Computational Screening and Experimental Validation on Multicomponent Crystals of a New Class of Janus Kinase (JAK) Inhibitor Drug with Improved Solubility
Abstract
1. Introduction
2. Theoretical Prediction Models
3. Experimental Section
3.1. Materials
3.2. Preparation of Multicomponent Crystal Forms
3.3. Virtual Coformer Screening
3.4. Powder X-ray Diffraction (PXRD)
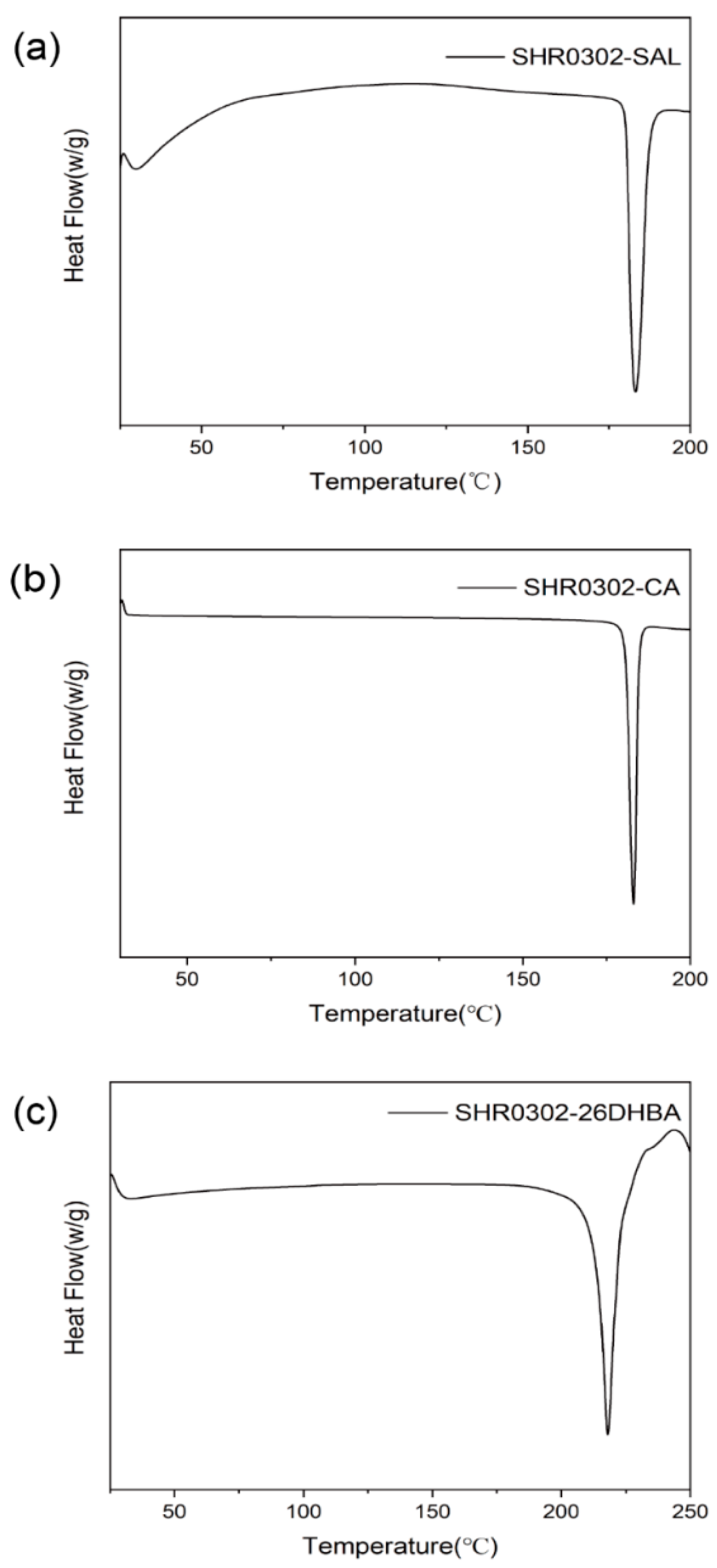
3.5. Thermal Analysis
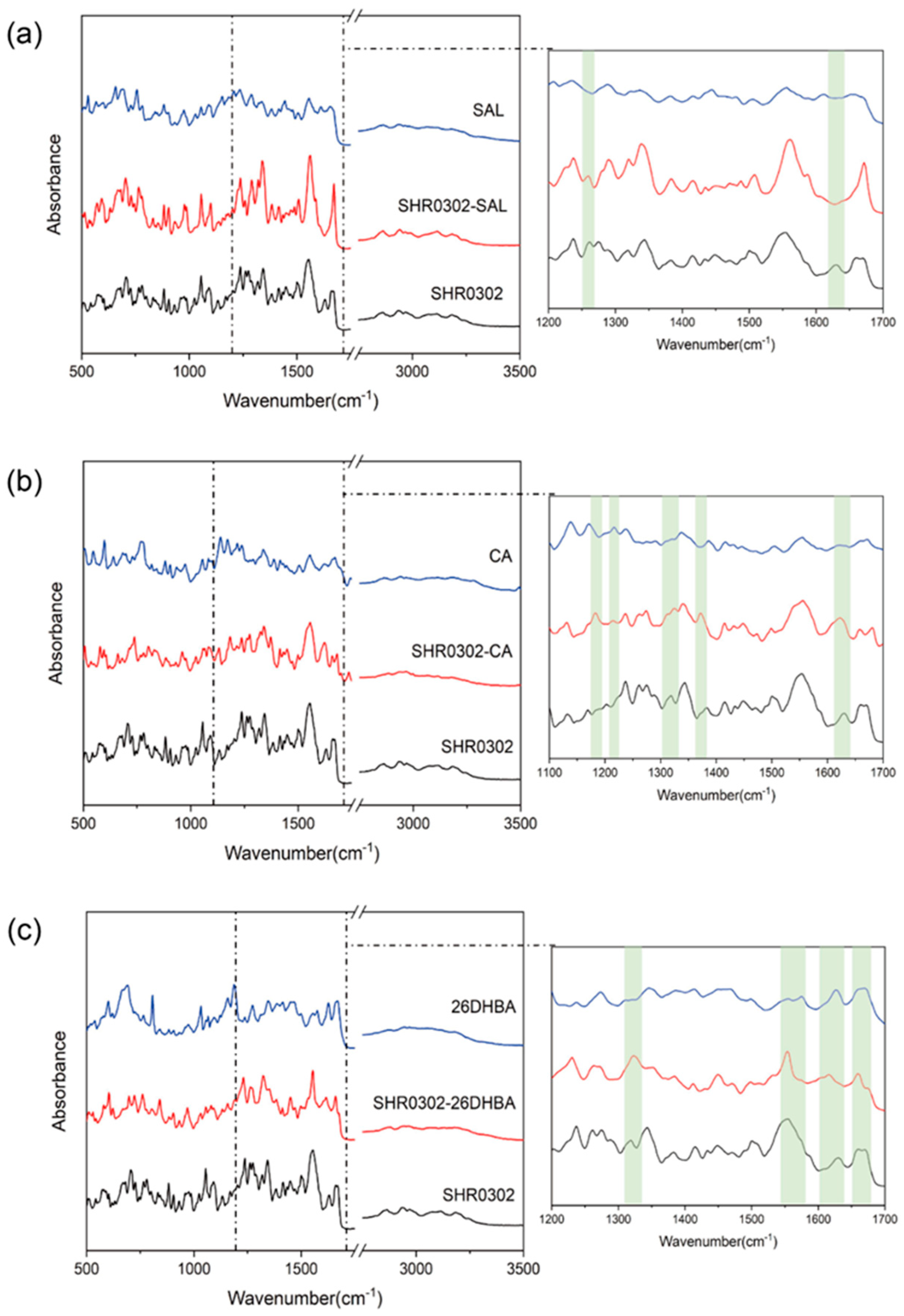
3.6. Fourier-Transformed Infrared Spectrometer (FTIR)
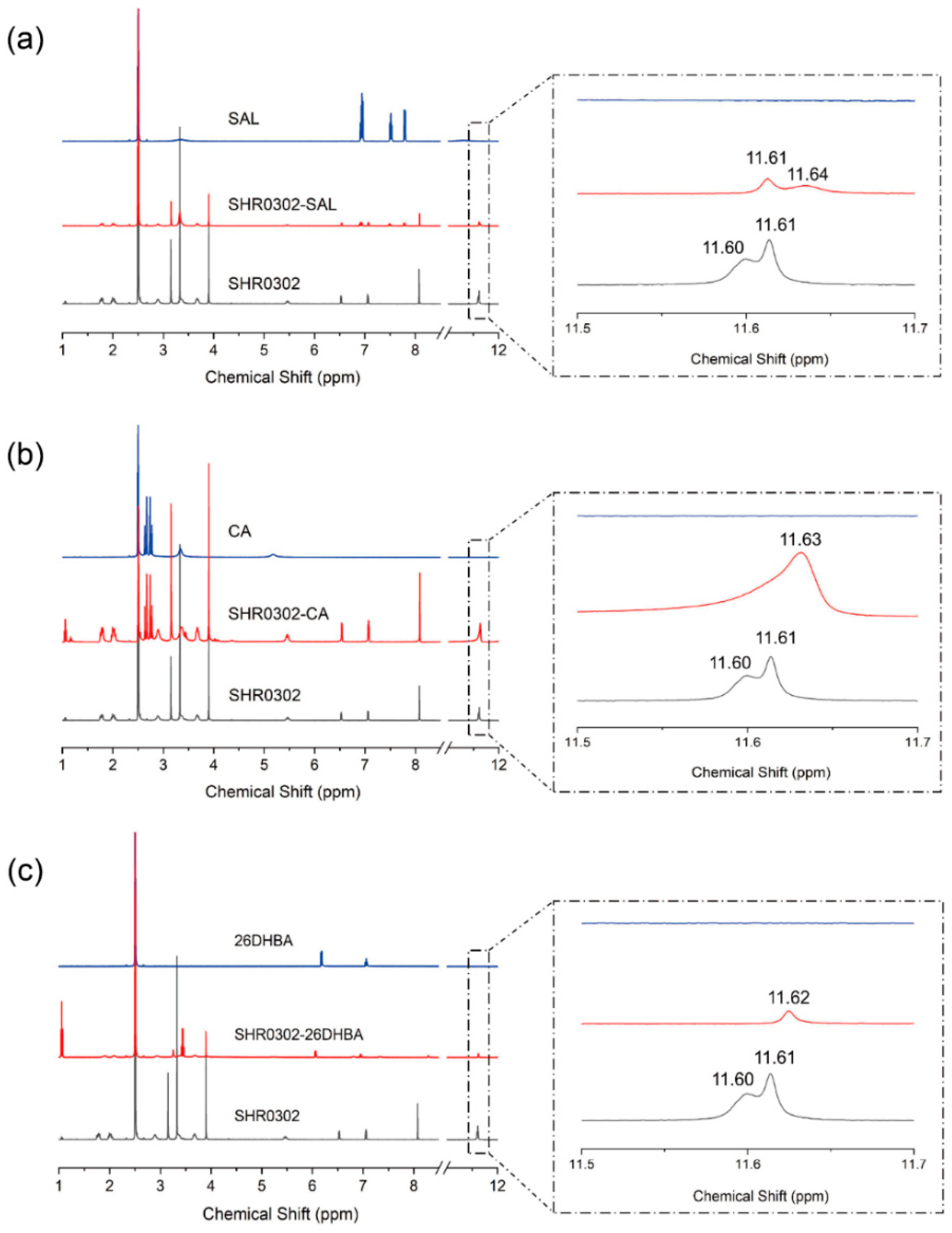
3.7. NMR Spectroscopy
3.8. Solubility Measurement
4. Results and Discussion
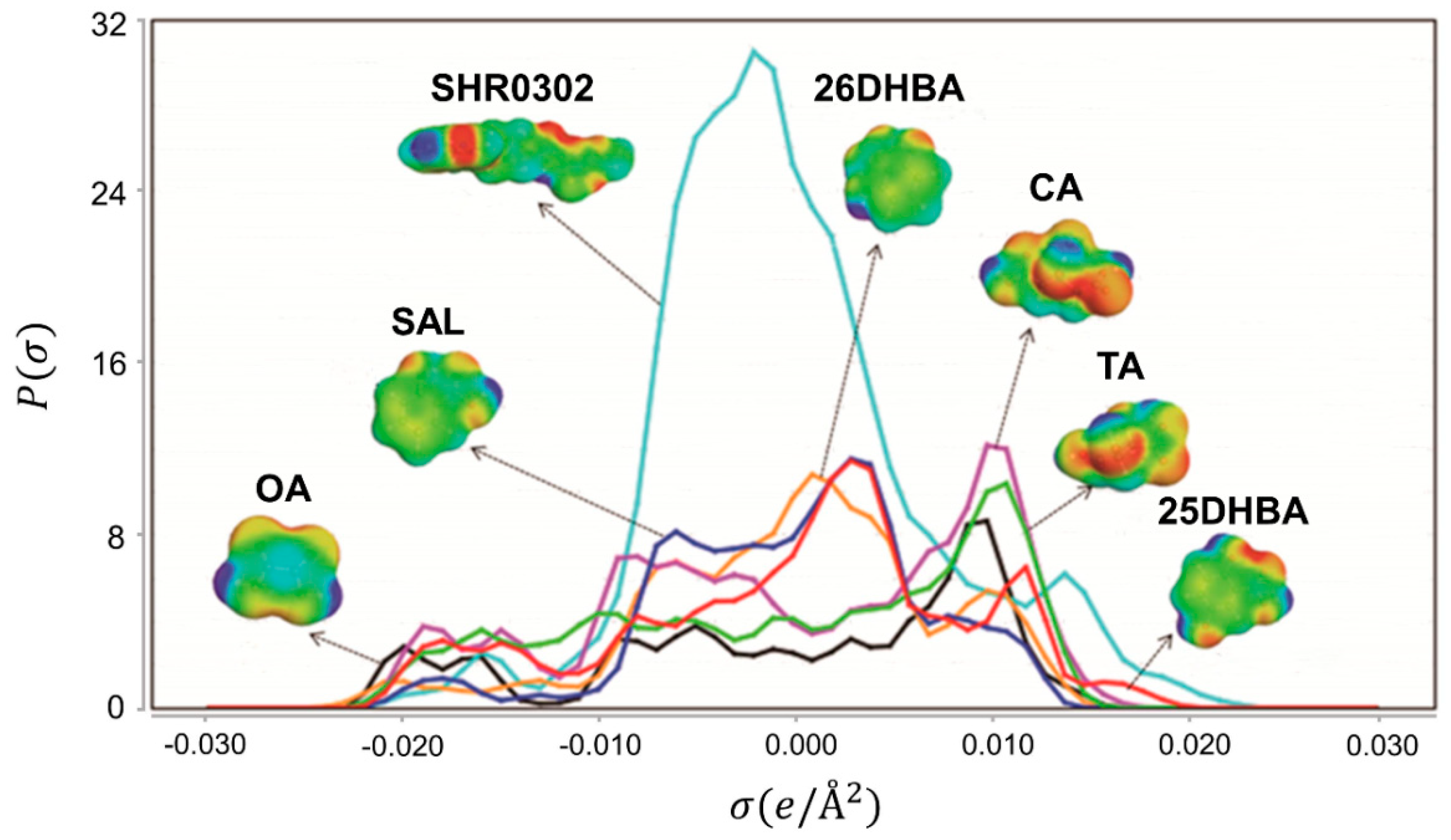
4.1. Virtual Coformer Screening
4.2. Solid-State Characterization
4.2.1. Power X-ray Diffraction (PXRD) Analysis
4.2.2. Thermal Analysis
4.2.3. FTIR Spectroscopy
4.2.4. 1H-NMR Spectroscopy
4.2.5. Intermolecular Interaction Analysis
4.3. Solubility Properties of SHR0302 Multicomponent Crystalline Forms
4.4. Evaluation of COSMO-RS Prediction Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef]
