The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
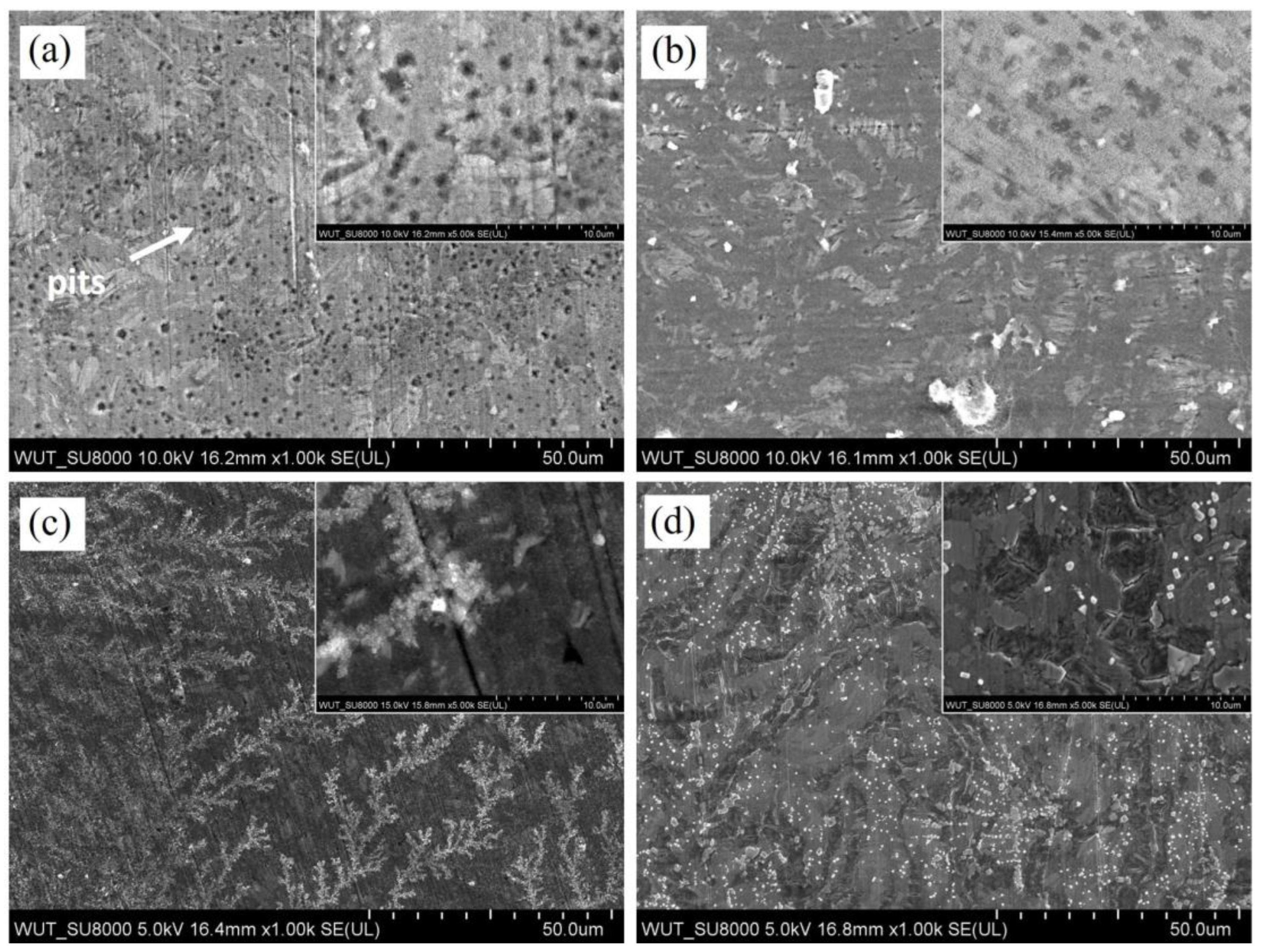
3.1. Microstructure Characterization
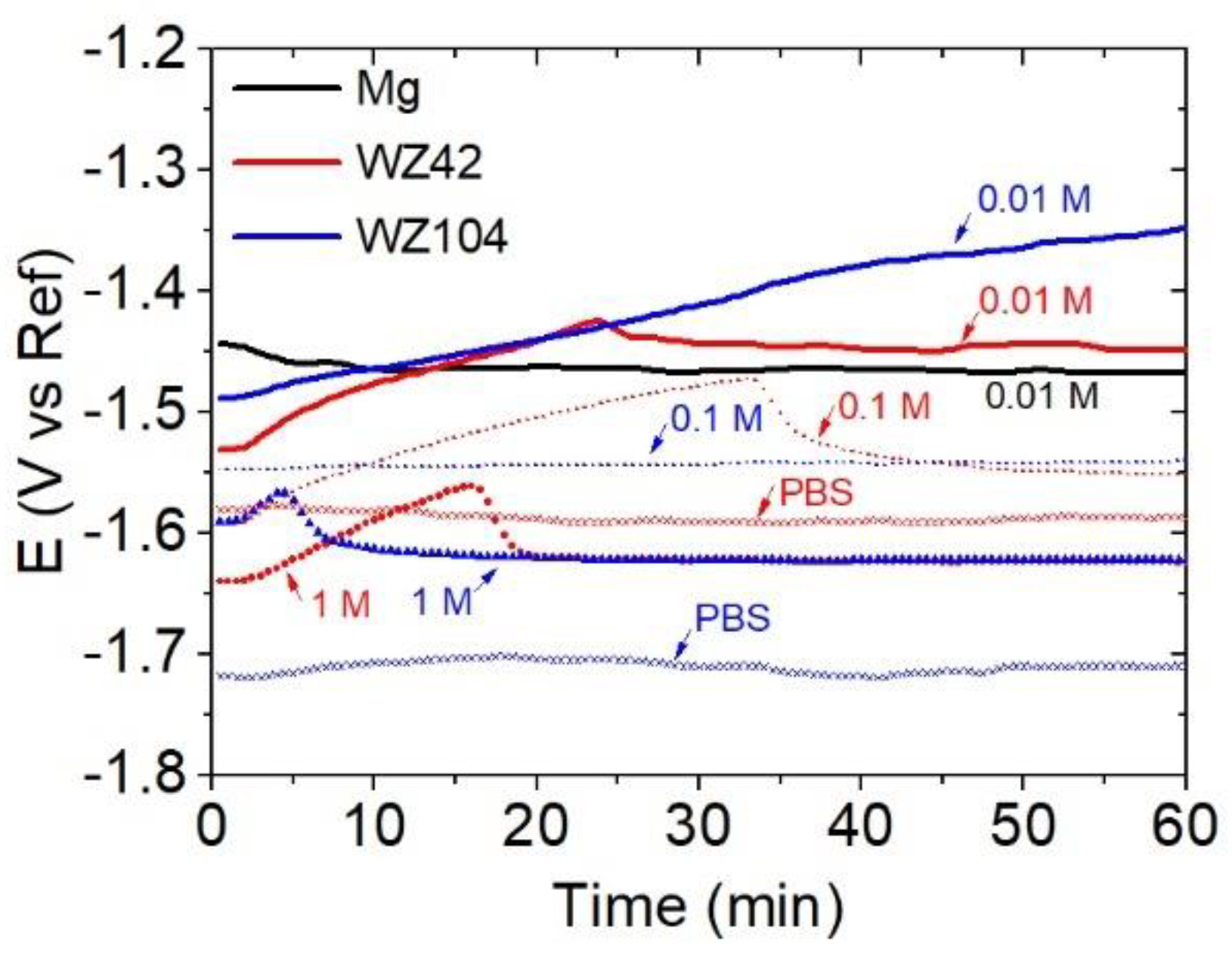
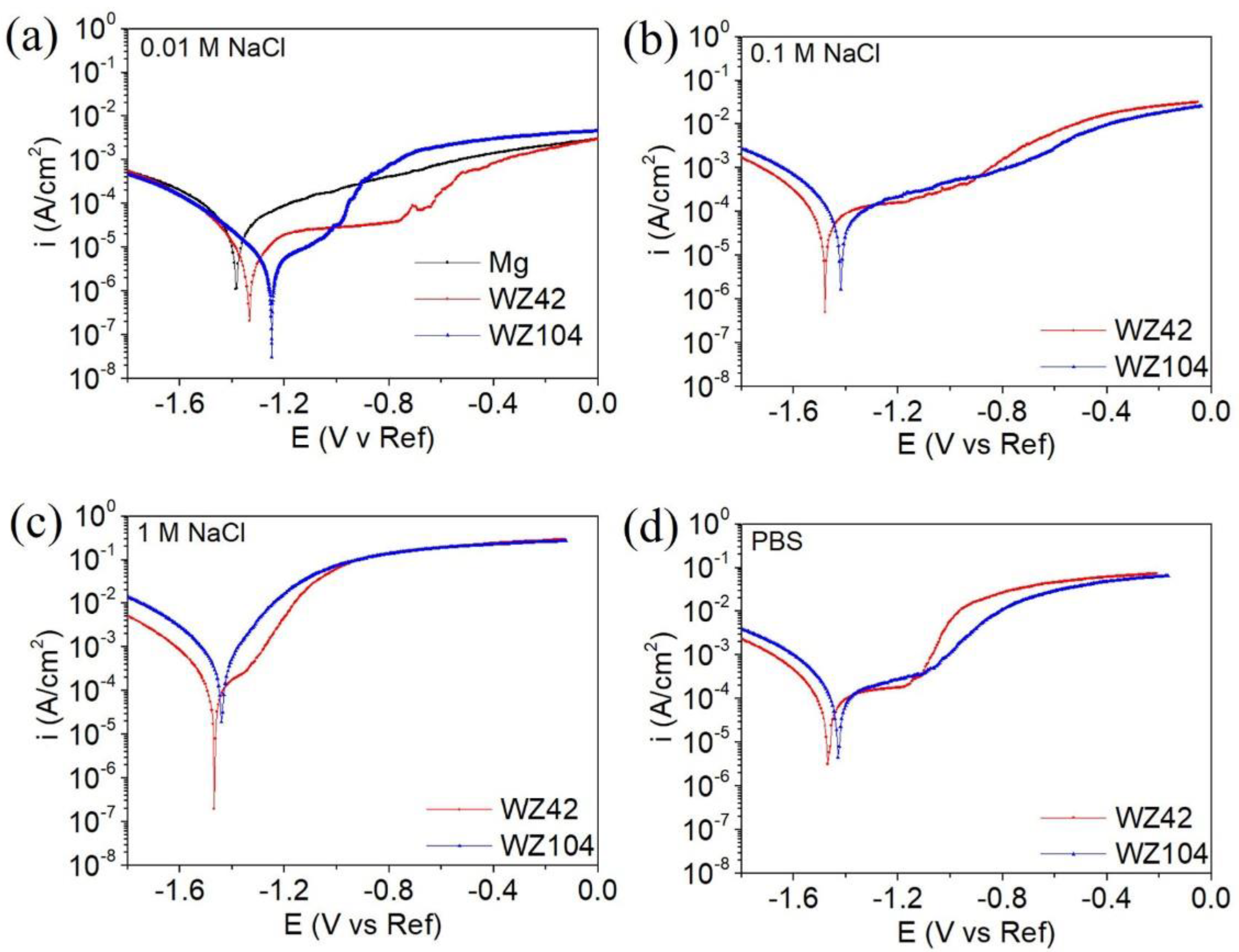
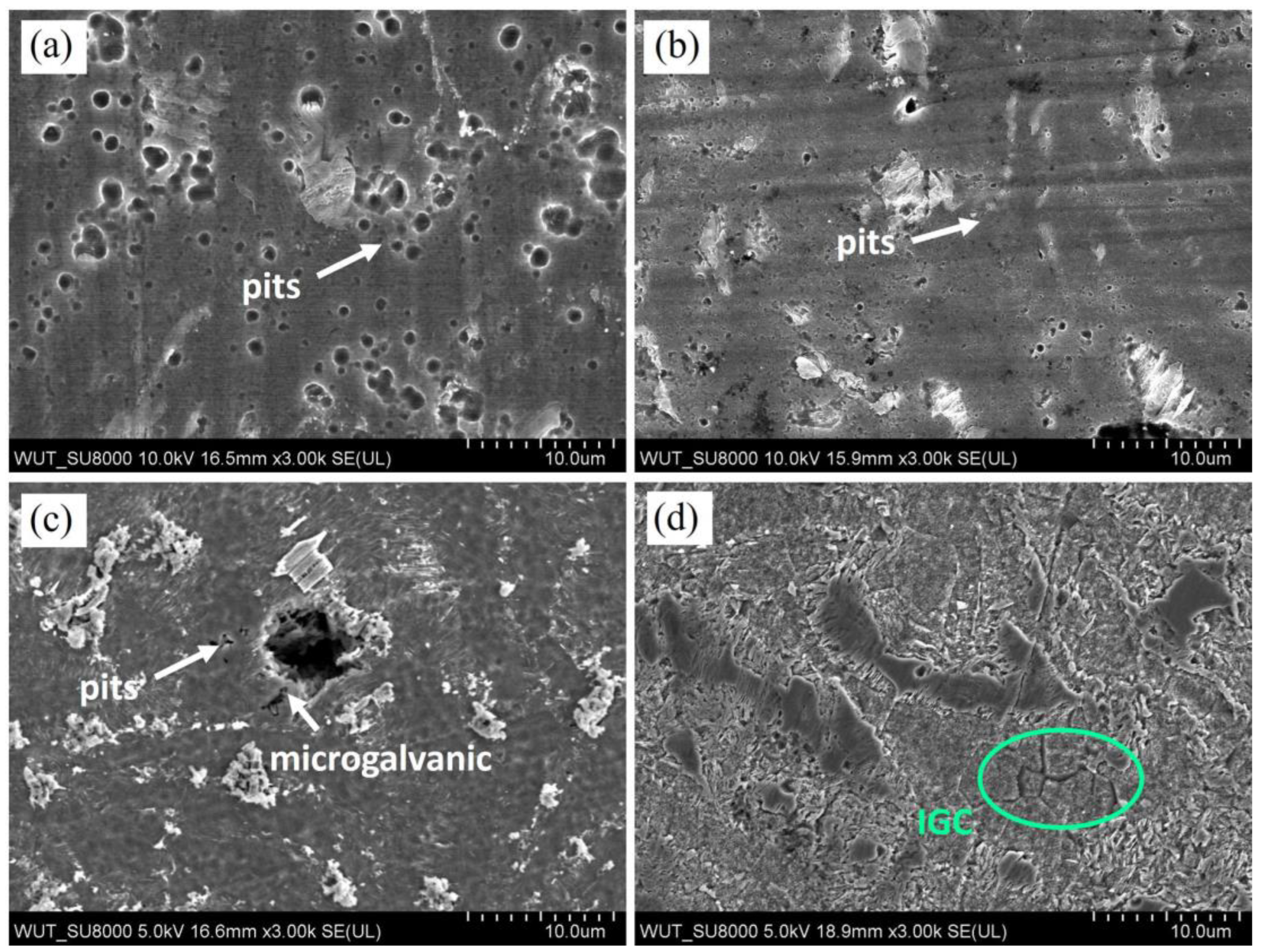
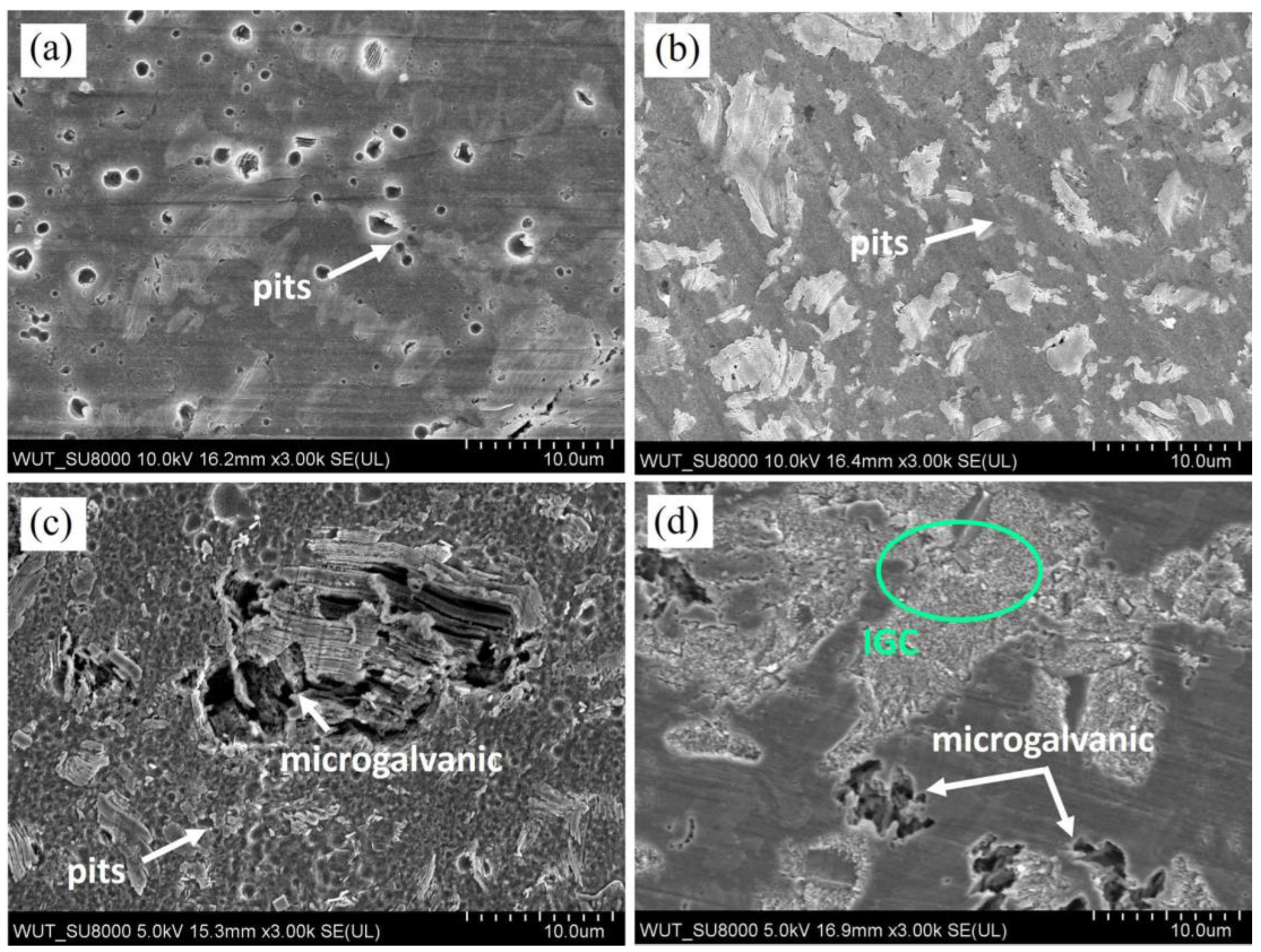
3.2. Corrosion Testing
4. Discussion
5. Conclusions
- The corrosion mechanisms occurring in the case Mg-LPSO alloys are dependent on the volume fraction of LPSO phase, its distribution, and corrosion conditions (corrosive medium);
- Depending on the corrosion medium, various corrosion mechanisms dominate. In 0.01 M NaCl and 0.1 M NaCl pitting is the dominant mechanism, while in 1M NaCl, pitting and microgalvanic corrosion between LPSO phase-Mg matrix occur. In PBS, microgalvanic and intragranular corrosion formed; however, their attack was partially slowed down by the formation of corrosion products containing P;
- Although PBS is a more complex medium for unveiling mechanistic information regarding the degradation of Mg-LPSO alloys, it gives additional protection and thus more realistic results when the purpose of the work is the development of materials for biomedical applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yokota, A.; Matsushita, M.; Geshi, N.; Yamasaki, D.; Shinmei, T.; Yamasaki, M.; Kawamura, Y. Formation process of long-period stacking-ordered structures in Mg97Zn1Y2 alloy comprising HCP and cubic phases fabricated by high-pressure high-temperature annealing. Metals 2021, 11, 1031. