Development and Testing of Zinc Oxide Embedded Sulfonated Poly (Vinyl Alcohol) Nanocomposite Membranes for Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of ZnO NP’s by Sol-Gel Method
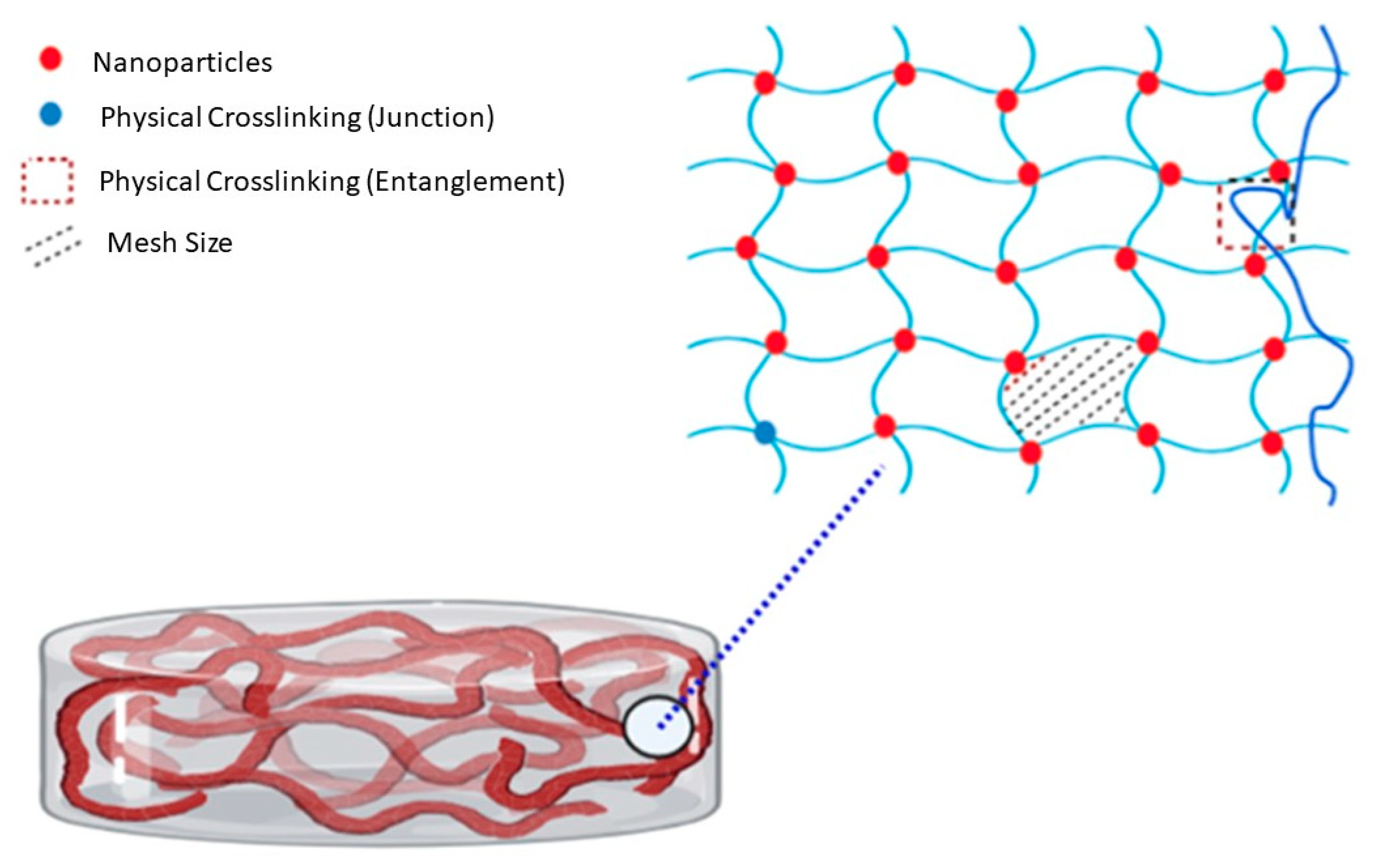
2.3. Preparation of Crosslinked Nanocomposite Membranes
2.4. Sulfonation of the Membranes
2.5. Membrane Characterization
2.5.1. Fourier Transmission Infrared (FTIR) Spectroscopic Studies