- Rajput, L.; Sanphui, P.; Desiraju, G.R. New solid forms of the anti-HIV drug etravirine: Salts, cocrystals, and solubility. Cryst. Growth Des. 2013, 13, 3681–3690. [Google Scholar] [CrossRef]
- Kaplinsky, E. Sacubitril/valsartan in heart failure: Latest evidence and place in therapy. Ther. Adv. Chronic Dis. 2016, 7, 278–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Wang, H.; He, Z.; Liu, H. Sacubitril-valsartan cocrystal revisited: Role of polymer excipients in the formulation. Expert Opin. Drug Deliv. 2021, 18, 515–526. [Google Scholar] [CrossRef]
- Almansa, C.; Merce, R.; Tesson, N.; Farran, J.; Tomas, J.; Plata-Salaman, C.R. Co-Crystal of Tramadol hydrochloride–Celecoxib (CTC): A novel API–API co-crystal for the treatment of pain. Cryst. Growth Des. 2017, 17, 1884–1892. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Liongue, C.; Sertori, R.; Ward, A.C. Evolution of cytokine receptor signaling. J. Immunol. 2016, 197, 11–18. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and future status of JAK inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef]
- Wu, H.; Yan, S.; Chen, J.; Luo, X.; Li, P.; Jia, X.; Dai, X.; Wang, C.; Huang, Q.; Liu, L.; et al. JAK1-STAT3 blockade by JAK inhibitor SHR0302 attenuates inflammatory responses of adjuvant-induced arthritis rats and decreases Th17 and total B cells. Jt. Bone Spine 2016, 83, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Ding, Y.; Tao, X.; Ji, C.; Dong, X.; Lu, J.; Wu, L.; Wang, R.; Lu, Q.; et al. Efficacy and Safety of SHR0302, a Highly Selective Janus Kinase 1 Inhibitor, in Patients with Moderate to Severe Atopic Dermatitis: A Phase II Randomized Clinical Trial. Am. J. Clin. Dermatol. 2021, 22, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, Q.; Yang, J.; Wang, A.; Zhang, F.; Qiu, H.; Zhou, K.; Wang, P.; Ding, X.; Yuan, X.; et al. Preventive and Therapeutic Effects of a Novel JAK Inhibitor SHR0302 in Acute Graft-Versus-Host Disease. Cell Transplant. 2021, 30, 9636897211033778. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E. Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization; Academic Press: San Diego, CA, USA, 2008; pp. 61–92. [Google Scholar]