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Sun, M.; Ding, W. Recent developments and applications on high-performance cast magnesium rare-earth alloys. J. Magnes. Alloy 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Meier, J.M.; Miao, J.; Liang, S.M.; Zhu, J.; Zhang, C.; Caris, J.; Luo, A.A. Phase equilibria and microstructure investigation of Mg-Gd-Y-Zn alloy system. J. Magnes. Alloy 2021, 10, 689–696. [Google Scholar] [CrossRef]
- Li, J.; Albu, M.; Wu, Y.; Peng, L.; Dienstleder, M.; Kothleitner, G.; Hofer, F.; Schumacher, P. Precipitation of long-period stacking ordered structure in Mg-Gd-Zn-Mn Alloy. Adv. Eng. Mater. 2017, 19, 1600705. [Google Scholar] [CrossRef]
- Lu, F.; Ma, A.; Jiang, J.; Yang, D.; Zhou, Q. Review on long-period stacking-ordered structures in Mg-Zn-RE alloys. Rare Met. 2012, 31, 303–310. [Google Scholar] [CrossRef]
- Nie, Y.; Dai, J.; Li, X.; Zhang, X. Recent developments on corrosion behaviors of Mg alloys with stacking fault or long period stacking ordered structures. J. Magnes. Alloy 2021, 9, 1123–1146. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Feyerabend, F.; Willumeit, R.; Mendis, C.; Kainer, K.U.; Hort, N. Microstructure, mechanical and corrosion properties of Mg–Dy–Gd–Zr alloys for medical applications. Acta Biomater. 2013, 9, 8499–8508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ba, Z.; Wang, Z.; Wu, Y.; Xue, Y. Effect of LPSO structure on mechanical properties and corrosion behavior of as-extruded GZ51K magnesium alloy. Mater. Lett. 2016, 163, 250–253. [Google Scholar] [CrossRef]
