Surfactant Provided Control of Crystallization Polymorphic Outcome and Stabilization of Metastable Polymorphs of 2,6-Dimethoxyphenylboronic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solubility Measurements
2.2. Crystallization Experiments
2.3. Solid Phase Characterization
2.4. Phenylboronic Acid Derivative CSD Structure Analysis and Theoretical Calculations
3. Results and Discussion
3.1. Crystallization from Pure Solvents
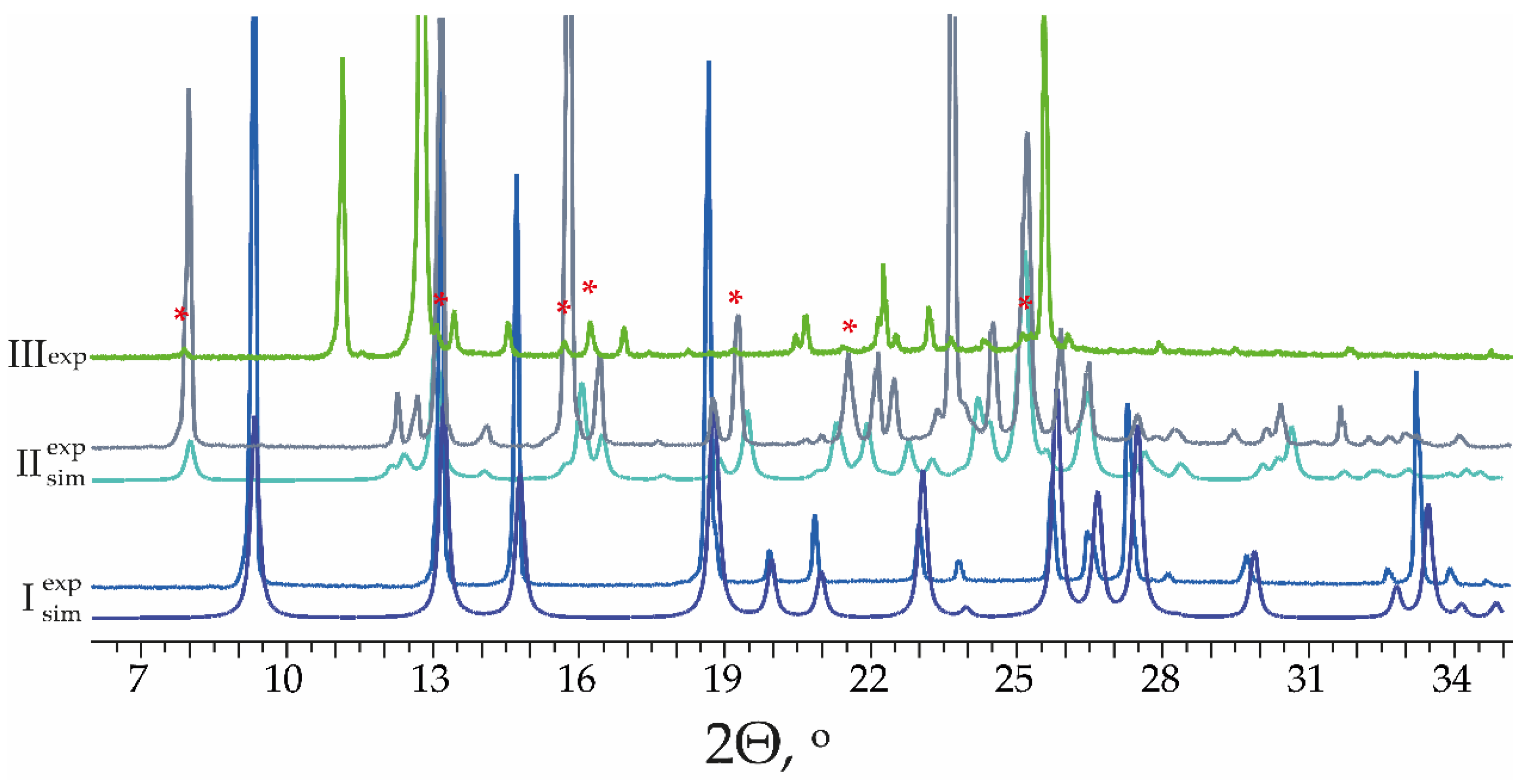
3.2. Characterization of MPBA Polymorphs
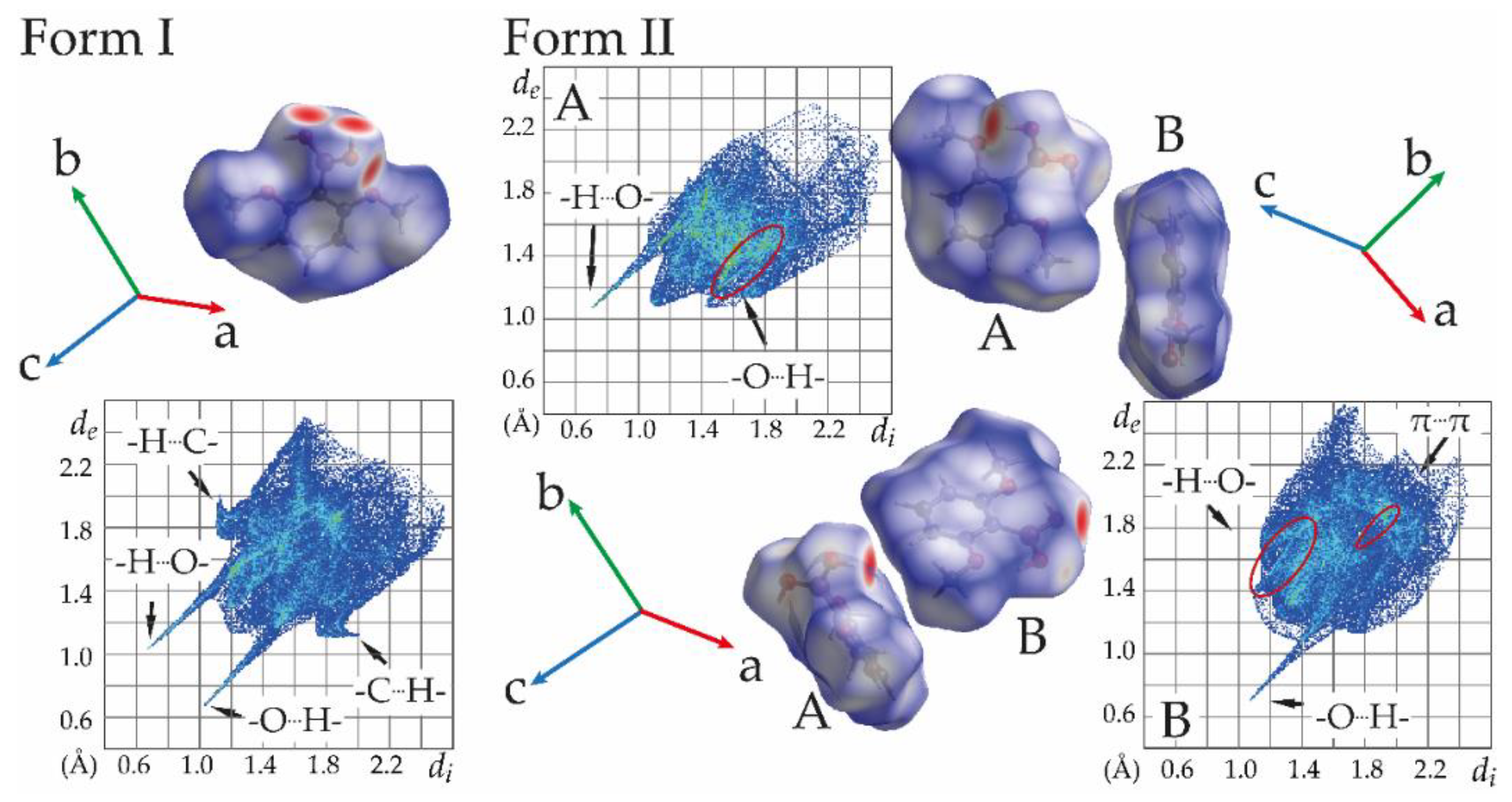