- Marques, M.R.; Choo, Q.; Ashtikar, M.; Rocha, T.C.; Bremer-Hoffmann, S.; Wacker, M.G. Nanomedicines-tiny particles and big challenges. Adv. Drug Deliv. Rev. 2019, 151, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Fernández Casares, A.; Nap, W.M.; Ten Figás, G.; Huizenga, P.; Groot, R.; Hoffmann, M. An evaluation of salt screening methodologies. J. Pharm. Pharmacol. 2015, 67, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, B.; Jia, L.; Wang, Y.; Wang, M.; Yang, H.; Qiao, Y.; Gong, J.; Tang, W. Tuning physicochemical properties of antipsychotic drug aripiprazole with multicomponent crystal strategy based on structure and property relationship. Cryst. Growth Des. 2020, 20, 3747–3761. [Google Scholar] [CrossRef]
- Etter, M.C. Hydrogen bonds as design elements in organic chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the p K a rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. WIREs. Comput. Mol. Sci. 2011, 1, 699–709. [Google Scholar] [CrossRef]
- Loschen, C.; Klamt, A. Computational screening of drug solvates. Pharm. Res. 2016, 33, 2794–2804. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, J.; Xiao, Y.; Ji, X.; Li, C.; Zhang, B.; Hou, B.; Zhou, L.; Xie, C.; Gong, J.; et al. New salts and cocrystals of pymetrozine with improvements on solubility and humidity stability: Experimental and theoretical study. Cryst. Growth Des. 2021, 21, 2371–2388. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Wang, M.; Wang, Y.; Du, S.; Gong, J.; Wu, S. Intermolecular interactions and solubility behavior of multicomponent crystal forms of orotic acid: Prediction and experiments. Cryst. Growth Des. 2021, 21, 1473–1481. [Google Scholar] [CrossRef]
- Fábián, L. Cambridge structural database analysis of molecular complementarity in cocrystals. Cryst. Growth Des. 2009, 9, 1436–1443. [Google Scholar] [CrossRef]
- Loschen, C.; Klamt, A. Solubility prediction, solvate and cocrystal screening as tools for rational crystal engineering. J. Pharm. Pharmacol. 2015, 67, 803–811. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef]