2.5.2. X-ray Diffraction Studies
2.5.3. Scanning Electron Microscopy (SEM) Studies
2.5.4. Measurement of Particle Size
2.5.5. UV–Visible Analysis
2.5.6. Mechanical Properties
2.5.7. Ion Exchange Capacity (IEC)
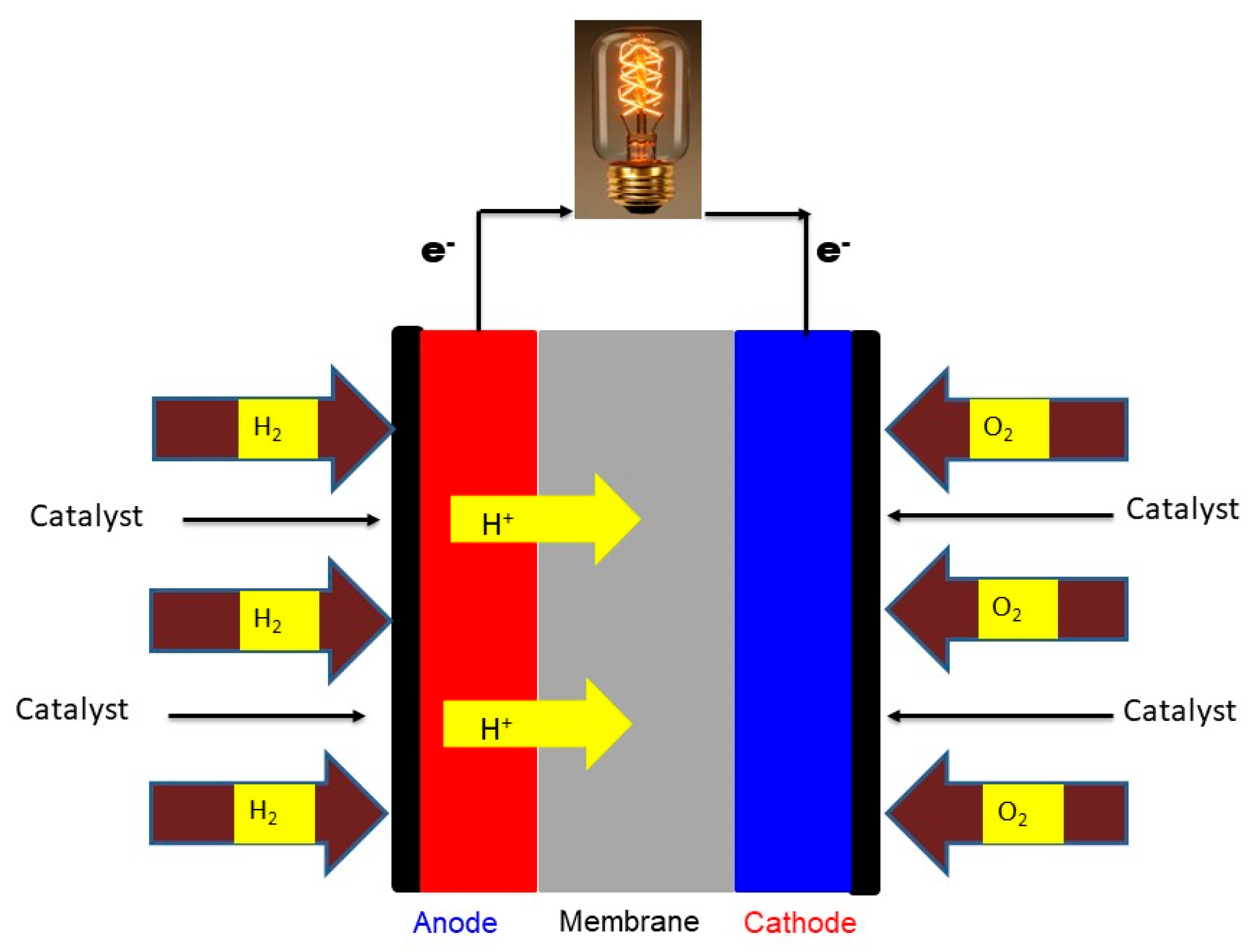
2.5.8. Fuel Cell Measurements
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy Study
3.2. X-ray Diffraction Studies
3.3. Scanning Electron Microscopy (SEM) Studies
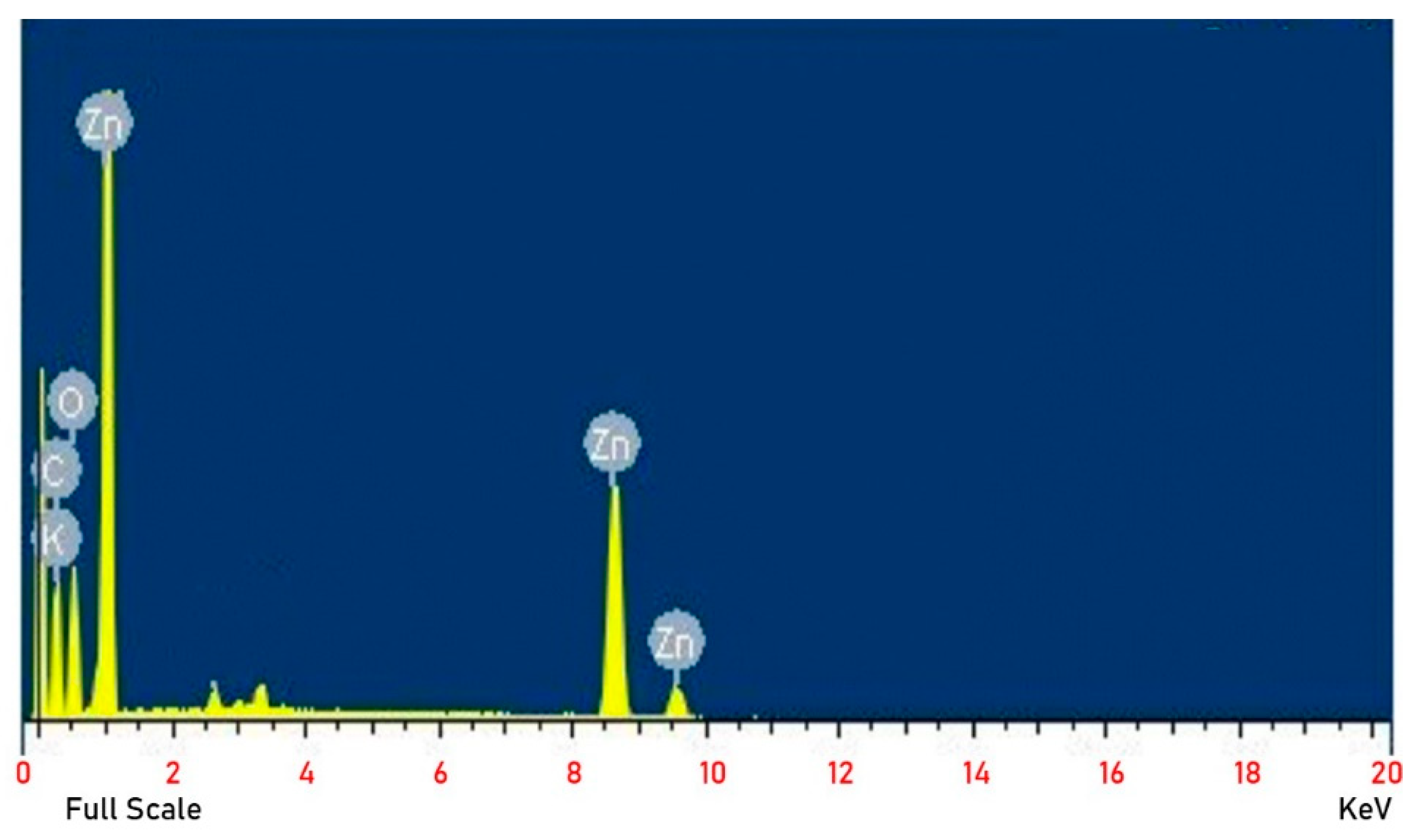