- Chen, R.; Sandlöbes, S.; Zeng, X.; Li, D.; Korte-Kerzel, S.; Raabe, D. Room temperature deformation of LPSO structures by non-basal slip. Mater. Sci. Eng. A 2017, 682, 354–358. [Google Scholar] [CrossRef]
- Wang, W.Y.; Shang, S.L.; Wang, Y.; Darling, K.A.; Kecskes, L.J.; Mathaudhu, S.N.; Hui, X.D.; Liu, Z.K. Electronic structures of long periodic stacking order structures in Mg: A first-principles study. J. Alloys Compd. 2014, 586, 656–662. [Google Scholar] [CrossRef]
- Briffod, F.; Shiraiwa, T.; Enoki, M. The effect of the 18R-LPSO phase on the fatigue behavior of extruded Mg/LPSO two-phase alloy through a comparative experimental-numerical study. J. Magnes. Alloy 2021, 9, 130–143. [Google Scholar] [CrossRef]
- Shao, J.; Chen, Z.; Chen, T.; Hu, Z.; Zhou, X.; Liu, C. The effect of LPSO on the deformation mechanism of Mg–Gd–Y–Zn–Zr magnesium alloy. J. Magnes. Alloy 2016, 4, 83–88. [Google Scholar] [CrossRef]
- Hagihara, K.; Okamoto, T.; Izuno, H.; Yamasaki, M.; Matsushita, M.; Nakano, T.; Kawamura, Y. Plastic deformation behavior of 10H-type synchronized LPSO phase in a Mg–Zn–Y system. Acta Mater. 2016, 109, 90–102. [Google Scholar] [CrossRef]
- Tahreen, N.; Chen, D.L. A critical review of Mg-Zn-Y series alloys containing I, W, and LPSO phases. Adv. Eng. Mater. 2016, 18, 1983–2002. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Zhang, J.; Zhang, L.; Zhang, J.; Xu, C.; Guo, W. Enhanced performance of Mg–Zn–Y–Mn Alloy via minor Ca addition. Adv. Eng. Mater. 2019, 21, 1900908. [Google Scholar] [CrossRef]