3.2.1. Structure Analysis of MPBA Polymorphs and Analysis of Phenylboronic Acid Moiety Interaction Preferences in CSD Structures
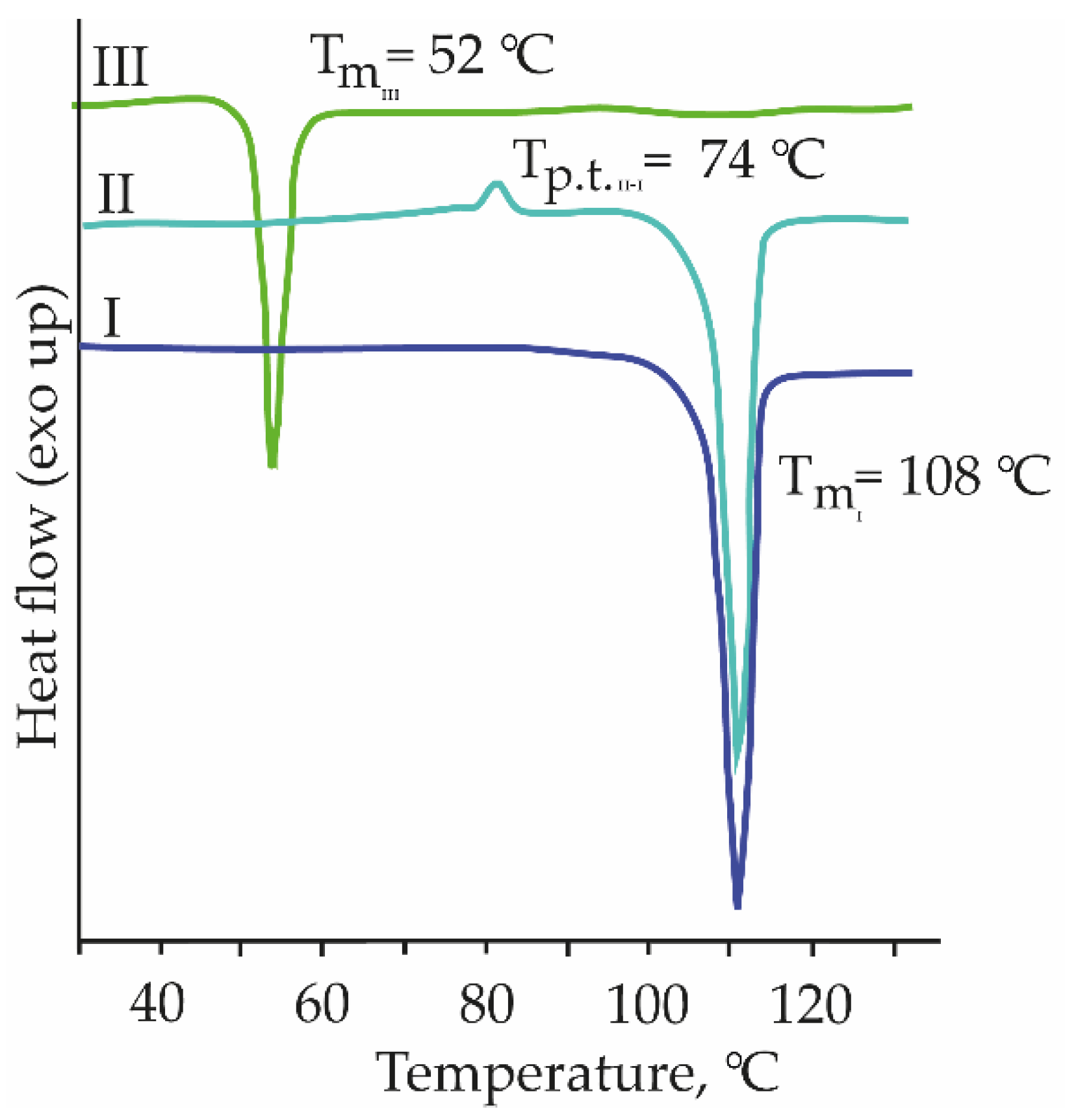
3.2.2. Thermal Characterization of MPBA Polymorphs
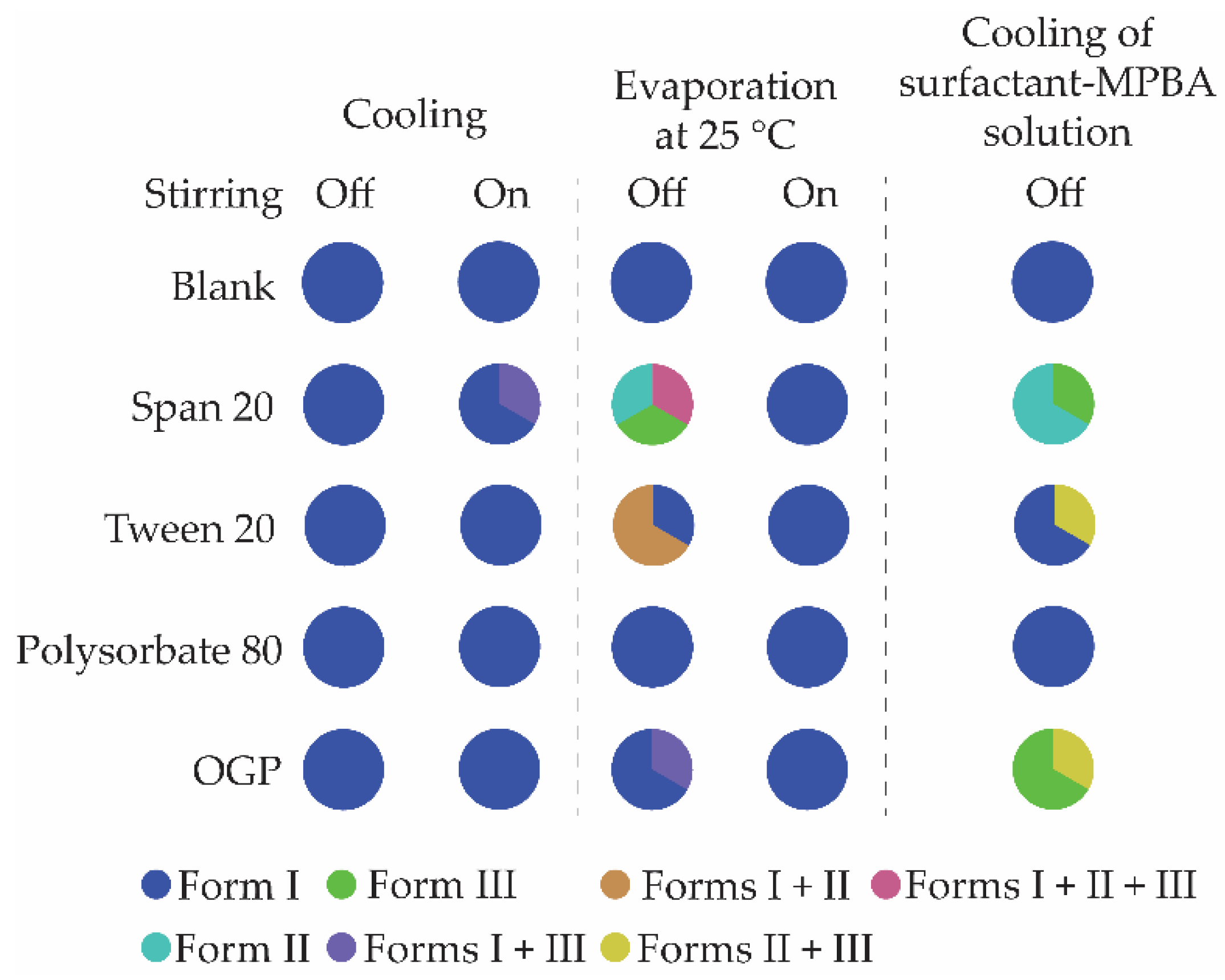
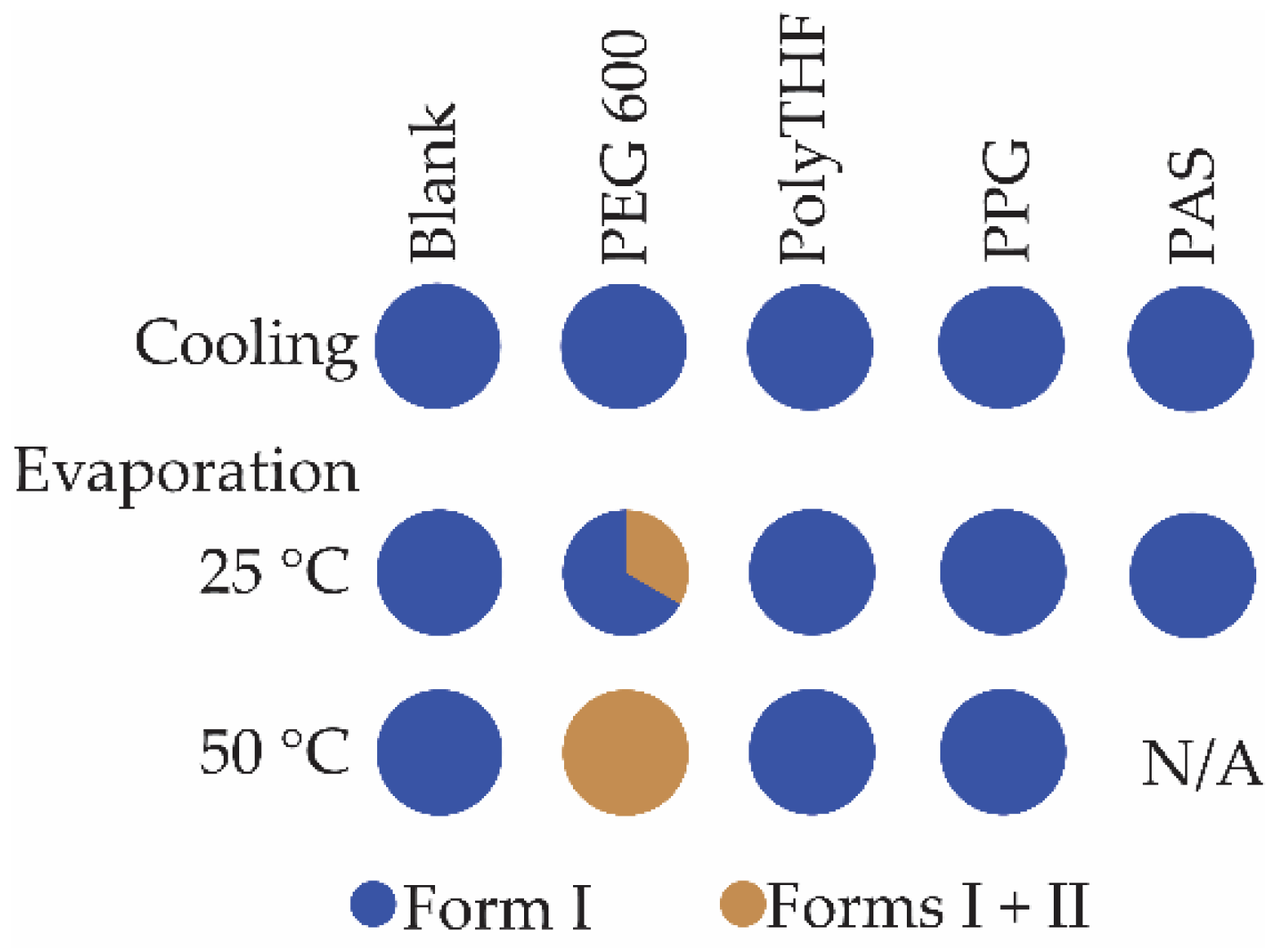
3.3. Crystallization in the Presence of Additives
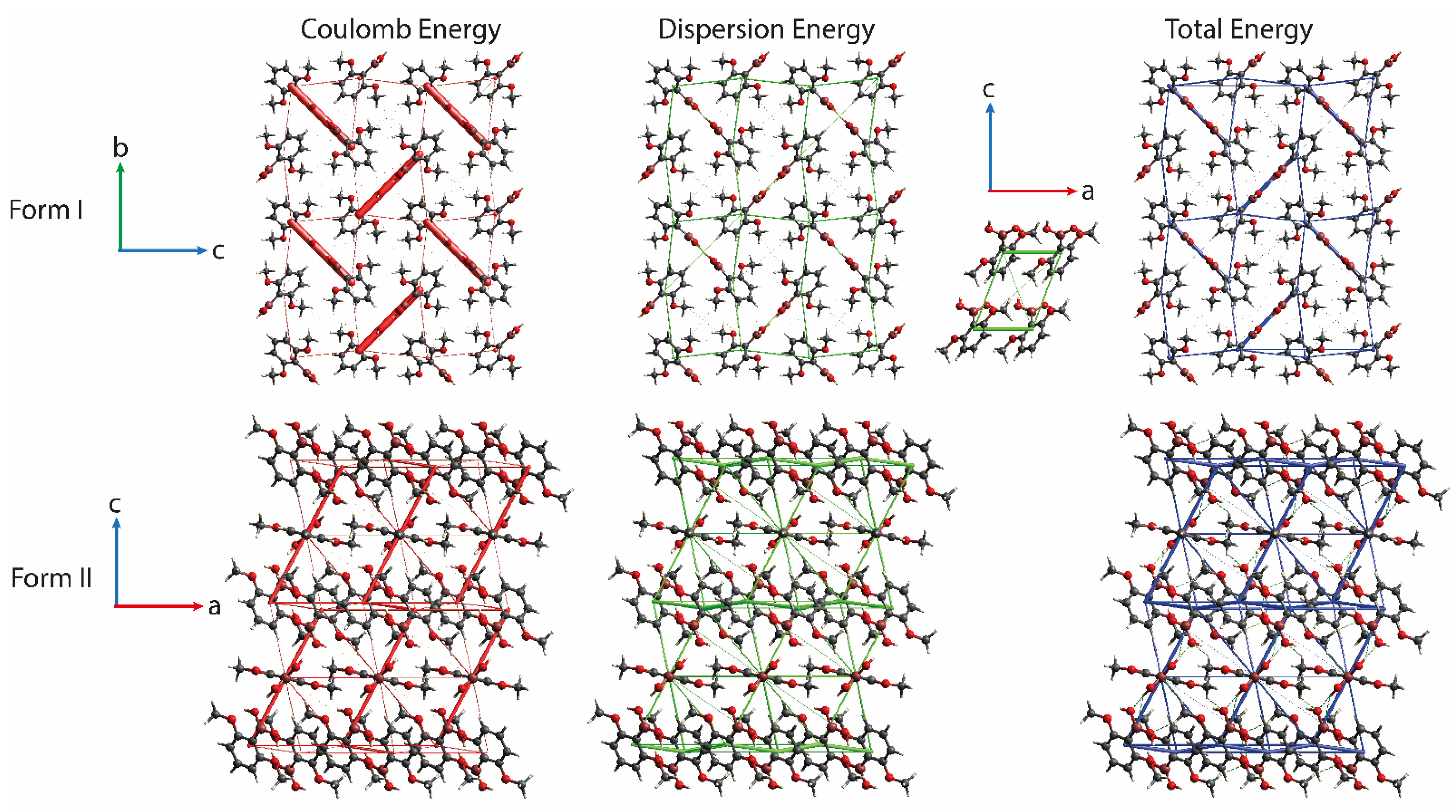
3.4. Theoretical Analysis of MPBA Crystal Structures
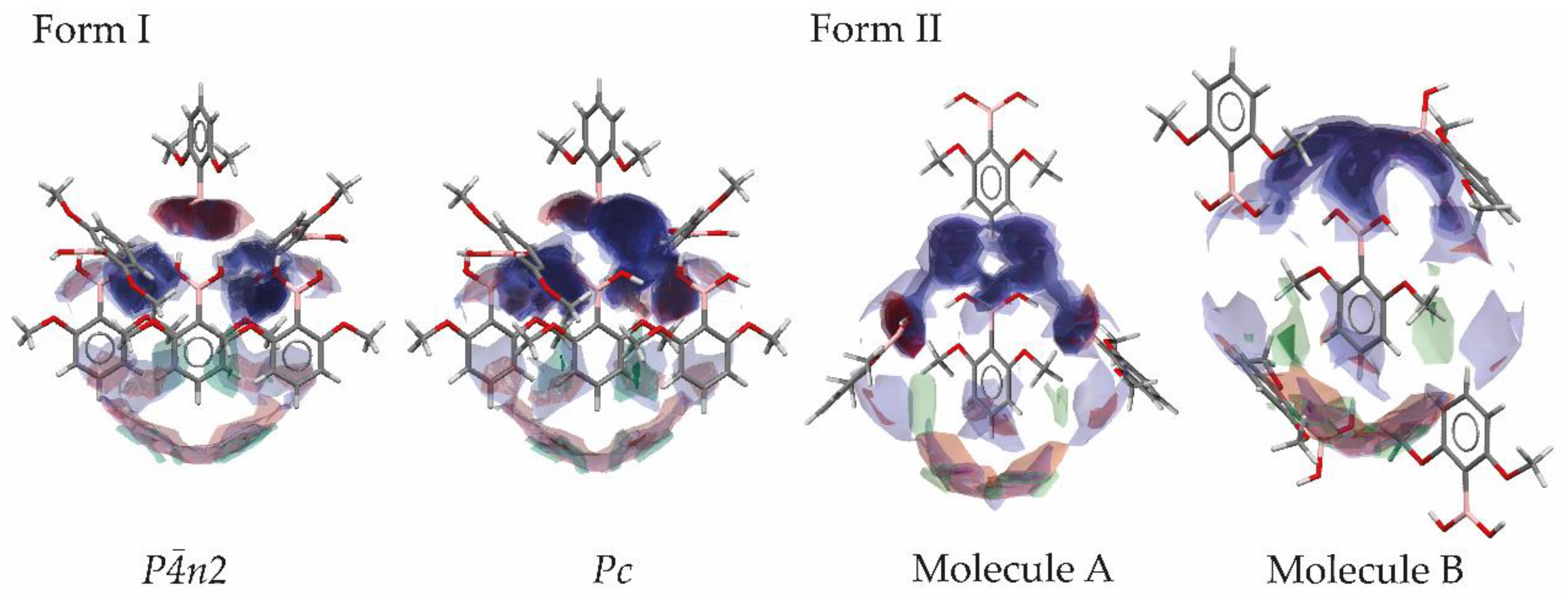
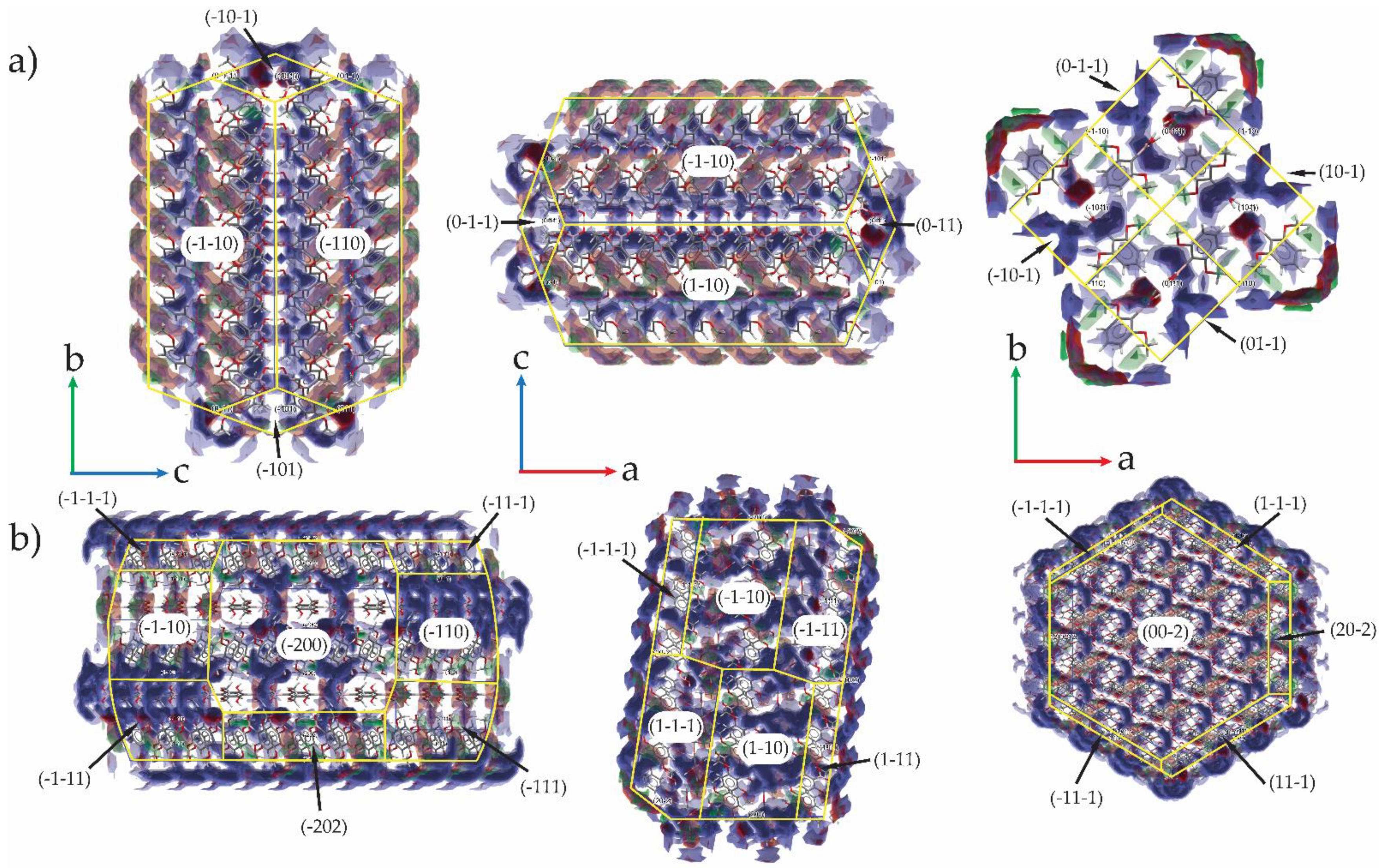