3.4. Composition
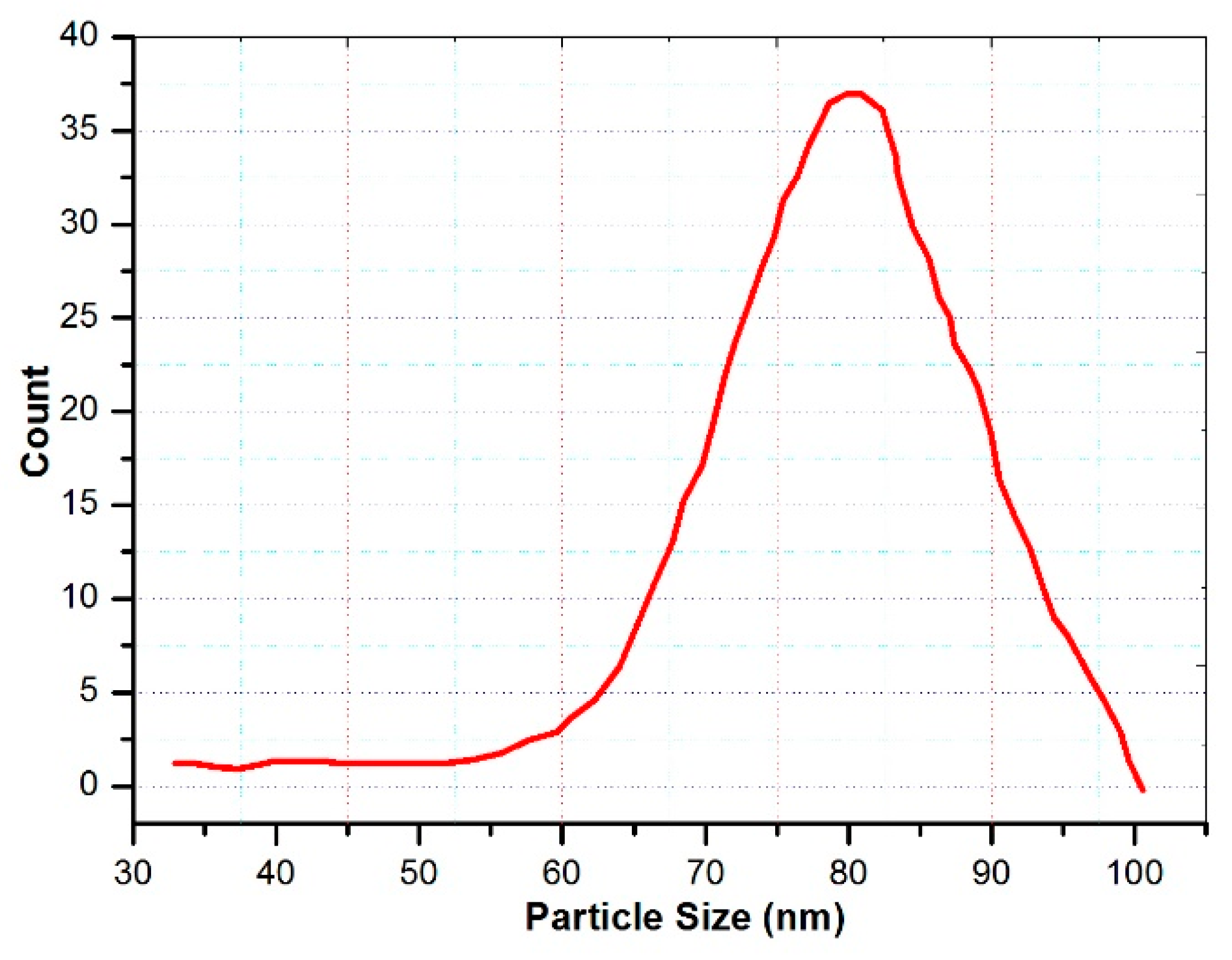
3.5. Particle Size and Distribution Analysis
3.6. UV–Visible Analysis
3.7. Mechanical Properties
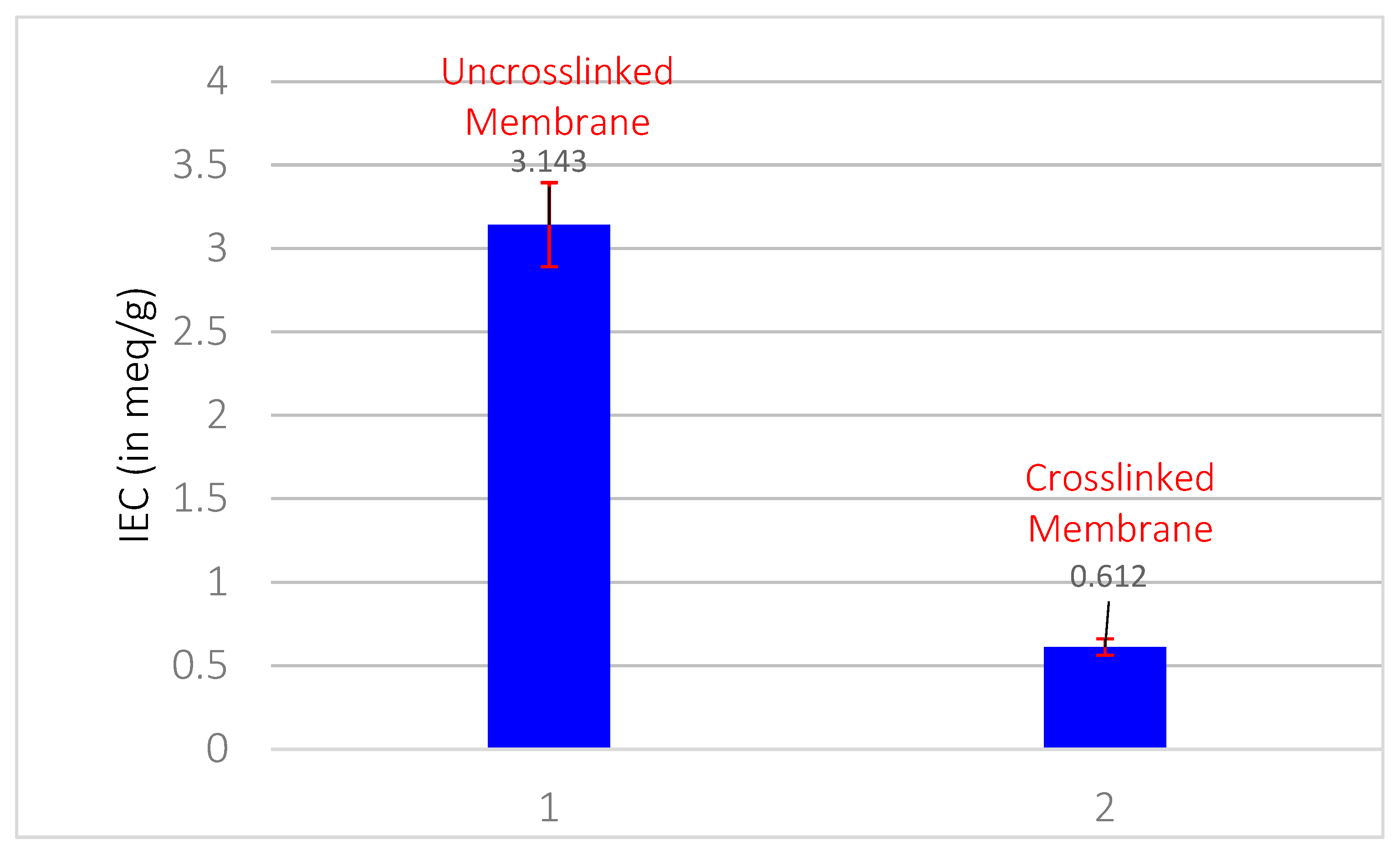
3.8. IEC Results
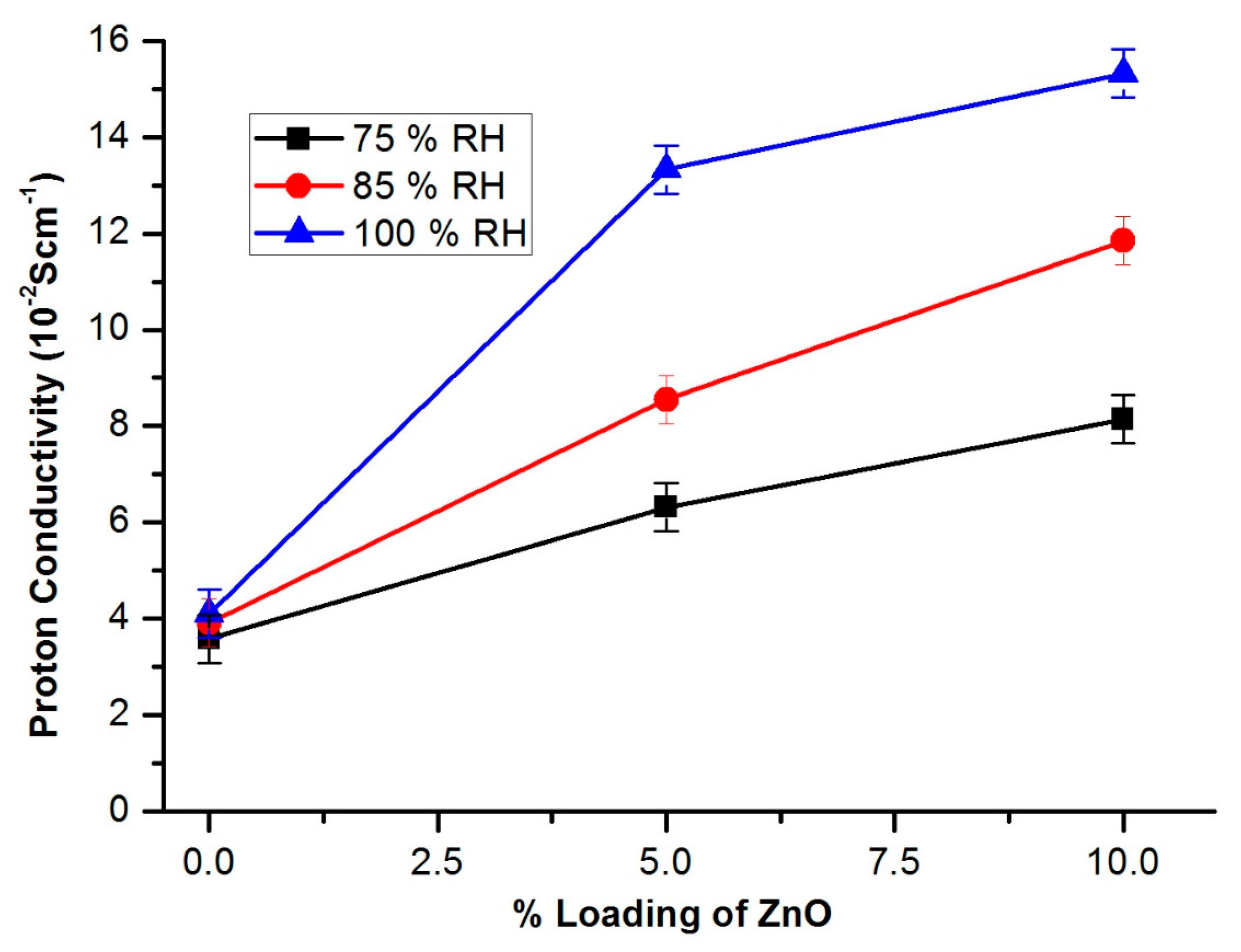
3.9. Fuel Cell Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K. MOFs as proton conductors-challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef] [PubMed]