- Horváth, K.; Drozdenko, D.; Daniš, S.; Garcés, G.; Máthis, K.; Kim, S.; Dobroň, P. Characterization of microstructure and mechanical properties of Mg-Y-Zn alloys with respect to different content of LPSO phase. Adv. Eng. Mater. 2018, 20, 1700396. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, C.; Chen, X.; Huang, Y.; Hort, N.; Gavras, S.; Pan, F. Compressive deformation of as-extruded LPSO-containing Mg alloys at different temperatures. J. Mater. Res. Technol. 2022, 16, 944–959. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Z.; Yan, K.; Yan, J.; Bai, J.; Jiang, J.; Ma, A. Effect of multi-pass equal channel angular pressing on the microstructure and mechanical properties of a heterogeneous Mg88Y8Zn4 Alloy. J. Mater. Sci. Technol. 2016, 32, 1274–1281. [Google Scholar] [CrossRef]
- Inoue, A.; Kawamura, Y.; Matsushita, M.; Hayashi, K.; Koike, J. Novel hexagonal structure and ultrahigh strength of magnesium solid solution in the Mg–Zn–Y system. J. Mater. Res. 2001, 16, 1894–1900. [Google Scholar] [CrossRef]
- Fekete, K.H.; Drozdenko, D.; Čapek, J.; Máthis, K.; Tolnai, D.; Stark, A.; Garcés, G.; Dobroň, P. Hot deformation of Mg-Y-Zn alloy with a low content of the LPSO phase studied by in-situ synchrotron radiation diffraction. J. Magnes. Alloy 2020, 8, 199–209. [Google Scholar] [CrossRef]