- Sarkar, N.; Gonnella, N.C.; Krawiec, M.; Xin, D.; Aakeröy, C.B. Evaluating the predictive abilities of protocols based on hydrogen-bond propensity, molecular complementarity, and hydrogen-bond energy for cocrystal screening. Cryst. Growth Des. 2020, 20, 7320–7327. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Pallipurath, A.R.; Civati, F.; Eziashi, M.; Omar, E.; McArdle, P.; Erxleben, A. Tailoring Cocrystal and Salt Formation and Controlling the Crystal Habit of Diflunisal. Cryst. Growth Des. 2016, 16, 6468–6478. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wu, G.; Gao, X.; Shen, L. A Bisulfate of JAK Kinase Inhibitor and Its Preparation Method. CN104470927B, 4 May 2016. [Google Scholar]
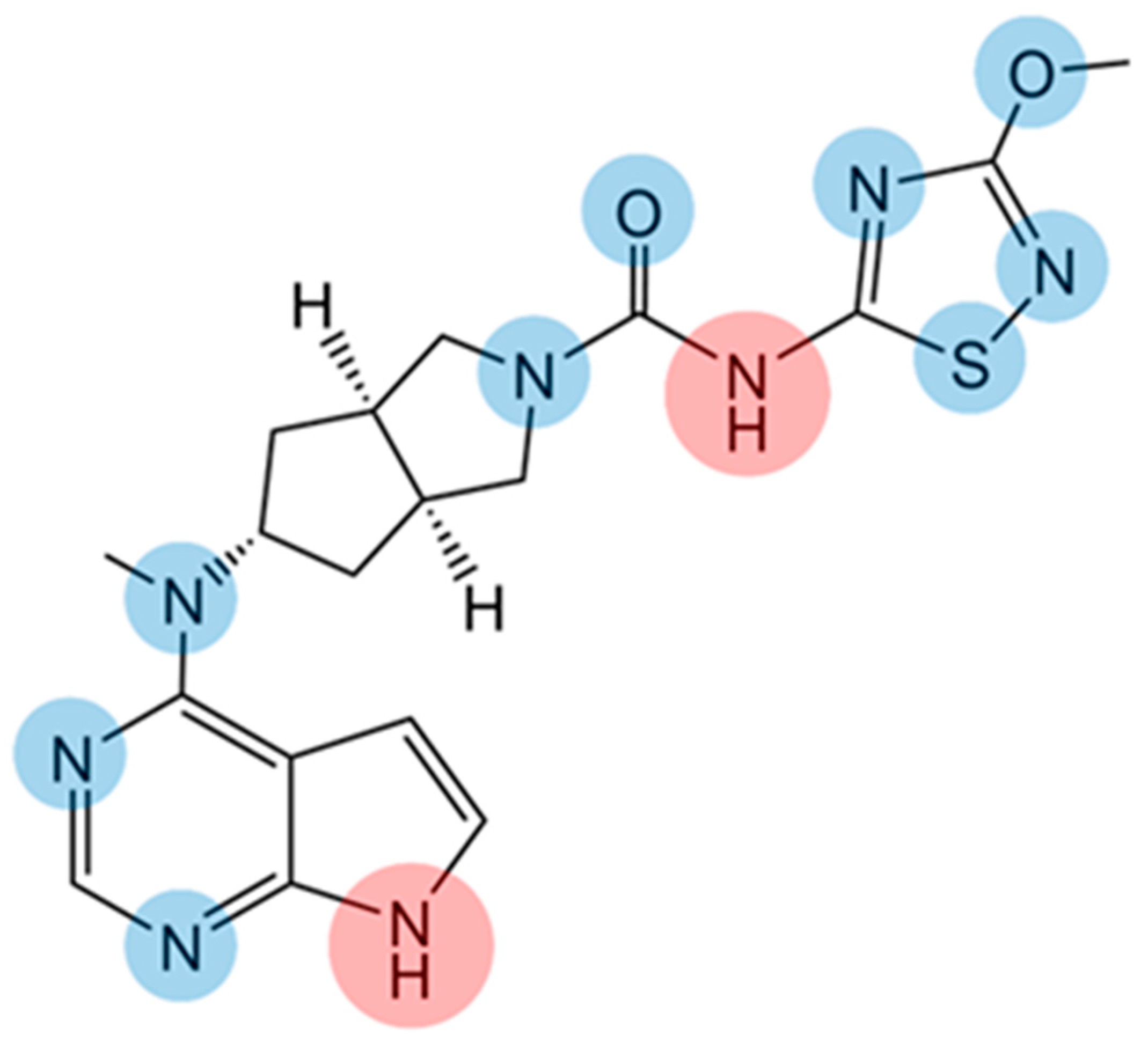




| Water | 0.1 M HCl | |
|---|---|---|
| SHR0302 | 0.10 | \ |
| SHR0302-SAL | 0.17 | 1.56 |
| SHR0302-CA | 0.33 | 1.30 |
| SHR0302-26DHBA | 0.02 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Shi, G.; Sun, J.; Li, S.; Gao, W.; Hu, Y.; Zu, C.; Tang, W.; Gong, J. Computational Screening and Experimental Validation on Multicomponent Crystals of a New Class of Janus Kinase (JAK) Inhibitor Drug with Improved Solubility. Crystals 2022, 12, 1722. https://doi.org/10.3390/cryst12121722
Xie Y, Shi G, Sun J, Li S, Gao W, Hu Y, Zu C, Tang W, Gong J. Computational Screening and Experimental Validation on Multicomponent Crystals of a New Class of Janus Kinase (JAK) Inhibitor Drug with Improved Solubility. Crystals. 2022; 12(12):1722. https://doi.org/10.3390/cryst12121722
Chicago/Turabian StyleXie, Yujiang, Genpei Shi, Jie Sun, Si Li, Wei Gao, Yimin Hu, Chang Zu, Weiwei Tang, and Junbo Gong. 2022. "Computational Screening and Experimental Validation on Multicomponent Crystals of a New Class of Janus Kinase (JAK) Inhibitor Drug with Improved Solubility" Crystals 12, no. 12: 1722. https://doi.org/10.3390/cryst12121722
APA StyleXie, Y., Shi, G., Sun, J., Li, S., Gao, W., Hu, Y., Zu, C., Tang, W., & Gong, J. (2022). Computational Screening and Experimental Validation on Multicomponent Crystals of a New Class of Janus Kinase (JAK) Inhibitor Drug with Improved Solubility. Crystals, 12(12), 1722. https://doi.org/10.3390/cryst12121722









