a rigid ice model of frost heave. Water Resour. Res. 1985, 21, 281–296. [Google Scholar] [CrossRef]
- Ishizaki, T.; Yoneyama, K.; Nishio, N. X-ray technique for observation of ice lens growth in partially frozen, saturated soil. Cold Reg. Sci. Technol. 1985, 11, 213–221. [Google Scholar] [CrossRef]
- Watanabe, K.; Mizoguchi, M. Ice configuration near a growing ice lens in a freezing porous medium consisting of micro glass particles. J. Cryst. Growth. 2000, 213, 135–140. [Google Scholar] [CrossRef]
- Azmatch, T.F.; Sego, D.C.; Arenson, L.U.; Biggar, K.W. New ice lens initiation condition for frost heave in fine-grained soils. Cold Reg. Sci. Technol. 2012, 82, 8–13. [Google Scholar] [CrossRef]
- Watanabe, K. Relationship between growth rate and supercooling in the formation of ice lenses in a glass powder. J. Cryst. Growth. 2002, 237, 2194–2198. [Google Scholar] [CrossRef]
- Martin, R.T. Rhythmic Ice Banding in Soil; Highway Research Board Bulletin: Washington, DC, USA, 1959; pp. 11–23. [Google Scholar]
- Konrad, J.M.; Morgenstern, N.R. A mechanistic theory of ice lens formation in fine-grained soils. Can. Geotech. J. 1980, 17, 473–486. [Google Scholar] [CrossRef]
- You, J.X.; Wang, J.C.; Wang, L.L.; Wang, Z.J.; Li, J.J.; Lin, X. Can secondary nucleation exist in ice banding of freezing colloidal suspensions? Chin. Phys. B 2016, 25, 128202. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: New York, NY, USA, 2010; pp. 190–195. [Google Scholar]
- Saarenketo, T. Electrical properties of water in clay and silty soils. J. Appl. Geophys. 1998, 40, 73–88. [Google Scholar] [CrossRef]
- Ma, W. Water-Solid Interface Chemistry and Adsorption Technology, 1st ed.; Metallurgical Industry Press: Beijing, China, 2011; pp. 92–95. ISBN 9787502457174. [Google Scholar]
- Yu, F.; Guo, P.; Lai, Y.M.; Stolle, D. Frost heave and thaw consolidation modelling. Part 1: A water flux function for frost heaving. Can. Geotech. J. 2020, 57, 1581–1594. [Google Scholar] [CrossRef]
- Ji, Y.K.; Zhou, G.Q.; Zhou, Y.; Vandeginste, V. Frost heave in freezing soils: A quasi-static model for ice lens growth. Cold Reg. Sci. Technol. 2019, 158, 10–17. [Google Scholar] [CrossRef]
- Saruya, T.; Kurita, K.; Rempel A., W. Experimental constraints on the kinetics of ice lens initiation and growth. Phys. Rev. E 2013, 87, 032404. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, H.Q.; Cao, G.Y.; Rong, C.X.; Yao, Z.S.; Cai, H.B. Migration mechanism of capillary-film water in frozen soil and its experimental verification. Chin. J. Geotech. Eng. 2020, 42, 1790–1799. [Google Scholar]
- Dobson, M.C.; Ulaby, F.T.; Hallikainen, M.T.; El-rayes, M.A. Microwave dielectric behavior of wet soil-Part II: Dielectric mixing models. IEEE. T. Geosci. Remote 1985, 23, 35–46. [Google Scholar] [CrossRef]
- Jin, X.; Yang, W.; Meng, X.H.; Lei, L.L. Deduction and application of unfrozen water content in soil based on electrical double-layer theory. Rock Soil Mech. 2019, 40, 1449–1456. [Google Scholar] [CrossRef]
- Gonçalvès, J.; Rousseau-Gueutin, P.; De Marsily, G.; Cosenza, P.; Violette, S. What is the significance of pore pressure in a saturated shale layer? Water Resour. Res. 2010, 46, W04514. [Google Scholar] [CrossRef]
- Kong, B.W.; Ding, Z.; He, S.H.; Zhuang, J.H. Experimental study on pore features and dynamic behaviors of soft clay under different confine pressures during freezing. Chin. J. Rock Mech. Eng. 2020, 39, 2328–2340. [Google Scholar] [CrossRef]
- Style, R.W.; Peppin, S.S.L. The kinetics of ice-lens growth in porous media. J. Fluid. Mech. 2012, 692, 482–498. [Google Scholar] [CrossRef] [Green Version]
- Maeno, N.; Nishimura, H. The electrical properties of ice surfaces. J. Glaciol. 1978, 21, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Everett, D.H. The thermodynamics of frost damage to porous solids. Trans. Faraday Soc. 1961, 57, 1541–1551. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cement. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Takagi, S. The adsorption force theory of frost heaving. Cold Reg. Sci. Technol. 1980, 3, 57–81. [Google Scholar] [CrossRef]
- Rosenberg, R. Why is ice slippery? Phys. Today 2005, 58, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, Y.; Ishikawa, I. Direct evidence for melting transition at interface between ice crystal and glass substrate. J. Cryst.Growth 1993, 128, 1137–1142. [Google Scholar] [CrossRef]