3.5. Use of Full Interaction Maps to Understand Polymorph Stability and Effect of Crystallization Additives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hilfiker, R. (Ed.) Polymorphism: In the Pharmaceutical Industry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 9783527311460. [Google Scholar]
- Aitipamula, S.; Nangia, A. Polymorphism: Fundamentals and Applications. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012; ISBN 9780470661345. [Google Scholar]
- Lu, J.; Rohani, S. Polymorphism and Crystallization of Active Pharmaceutical Ingredients (APIs). Curr. Med. Chem. 2009, 16, 884–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H. A Practical Guide to Pharmaceutical Polymorph Screening & Selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Pudipeddi, M.; Serajuddin, A.T.M. Trends in Solubility of Polymorphs. J. Pharm. Sci. 2005, 94, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Nicoud, L.; Licordari, F.; Myerson, A.S. Estimation of the Solubility of Metastable Polymorphs: A Critical Review. Cryst. Growth Des. 2018, 18, 7228–7237. [Google Scholar] [CrossRef]
- Censi, R.; di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, B.A.; Castiglioni, C.; Fausto, R. Color Polymorphism in Organic Crystals. Commun. Chem. 2020, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.A.; van de Streek, J. How Many Ritonavir Cases Are There Still out There? Faraday Discuss. 2018, 211, 441–458. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency Public Statement: Suply of Norvir Hard Capsules. Available online: https://www.ema.europa.eu/en/documents/public-statement/public-statement-supply-norvir-hard-capsules_en.pdf (accessed on 30 January 2022).