- Carrette, L.; Friedrich, K.L.; Stimming, U. Fuel cells-fundamentals and applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Mahreni, A.; Mohamad, A.B.; Kadhum, A.A.H.; Daud, W.R.W.; Iyuke, S.E. Nafion/silicon oxide/phosphotungstic acid nanocomposite membrane with enhanced proton conductivity. J. Membr. Sci. 2009, 327, 32–40. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, F.; Feng, W.; Zou, X.; Zhao, C.; Na, H.; Liu, C.; Sun, F.; Zhu, G. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Road Transport: EU-Wide Carbon Dioxide Emissions Have Increased by 24% Since 1990, Statistisches Bundesamt (Destatis). Available online: https://www.destatis.de/Europa/EN/Topic/Environment-energy/CarbonDioxideRoadTransport.html (accessed on 2 November 2022).
- Slowik, P.; Hall, D.; Lutsey, N.; Nicholas, M.; Wappelhorst, S. Funding the Transition to All Zero-Emission Vehicles. ICCT White Paper. Available online: https://theicct.org/wp-content/uploads/2021/06/Funding_transition_ZEV_20191014.pdf (accessed on 2 November 2022).
- Carey, N.; Steitz, C. EU Proposes Effective Ban for New Fossil-Fuel Cars from 2035. Available online: https://www.reuters.com/business/retail-consumer/eu-proposes-effective-ban-new-fossil-fuel-car-sales-2035-2021-07-14/ (accessed on 2 November 2022).