- Pérez, P.; Cabeza, S.; Garcés, G.; Adeva, P. Influence of long period stacking ordered phase arrangements on the corrosion behaviour of extruded Mg97Y2Zn1 alloy. Corros. Sci. 2016, 107, 107–112. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Zeng, Z.R.; Wang, B.J.; Sheng, L.Y.; Chen, X.-B.; Han, E.H. Effect of volume fraction of LPSO phases on corrosion and mechanical properties of Mg-Zn-Y alloys. Mater. Des. 2017, 121, 430–441. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, P.; Zhang, D.; Yu, B.; Xu, Z.; Jiang, H. Effect of LPSO phases and aged-precipitations on corrosion behavior of as-forged Mg–6Gd–2Y–1Zn–0.3Zr alloy. J. Mater. Res. Technol. 2020, 9, 7087–7099. [Google Scholar] [CrossRef]
- Wang, L.S.; Jiang, J.H.; Saleh, B.; Xie, Q.Y.; Xu, Q.; Liu, H.; Ma, A.B. Controlling Corrosion Resistance of a Biodegradable Mg–Y–Zn Alloy with LPSO Phases via Multi-pass ECAP Process. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 1180–1190. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Kurzydlowski, K.J. Stereology of grains in nano-crystals. Solid State Phenom. 2003, 94, 221–228. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Lewandowska, M.; Kurzydłowski, K.J. Stereology of nano-materials. Image Anal. Stereol. 2010, 29, 1–12. [Google Scholar] [CrossRef]
- ASTM G1; Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 1999.
- Abe, E.; Ono, A.; Itoi, T.; Yamasaki, M.; Kawamura, Y. Polytypes of long-period stacking structures synchronized with chemical order in a dilute Mg–Zn–Y alloy. Philos. Mag. Lett. 2011, 91, 690–696. [Google Scholar] [CrossRef]