- Xu, S.; Cao, D.; Liu, Y.; Wang, Y. Role of Additives in Crystal Nucleation from Solutions: A Review. Cryst. Growth Des. 2021, 22, 2001–2022. [Google Scholar] [CrossRef]
- Kras, W.; Carletta, A.; Montis, R.; Sullivan, R.A.; Cruz-Cabeza, A.J. Switching Polymorph Stabilities with Impurities Provides a Thermodynamic Route to Benzamide Form III. Commun. Chem. 2021, 4, 38. [Google Scholar] [CrossRef]
- Bērziņš, A.; Trimdale-Deksne, A.; Belyakov, S.; ter Horst, J.H. Reversing the Polymorphic Outcome of Crystallization and the Apparent Relative Stability of Nitrofurantoin Polymorphs Using Additives, University of Latvia, Faculty of Chemistry, Riga, Latvia. 2022; manuscript in preparation; to be submitted. [Google Scholar]
- Poornachary, S.K.; Han, G.; Kwek, J.W.; Chow, P.S.; Tan, R.B.H. Crystallizing Micronized Particles of a Poorly Water-Soluble Active Pharmaceutical Ingredient: Nucleation Enhancement by Polymeric Additives. Cryst. Growth Des. 2016, 16, 749–758. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Liu, Q.; Zong, S.; Tian, B.; Huang, X.; Wang, T.; Yin, Q.; Hao, H. Influences and the Mechanism of Additives on Intensifying Nucleation and Growth of P-Methylacetanilide. Cryst. Growth Des. 2020, 20, 973–983. [Google Scholar] [CrossRef]
- Quan, Y.; Yang, Y.; Xu, S.; Zhu, P.; Liu, S.; Jia, L.; Gong, J. Insight into the Role of Piperazine in the Thermodynamics and Nucleation Kinetics of the Triethylenediamine-Methyl Tertiary Butyl Ether System. CrystEngComm 2019, 21, 948–956. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, J.H.; Shim, H.M.; Koo, K.K. Effect of Amphiphilic Additives on Nucleation of Hexahydro-1,3,5-Trinitro- 1,3,5-Triazine. Cryst. Growth Des. 2013, 13, 4688–4694. [Google Scholar] [CrossRef]
- Pino-García, O.; Rasmuson, Å.C. Influence of Additives on Nucleation of Vanillin: Experiments and Introductory Molecular Simulations. Cryst. Growth Des. 2004, 4, 1025–1037. [Google Scholar] [CrossRef]
- Poon, G.G.; Seritan, S.; Peters, B. A Design Equation for Low Dosage Additives That Accelerate Nucleation. Faraday Discuss. 2015, 179, 329–341. [Google Scholar] [CrossRef]
- Lin, J.; Shi, P.; Wang, Y.; Wang, L.; Ma, Y.; Liu, F.; Wu, S.; Gong, J. Template Design Based on Molecular and Crystal Structure Similarity to Regulate Conformational Polymorphism Nucleation: The Case of α,ω-Alkanedicarboxylic Acids. IUCrJ 2021, 8, 814–822. [Google Scholar] [CrossRef]
- Singh, M.K. Controlling the Aqueous Growth of Urea Crystals with Different Growth Inhibitors: A Molecular-Scale Study. RSC Adv. 2021, 11, 12938–12950. [Google Scholar] [CrossRef]
- Simone, E.; Steele, G.; Nagy, Z.K. Tailoring Crystal Shape and Polymorphism Using Combinations of Solvents and a Structurally Related Additive. CrystEngComm 2015, 17, 9370–9379. [Google Scholar] [CrossRef]
- Urwin, S.J.; Yerdelen, S.; Houson, I.; ter Horst, J.H. Impact of Impurities on Crystallization and Product Quality: A Case Study with Paracetamol. Crystals 2021, 11, 1344. [Google Scholar] [CrossRef]
- Agnew, L.R.; Cruickshank, D.L.; McGlone, T.; Wilson, C.C. Controlled Production of the Elusive Metastable Form II of Acetaminophen (Paracetamol): A Fully Scalable Templating Approach in a Cooling Environment. ChemComm 2016, 52, 7368–7371. [Google Scholar] [CrossRef]
- Shi, P.; Xu, S.; Yang, H.; Wu, S.; Tang, W.; Wang, J.; Gong, J. Use of Additives to Regulate Solute Aggregation and Direct Conformational Polymorph Nucleation of Pimelic Acid. IUCrJ 2021, 8, 161–167. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Council of Europe Control of Impurities in Substances for Pharmaceutical Use. In European Pharmacopoeia, Supplement 10.8; European Medicines Agency: Strasbourg, France, 2022; pp. 763–765. [Google Scholar]