- Breed, A.K.; Speth, D.; Plötz, P. CO2 fleet regulation and the future market diffusion of zero-emission trucks in Europe. Energy Policy 2021, 159, 112640. [Google Scholar] [CrossRef]
- Samsun, R.C.; Rex, M.; Antoni, L.; Stolten, D. Deployment of Fuel Cell Vehicles and Hydrogen Refueling Station Infrastructure: A Global Overview and Perspectives. Energies 2022, 15, 4975. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Study on the Preparation and Lipophilic Properties of Polyvinyl Alcohol (PVA) Nanofiber Membranes via Green Electrospinning. Nanomaterials 2021, 11, 2514. [Google Scholar] [CrossRef]
- Safi, M.A.; Mantzaras, J.; Prasianakis, N.I.; Lamibrac, A.; Büchi, F.N. A Pore-Level Direct Numerical Investigation of Water Evaporation Characteristics under Air and Hydrogen in the Gas Diffusion Layers of Polymer Electrolyte Fuel Cells. Int. J. Heat Mass Transf. 2019, 129, 1250–1262. [Google Scholar] [CrossRef]
- van Rooij, S.; Magnini, M.; Matar, O.K.; Haussener, S. Numerical Optimization of Evaporative Cooling in Artificial Gas Diffusion Layers. Appl. Therm. Eng. 2021, 186, 116460. [Google Scholar] [CrossRef]
- Qin, C.; Wang, J.; Yang, D.; Li, B.; Zhang, C. Proton Exchange Membrane Fuel Cell Reversal: A Review. Catalysts 2016, 6, 197. [Google Scholar] [CrossRef] [Green Version]
- Herrendörfer, R.; Cochet, M.; Schumacher, J.O. Simulation of Mass and Heat Transfer in an Evaporatively Cooled PEM Fuel Cell. Energies 2022, 15, 2734. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Guan, F.; Liu, Y.; Yang, Q.; Zhang, X.; Xu, Y. Preparation of Electrospun Polyvinyl Alcohol/Nanocellulose Composite Film and Evaluation of Its Biomedical Performance. Gels 2021, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, Q.; Christodoulatos, C.; Korfiatis, G.; Meng, X. Adsorptive filtration of lead by electrospun PVA/PAA nanofiber membranes in a fixed-bed column. Chem. Eng. J. 2019, 370, 1262–1273. [Google Scholar] [CrossRef]
- Zhu, M.; Hua, D.; Pan, H.; Wang, F.; Manshian, B.; Soenen, S.J.; Xiong, R.; Huang, C. Green electrospun and crosslinked poly (vinyl alcohol)/poly (acrylic acid) composite membranes for antibacterial effective air filtration. J. Colloid Interface Sci. 2018, 511, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, X.-Z.; Huang, T.; Zhang, N.; Qi, X.-D.; Yang, J.-H.; Zhou, Z.-W.; Wang, Y. Electrospun fibrous membranes with dual-scaled porous structure: Super hydrophobicity, super lipophilicity, excellent water adhesion, and anti-icing for highly efficient oil adsorption/separation. ACS Appl. Mater. Interfaces 2019, 11, 5073–5083. [Google Scholar] [CrossRef]
- Bazargan, A.M.; Fateminia, S.M.A.; Ganji, M.E.; Bahrevar, M.A. Electrospinning preparation and characterization of cadmium oxide nanofibers. Chem. Eng. J. 2009, 155, 523e7. [Google Scholar] [CrossRef]
- Patil, M. Synthesis and Characterization of Zinc Oxide based Poly Vinylalcohol nanocomposite Membranes and their Fuel Cell Measurement. Rev. Roum. Chim. 2021, 66, 391–397. [Google Scholar]
- Patil, M.; Mathad, S.N.; Patil, A.Y.; Arshad, M.N.; Alorfi, H.S.; Puttegowda, M.; Asiri, A.M.; Khan, A.; Azum, N. Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starchmethacrylate and Their Evaluation for Gas Transport Properties. Polymers 2022, 14, 350. [Google Scholar] [CrossRef]
- Patil, M.B. Synthesis and Characterization of heteropolyacid (H3PW12O40) embedded Poly (vinyl alcohol)-g-Acrylamide Copolymeric Membranes and their Evaluation for Proton Exchange Membrane Fuel Cells. Mater. Sci. Energy Technol. 2020, 3, 846–852. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Srinivasa Rao, S.N.; Basaveswara Rao, M.V. Structural and Optical Investigation of ZnO Nanopowders Synthesized from Zinc Chloride and Zinc Nitrate. Am. J. Mater. Sci. 2015, 5, 66–68. [Google Scholar]