- Fang, Y.; Yao, Z.S.; Li, X.W.; Xu, Y.J.; Huang, X.W. Durability of reactive powder concrete of drilling shaft and triaxial compression damage constitutive model under composite salt erosion. J. Build. Eng. 2022, 62, 105395. [Google Scholar] [CrossRef]
- Graham, J.; Houlsby, G.T. Anisotropic elasticity of a natural clay. Géotechnique 1983, 33, 165–180. [Google Scholar] [CrossRef]
- Liu, X.Y.; Cheng, H.; Chen, H.Q.; Guo, L.H.; Fang, Y.; Wang, X.S. Theoretical Study on Freezing Separation Pressure of Clay Particles with Surface Charge Action. Crystals 2022, 12, 1304. [Google Scholar] [CrossRef]
- Gilpin, R.R. A model for the prediction of ice lensing and frost heave in soils. Water Resour. Res. 1980, 16, 918–930. [Google Scholar] [CrossRef]
- Gray, C.W.; Allbrook, R. Relationships between shrinkage indices and soil properties in some New Zealand soils. Geoderma 2002, 108, 287–299. [Google Scholar] [CrossRef]
- Murton, J.B.; Peterson, R.; Ozouf, J.C. Bedrock fracture by ice segregation in cold regions. Science 2006, 314, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.F.; Hou, M.S. Engineering Fracture Mechanics; China University of Petroleum Press: Shandong, China, 2006; pp. 10–20. [Google Scholar]
- Atkinson, B.K.; Rawlings, R.D. Acoustic emission during stress corrosion cracking in rocks. Earthq. Predict. Int. Rev. 1981, 4, 605–616. [Google Scholar] [CrossRef]
- Segall, P. Rate-dependent extensional deformation resulting from crack growth in rock. J. Geophys. Res.-Solid Earth 1984, 89, 4185–4195. [Google Scholar] [CrossRef]
- Rice, J.R. Thermodynamics of the quasi-static growth of Griffith cracks. J. Mech. Phys. Solids 1978, 26, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Azmatch, T.F.; Sego, D.C.; Arenson, L.U.; Biggar, K.W. Tensile strength and stress–strain behaviour of Devon silt under frozen fringe conditions. Cold Reg. Sci. Technol. 2011, 68, 85–90. [Google Scholar] [CrossRef]
- Akagawa, S.; Satoh, M.; Kanie, S.; Mikami, T. Effect of tensile strength on ice lens initiation temperature. In Proceedings of the 13th International Conference on Cold Regions Engineering: Current Practices in Cold Regions Engineering, Orono, ME, USA, 23–26 July 2006; pp. 1–12. [Google Scholar] [CrossRef]
- Swainson, I.P.; Schulson, E.M. Invasion of ice through rigid anisotropic porous media. J. Geophys. Res.-Solid Earth 2004, 109, B12205. [Google Scholar] [CrossRef]
- Akagawa, S.; Nishisato, K. Tensile strength of frozen soil in the temperature range of the frozen fringe. Cold Reg. Sci. Technol. 2009, 57, 13–22. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Carbee, D.L. Tensile strength of frozen silt. J. Glaciol. Geocryol. 1986, 15–28. [Google Scholar]

| D (nm) | <3 | 3~100 | >100 |
|---|---|---|---|
| Dbef (%) | 96.91 | 2.85 | 0.24 |
| Dmel (%) | 95.44 | 4.09 | 0.47 |
| Soil Type | Sandy Soil/% | Silt/% | Clay/% | n0 (mol/m3) | 1/(k/m) × 10−9 | |
|---|---|---|---|---|---|---|
| Sandy loam | 51.51 | 35.06 | 13.43 | 0.152 | 5.35 | 3.99 |
| loam | 41.96 | 49.51 | 8.53 | 0.150 | 3.96 | 4.63 |
| Silty loam 1 | 30.63 | 55.89 | 13.48 | 0.166 | 5.40 | 3.97 |
| Silty loam 2 | 17.16 | 63.84 | 19.00 | 0.166 | 5.77 | 3.84 |
| Silty clay | 5.02 | 47.06 | 47.38 | 0.133 | 4.96 | 4.26 |
| Freezing Temperature (°C) | −0.15 | −0.39 | −0.49 | −0.64 | −0.79 | −0.85 |
|---|---|---|---|---|---|---|
| A1 (kPa) | 164 | 417 | 453 | 439 | 459 | 499 |
| Z1 (kPa) | 536 | 741 | 800 | 876 | 941 | 965 |
| Cal (kPa) | 169 | 440 | 552 | 722 | 891 | 959 |
| Er1 (%) | 3 | 5.5 | 21.8 | 64.5 | 94.1 | 92.2 |
| Er2 (%) | 226.8 | 77.7 | 76.6 | 99.5 | 105 | 93.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cheng, H.; Chen, H.; Wang, X.; Guo, L. Growth Mechanism of Ice Lens in Saturated Clay Considering Surface Charge. Crystals 2022, 12, 1743. https://doi.org/10.3390/cryst12121743
Liu X, Cheng H, Chen H, Wang X, Guo L. Growth Mechanism of Ice Lens in Saturated Clay Considering Surface Charge. Crystals. 2022; 12(12):1743. https://doi.org/10.3390/cryst12121743
Chicago/Turabian StyleLiu, Xiaoyan, Hua Cheng, Hanqing Chen, Xiaoyun Wang, and Longhui Guo. 2022. "Growth Mechanism of Ice Lens in Saturated Clay Considering Surface Charge" Crystals 12, no. 12: 1743. https://doi.org/10.3390/cryst12121743
APA StyleLiu, X., Cheng, H., Chen, H., Wang, X., & Guo, L. (2022). Growth Mechanism of Ice Lens in Saturated Clay Considering Surface Charge. Crystals, 12(12), 1743. https://doi.org/10.3390/cryst12121743