- Oñorbe, E.; Garcés, G.; Pérez, P.; Adeva, P. Effect of the LPSO volume fraction on the microstructure and mechanical properties of Mg-Y 2X -Zn X alloys. J. Mater. Sci. 2012, 47, 1085–1093. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk-Cieślak, B.; Koralnik, M.; Chromiński, W.; Kubasek, J.; Ciftci, J.; Kuc, D.; Mizera, J. Corrosion behavior of fine-grained Mg-7.5Li-3Al-1Zn fabricated by extrusion with a forward-backward rotating die (KoBo). J. Magnes. Alloy 2021, 10, 811–820. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Q.; Zheng, M.; Hou, B.; Li, Y. Corrosion behavior of Mg-8Li-3Zn-Al alloy in neutral 3.5% NaCl solution. J. Magnes. Alloy 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Poorqasemi, E.; Abootalebi, O.; Peikari, M.; Haqdar, F. Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 2009, 51, 1043–1054. [Google Scholar] [CrossRef]
- Leleu, S.; Rives, B.; Causse, N.; Pébère, N. Corrosion rate determination of rare-earth Mg alloys in a Na2SO4 solution by electrochemical measurements and inductive coupled plasma-optical emission spectroscopy. J. Magnes. Alloy 2019, 7, 47–57. [Google Scholar] [CrossRef]
- Hara, N.; Kobayashi, Y.; Kagaya, D.; Akao, N. Formation and breakdown of surface films on magnesium and its alloys in aqueous solutions. Corros. Sci. 2007, 49, 166–175. [Google Scholar] [CrossRef]
- Ishizaki, T.; Masuda, Y.; Teshima, K. Composite film formed on magnesium alloy AZ31 by chemical conversion from molybdate/phosphate/fluorinate aqueous solution toward corrosion protection. Surf. Coat. Technol. 2013, 217, 76–83. [Google Scholar] [CrossRef]