- Tominov, R.V.; Vakulov, Z.E.; Avilov, V.I.; Khakhulin, D.A.; Fedotov, A.A.; Zamburg, E.G.; Smirnov, V.A.; Ageev, O.A. Synthesis and Memristor Effect of a Forming-Free ZnO Nanocrystalline Films. Nanomaterials 2020, 10, 1007. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc Oxide and Zinc Oxide Nanoparticles Impact on In Vitro Germination and Seedling Growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Bigdeli, F.; Morsali, A.; Retalleau, P. Synthesis and Characterization of Different zinc (II) Oxide Nano-Structures from Direct Thermal Decomposition of ID Coordination Polymers. Polyhedron 2010, 29, 801–806. [Google Scholar] [CrossRef]
- Beams, R.; Woodcock, J.W.; Gilman, J.W.; Stranick, S.J. Phase Mask-Based Multimodal Superresolution Microscopy. Photonics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of Nanocomposite Film Comprising of Polyvinyl Alcohol (PVA) Incorporated with Bacterial Cellulose Nanocrystals and Magnetite Nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef] [PubMed]
- Fuku, X.; Diallo, A.; Maaza, M. Nanoscaled electrocatalytic optically modulated ZnO nanoparticles through green process of Punica granatum L. and their antibacterial activities. Int. J. Electrochem. 2016, 2016, 4682967. [Google Scholar] [CrossRef]
- Fuku, X.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Punicalagin green functionalized Cu/Cu2O/ZnO/CuO nanocomposite for potential electrochemical transducer and catalyst. Nanoscale Res. Lett. 2016, 11, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Patil, M.B.; Rajamani, S.B.; Mathad, S.N.; Patil, A.Y.; Hussain, M.A.; Alorfii, H.S.; Khan, A.; Asiri, A.M.; Khan, I.; Puttegowda, M. Microwave-assisted synthesis of poly (acrylamide-co-2-hydroxyethyl methacrylate)/chitosan semi-IPN ZnO nanocomposite membranes for food packaging applications. J. Mater. Res. Technol. 2022, 20, 3537–3548. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Mareev, S. Ion-Exchange Membranes and Processes (Volume II). Membranes 2021, 11, 816. [Google Scholar] [CrossRef] [PubMed]






| Sl. No. | ZnO (gm) | ZnO (%) | Name |
|---|---|---|---|
| 1 | 0.15 | 2.5 | M_1 |
| 2 | 0.3 | 5 | M_2 |
| 3 | 0.6 | 10 | M_3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaibi, A.A.; Patil, M.B.; Rajamani, S.B.; Mathad, S.N.; Patil, A.Y.; Amshumali, M.K.; Shaik, J.P.; Asiri, A.M.; Khan, A. Development and Testing of Zinc Oxide Embedded Sulfonated Poly (Vinyl Alcohol) Nanocomposite Membranes for Fuel Cells. Crystals 2022, 12, 1739. https://doi.org/10.3390/cryst12121739
Otaibi AA, Patil MB, Rajamani SB, Mathad SN, Patil AY, Amshumali MK, Shaik JP, Asiri AM, Khan A. Development and Testing of Zinc Oxide Embedded Sulfonated Poly (Vinyl Alcohol) Nanocomposite Membranes for Fuel Cells. Crystals. 2022; 12(12):1739. https://doi.org/10.3390/cryst12121739
Chicago/Turabian StyleOtaibi, Ahmed Al, Mallikarjunagouda B. Patil, Shwetarani B. Rajamani, Shridhar N. Mathad, Arun Y. Patil, M. K. Amshumali, Jilani Purusottapatnam Shaik, Abdullah M. Asiri, and Anish Khan. 2022. "Development and Testing of Zinc Oxide Embedded Sulfonated Poly (Vinyl Alcohol) Nanocomposite Membranes for Fuel Cells" Crystals 12, no. 12: 1739. https://doi.org/10.3390/cryst12121739
APA StyleOtaibi, A. A., Patil, M. B., Rajamani, S. B., Mathad, S. N., Patil, A. Y., Amshumali, M. K., Shaik, J. P., Asiri, A. M., & Khan, A. (2022). Development and Testing of Zinc Oxide Embedded Sulfonated Poly (Vinyl Alcohol) Nanocomposite Membranes for Fuel Cells. Crystals, 12(12), 1739. https://doi.org/10.3390/cryst12121739