- Giordani, C.F.A.; Campanharo, S.; Wingert, N.R.; Bueno, L.M.; Manoel, J.W.; Costa, B.; Cattani, S.; Arbo, M.D.; Garcia, S.C.; Garcia, C.V.; et al. In Vitro Toxic Evaluation of Two Gliptins and Their Main Impurities of Synthesis. BMC Pharmacol. Toxicol. 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Agrawal, Y.K. Analysis and Impurity Identification in Pharmaceuticals. Rev. Anal. Chem. 2014, 33, 123–133. [Google Scholar] [CrossRef]
- Abdin, A.Y.; Yeboah, P.; Jacob, C. Chemical Impurities: An Epistemological Riddle with Serious Side Effects. Int. J. Environ. Res. Public Health 2020, 17, 1030. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing… Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Sinko, P.J. Pharmaceutical Polymers. In Martin’s Physical Pharmacy and Pharmaceutical Sciences; Troy, D.B., Ed.; Wolters Kluwer, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2016; pp. 508–514. ISBN 9781451191455. [Google Scholar]
- Tadros, T.F. Surfactants in Pharmaceutical Formulations. In Applied Surfactants: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2005; pp. 433–501. ISBN 9783527306299. [Google Scholar]
- Tadros, T.F. Surfactants, Industrial Applications. In Encyclopedia of Physical Science Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2003; Volume 16, pp. 423–438. ISBN 9780122274107. [Google Scholar] [CrossRef]
- Garti, N.; Zour, H. The Effect of Surfactants on the Crystallization and Polymorphic Transformation of Glutamic Acid. J. Cryst. Growth 1997, 172, 486–498. [Google Scholar] [CrossRef]
- Feng, Y.; Meng, Y.; Tan, F.; Lv, L.; Li, Z.; Wang, Y.; Yang, Y.; Gong, W.; Yang, M. Effect of Surfactants and Polymers on the Dissolution Behavior of Supersaturable Tecovirimat-4-Hydroxybenzoic Acid Cocrystals. Pharmaceutics 2021, 13, 1772. [Google Scholar] [CrossRef]
- Cyrański, M.K.; Klimentowska, P.; Rydzewska, A.; Serwatowski, J.; Sporzyński, A.; Stȩpień, D.K. Towards a Monomeric Structure of Phenylboronic Acid: The Influence of Ortho-Alkoxy Substituents on the Crystal Structure. CrystEngComm 2012, 14, 6282–6294. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Gozdalik, J.T.; Kaczorowska, E.; Durka, K.; Wieczorek, D.; Zarzeczańska, D.; Sporzyński, A. (Trifluoromethoxy)Phenylboronic Acids: Structures, Properties, and Antibacterial Activity. Molecules 2021, 26, 2007. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Gozdalik, J.T.; Wieczorek, D.; Madura, I.D.; Kaczorowska, E.; Brzezińska, E.; Sporzyński, A.; Lipok, J. Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-Formylphenylboronic Acid. Molecules 2020, 25, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borys, K.M.; Wieczorek, D.; Pecura, K.; Lipok, J.; Adamczyk-Woźniak, A. Antifungal Activity and Tautomeric Cyclization Equilibria of Formylphenylboronic Acids. Bioorg. Chem. 2019, 91, 103081. [Google Scholar] [CrossRef] [PubMed]
- Hiller, N.D.J.; do Amaral e Silva, N.A.; Tavares, T.A.; Faria, R.X.; Eberlin, M.N.; de Luna Martins, D. Arylboronic Acids and Their Myriad of Applications Beyond Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 4841–4877. [Google Scholar] [CrossRef]
- Cruz, C.D.; Wrigstedt, P.; Moslova, K.; Iashin, V.; Mäkkylä, H.; Ghemtio, L.; Heikkinen, S.; Tammela, P.; Perea-Buceta, J.E. Installation of an Aryl Boronic Acid Function into the External Section of -Aryl-Oxazolidinones: Synthesis and Antimicrobial Evaluation. Eur. J. Med. Chem. 2021, 211, 113002. [Google Scholar] [CrossRef]
- Silva, M.P.; Saraiva, L.; Pinto, M.; Sousa, M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New Software for Searching the Cambridge Structural Database and Visualizing Crystal Structures. Acta Crystallogr. B 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Stokes, H.T.; Hatch, D.M.; Campbell, B.J. ISOCIF, ISOTROPY Software Suite, Department of Physics and Astronomy, Brigham Young University, Provo, Utah, 84602, USA. 2022. Available online: iso.byu.edu/iso/isocif.php (accessed on 21 June 2022).