- Dinodi, N.; Nityananda Shetty, A. Electrochemical investigations on the corrosion behaviour of magnesium alloy ZE41 in a combined medium of chloride and sulphate. J. Magnes. Alloy 2013, 1, 201–209. [Google Scholar] [CrossRef]
- Izumi, S.; Yamasaki, M.; Kawamura, Y. Relation between corrosion behavior and microstructure of Mg–Zn–Y alloys prepared by rapid solidification at various cooling rates. Corros. Sci. 2009, 51, 395–402. [Google Scholar] [CrossRef]
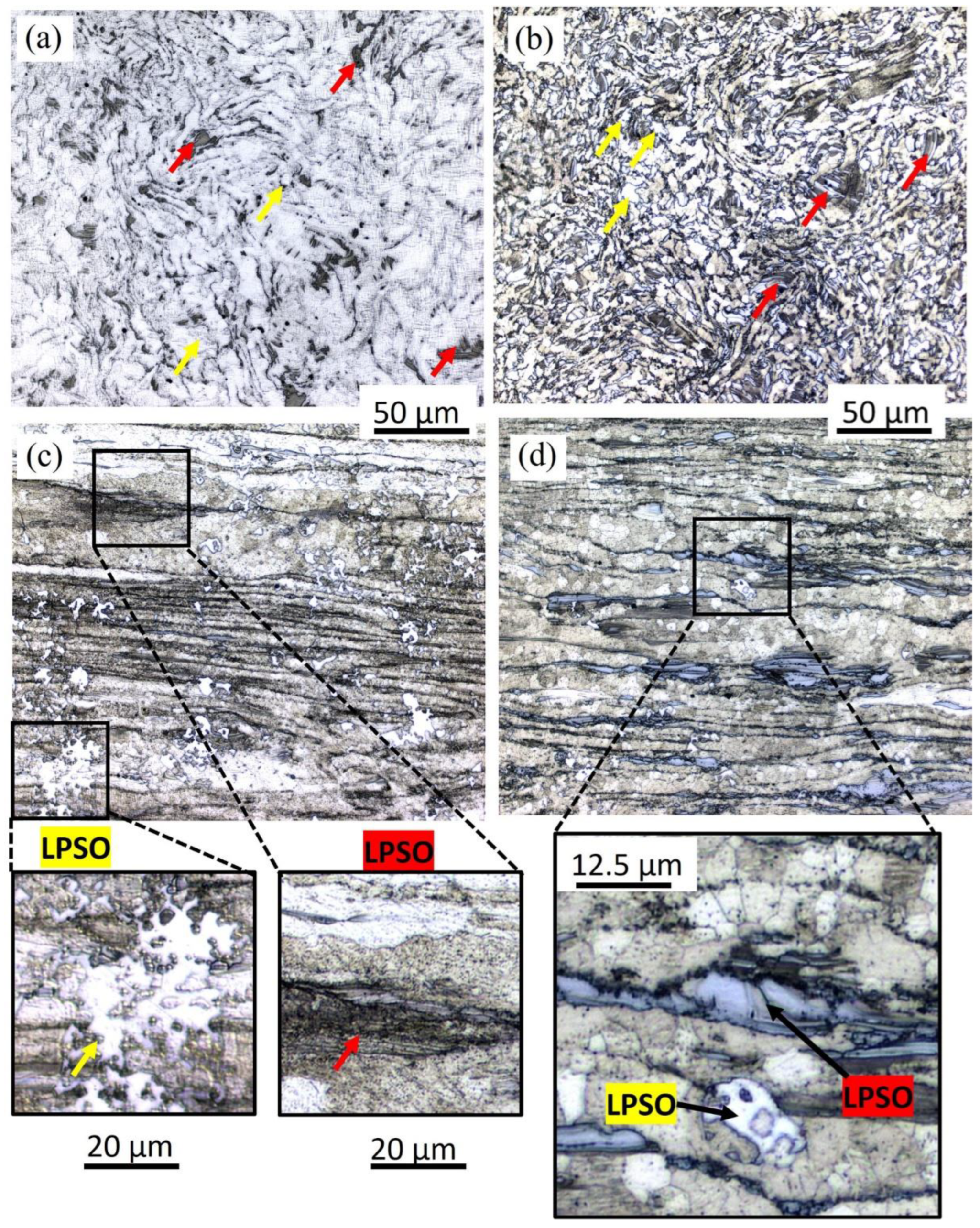

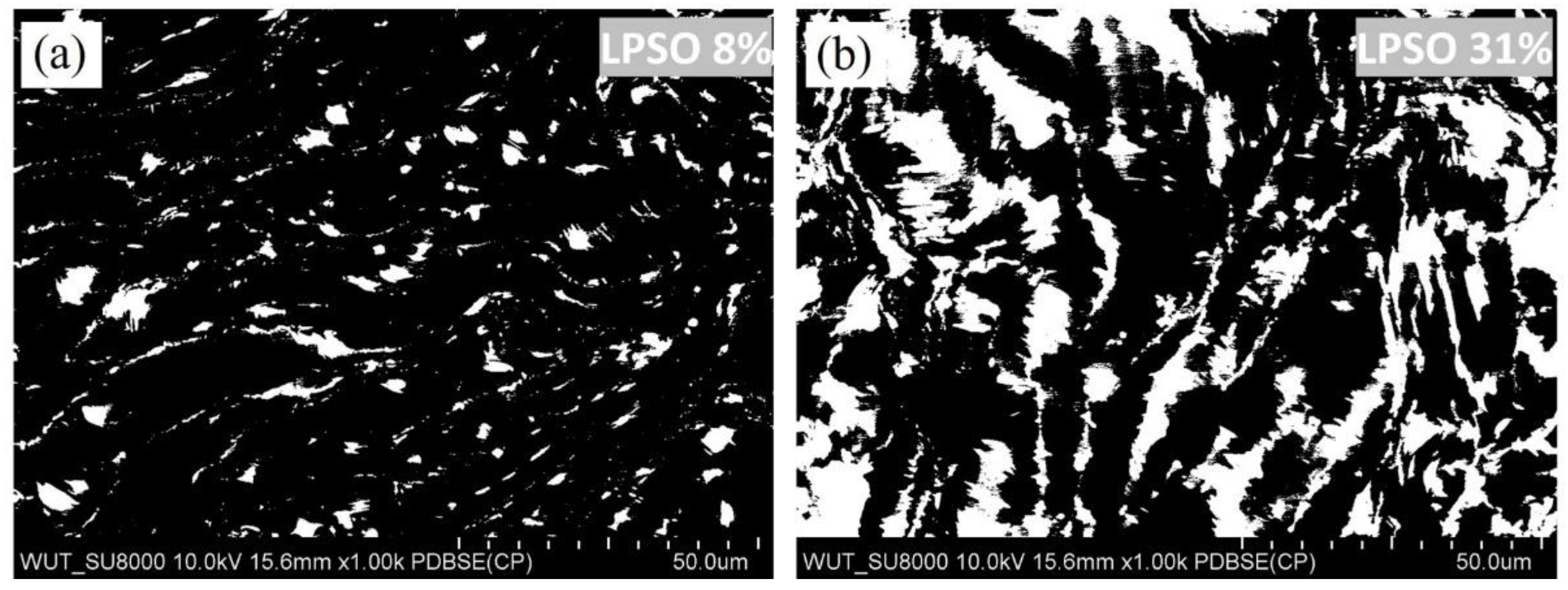



| Elements (wt.%) | Mg | Y | Zn |
|---|---|---|---|
| WZ42 | 94.9 | 3.5 | 1.6 |
| WZ104 | 86.3 | 10.0 | 3.7 |
| Ecorr (V/Ref) | icorr (µA/cm2) | Eb (V/Ref) | βc (V/Decade) | Ecorr (V/Ref) | icorr (µA/cm2) | Eb (V/Ref) | βc (V/Decade) | |
|---|---|---|---|---|---|---|---|---|
| 0.01 M NaCl | 0.1 M NaCl | |||||||
| Mg | −1.37 | 64 | n/a | 0.10 | n/a | n/a | n/a | n/a |
| WZ42 | −1.38 | 14 | −0.76 | 0.31 | −1.48 | 95 | −1.16 | 0.22 |
| WZ104 | −1.25 | 22 | −1.00 | 0.47 | −1.42 | 124 | n/a | 0.25 |
| 1 M NaCl | PBS | |||||||
| WZ42 | −1.47 | 155 | −1.35 | 0.18 | −1.47 | 276 | −1.18 | 0.34 |
| WZ104 | −1.44 | 738 | n/a | 0.27 | −1.43 | 389 | −1.10 | 0.38 |
| Rs (Ω∙cm2) | Rf (Ω∙cm2) | CPEf µSsa/cm2 | n1 | Rct (Ω∙cm2) | CPEct µSsa/cm2 | n2 | RL (Ω∙cm2) | L (H∙cm2) | |
|---|---|---|---|---|---|---|---|---|---|
| 0.01 M NaCl | |||||||||
| Mg | 309 | 202 | 11 | 0.91 | 254 | 29 | 0.99 | 216 | 987 |