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Frisch, J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09W, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Karamertzanis, P.G.; Day, G.M.; Welch, G.W.A.; Kendrick, J.; Leusen, F.J.J.; Neumann, M.A.; Price, S.L. Modeling the Interplay of Inter- and Intramolecular Hydrogen Bonding in Conformational Polymorphs. J. Chem. Phys. 2008, 128, 244708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, P.A.; Olsson, T.S.G.; Cole, J.C.; Cottrell, S.J.; Feeder, N.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Evaluation of Molecular Crystal Structures Using Full Interaction Maps. CrystEngComm 2013, 15, 65–72. [Google Scholar] [CrossRef]
- Ouyang, J.; Xing, X.; Chen, J.; Zhou, L.; Liu, Z.; Heng, J.Y.Y. Effects of Solvent, Supersaturation Ratio and Silica Template on Morphology and Polymorph Evolution of Vanillin during Swift Cooling Crystallization. Particuology 2022, 65, 93–104. [Google Scholar] [CrossRef]
- Ouyang, J.; Chen, J.; Rosbottom, I.; Chen, W.; Guo, M.; Heng, J.Y.Y. Supersaturation and Solvent Dependent Nucleation of Carbamazepine Polymorphs during Rapid Cooling Crystallization. CrystEngComm 2021, 23, 813–823. [Google Scholar] [CrossRef]
- Datta, S.; Grant, D.J.W. Effect of Supersaturation on the Crystallization of Phenylbutazone Polymorphs. Cryst. Res. Technol. 2005, 40, 233–242. [Google Scholar] [CrossRef]
- Bemisderfer, K.; Nazarenko, A.Y. Two Forms of (Naphthalen-1-Yl)Boronic Acid. Acta Crystallogr. E Crystallogr. Commun. 2016, 72, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, M.; Durka, K.; Luliński, S.; Serwatowski, J. (2,4-Dipropoxyphenyl)Boronic Acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o3455. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.Z.; Lou, X.H.; Li, H.M.; Yang, W.C.; Shi, H. Refinement of Crystal Structure of 2-Biphenylboronic Acid, C12H11BO2. Z. Krist.-New Cryst. Struct. 2010, 225, 295–296. [Google Scholar] [CrossRef]
- Durka, K.; Kliś, T.; Serwatowski, J. Crystal Structure of (2-Benzyloxypyrimidin-5-Yl)Boronic Acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, o1259–o1260. [Google Scholar] [CrossRef]
- Cárdenas-Valenzuela, A.J.; González-García, G.; Zárraga-Nuñez, R.; Höpfl, H.; Campos-Gaxiola, J.J.; Cruz-Enríquez, A. Crystal Structure and Hirshfeld Surface Analysis of 3-Cyanophenylboronic Acid. Acta Crystallogr. E Crystallogr. Commun. 2018, 74, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and Fictions about Polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef]
- Burger, A.; Ramberger, R. On the Polymorphism of Pharmaceuticals and Other Molecular Crystals. I. Mikrochim. Acta 1979, 72, 259–271. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Barrio, M.; Lloveras, P.; Céolin, R.; Tamarit, J.-L. Polymorphism of Spironolactone: An Unprecedented Case of Monotropy Turning to Enantiotropy with a Huge Difference in the Melting Temperatures. Int. J. Pharm. 2018, 552, 193–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semjonova, A.; Bērziņš, A. Controlling the Polymorphic Outcome of 2,6-Dimethoxybenzoic Acid Crystallization Using Additives. Crystals 2022, 12, 1161. [Google Scholar] [CrossRef]
- Larkin, J.D.; Bhat, K.L.; Markham, G.D.; Brooks, B.R.; Schaefer, H.F.; Bock, C.W. Structure of the Boronic Acid Dimer and the Relative Stabilities of Its Conformers. J. Phys. Chem. A 2006, 110, 10633–10642. [Google Scholar] [CrossRef] [PubMed]
- Nyman, J.; Day, G.M. Static and Lattice Vibrational Energy Differences between Polymorphs. CrystEngComm 2015, 17, 5154–5165. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Endo, S.; Nagayama, K. Stabilization of Protein Crystals by Electrostatic Interactions as Revealed by a Numerical Approach. J. Mol. Biol. 1993, 234, 421–432. [Google Scholar] [CrossRef]
- Takahashi, T. Significant Role of Electrostatic Interactions for Stabilization of Protein Assemblies. Adv. Biophys. 1997, 34, 41–54. [Google Scholar] [CrossRef]
- Wang, C.; Rosbottom, I.; Turner, T.D.; Laing, S.; Maloney, A.G.P.; Sheikh, A.Y.; Docherty, R.; Yin, Q.; Roberts, K.J. Molecular, Solid-State and Surface Structures of the Conformational Polymorphic Forms of Ritonavir in Relation to Their Physicochemical Properties. Pharm. Res. 2021, 38, 971–990. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Feeder, N.; Pidcock, E.; Reilly, A.M.; Sadiq, G.; Doherty, C.L.; Back, K.R.; Meenan, P.; Docherty, R. The Integration of Solid-Form Informatics into Solid-Form Selection. J. Pharm. Pharmacol. 2015, 67, 857–868. [Google Scholar] [CrossRef] [PubMed]


| Solvent | Cooling | Evaporation | |
|---|---|---|---|
| 25 °C | 50 °C | ||
| Acetone; acetonitrile; ethyl acetate; toluene; nitromethane; o–xylene; chloroform; 1,4-dioxane; methyl tert-butyl ether; dichloromethane; diethyl carbonate; tetrahydrofuran; methyl isobutyl ketone; cyclohexanol | I | I | I |
| 2,2,2-Trifluorethanol; water | I | I | I + II |
| Heptanol | I | II + III | I |
| Isopentanol | I | I + II | I |
| Isobutanol | I | I + II | II |
| Isopropanol | I | II/II + III | I + II |
| Methanol | II | II | I |
| Polymorph | Form I | Form II |
|---|---|---|
| CSD Ref. code | n2 structure) | UJACIT |
| Z/Z’ | n2 structure) 4/2 (for Pc structure) | 12/1.5 |
| Eintra, kJ·mol−1 | 15.2 | 6.0 |
| Einter, kJ·mol−1 | −144.4 | −135.9 |
| (Eele + Epol)/Edisp | 1.3 | 1.0 |
| Elattice, kJ·mol−1 | −129.2 | −129.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semjonova, A.; Bērziņš, A. Surfactant Provided Control of Crystallization Polymorphic Outcome and Stabilization of Metastable Polymorphs of 2,6-Dimethoxyphenylboronic Acid. Crystals 2022, 12, 1738. https://doi.org/10.3390/cryst12121738
Semjonova A, Bērziņš A. Surfactant Provided Control of Crystallization Polymorphic Outcome and Stabilization of Metastable Polymorphs of 2,6-Dimethoxyphenylboronic Acid. Crystals. 2022; 12(12):1738. https://doi.org/10.3390/cryst12121738
Chicago/Turabian StyleSemjonova, Aina, and Agris Bērziņš. 2022. "Surfactant Provided Control of Crystallization Polymorphic Outcome and Stabilization of Metastable Polymorphs of 2,6-Dimethoxyphenylboronic Acid" Crystals 12, no. 12: 1738. https://doi.org/10.3390/cryst12121738
APA StyleSemjonova, A., & Bērziņš, A. (2022). Surfactant Provided Control of Crystallization Polymorphic Outcome and Stabilization of Metastable Polymorphs of 2,6-Dimethoxyphenylboronic Acid. Crystals, 12(12), 1738. https://doi.org/10.3390/cryst12121738