| WZ42 | 243 | n/a | n/a | n/a | 1099 | 14 | 0.92 | 547 | 3180 |
| WZ104 | 293 | n/a | n/a | n/a | 954 | 24 | 0.88 | 272 | 1118 |
| 0.1 M NaCl | |||||||||
| WZ42 | 37 | n/a | n/a | n/a | 233 | 17 | 0.92 | 441 | 1204 |
| WZ104 | 37 | n/a | n/a | n/a | 138 | 37 | 0.89 | 198 | 757 |
| 1 M NaCl | |||||||||
| WZ42 | 5 | n/a | n/a | n/a | 468 | 22 | 0.91 | 2075 | 45 |
| WZ104 | 4 | n/a | n/a | n/a | 208 | 84 | 0.80 | 375 | 963 |
| PBS | |||||||||
| WZ42 | 20 | 365 | 35 | 0.88 | 749 | 1214 | 0.74 | 834 | 12,250 |
| WZ104 | 21 | 472 | 38 | 0.88 | 666 | 1568 | 0.87 | 846 | 25,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pałgan, D.; Dobkowska, A.; Zielińska, A.; Drozdenko, D.; Máthis, K.; Święszkowski, W. The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys. Crystals 2022, 12, 1723. https://doi.org/10.3390/cryst12121723
Pałgan D, Dobkowska A, Zielińska A, Drozdenko D, Máthis K, Święszkowski W. The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys. Crystals. 2022; 12(12):1723. https://doi.org/10.3390/cryst12121723
Chicago/Turabian StylePałgan, Daria, Anna Dobkowska, Aleksandra Zielińska, Daria Drozdenko, Kristián Máthis, and Wojciech Święszkowski. 2022. "The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys" Crystals 12, no. 12: 1723. https://doi.org/10.3390/cryst12121723
APA StylePałgan, D., Dobkowska, A., Zielińska, A., Drozdenko, D., Máthis, K., & Święszkowski, W. (2022). The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys. Crystals, 12(12), 1723. https://doi.org/10.3390/cryst12121723









