Understanding Cysteine Chemistry Using Conventional and Serial X-ray Protein Crystallography
Abstract
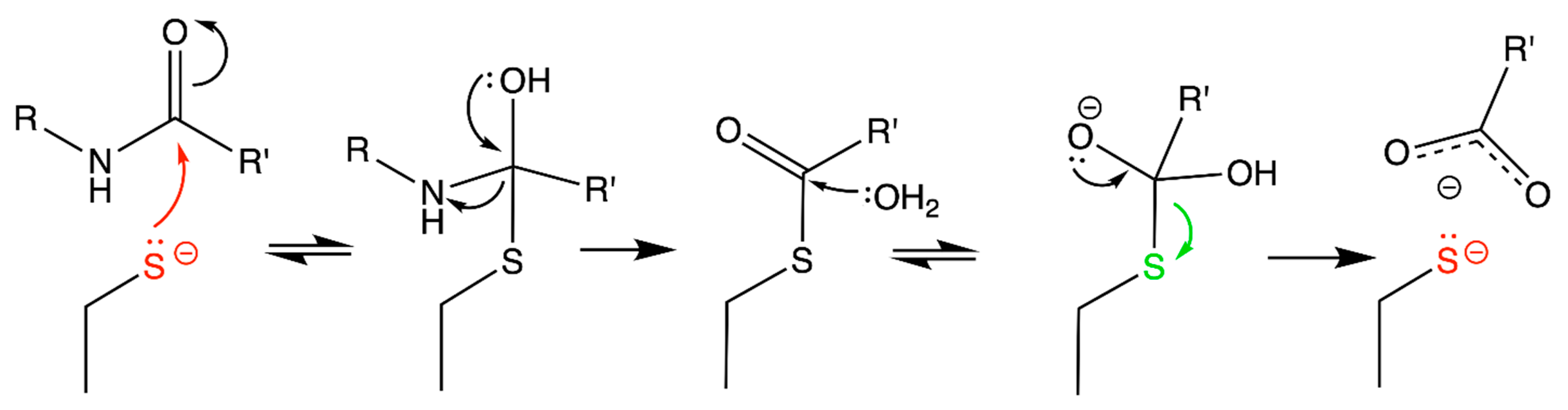
1. Why Are Cysteine Residues Interesting?
2. Cysteine Residues in Enzyme Catalysis: SARS-CoV-2 Proteases
2.1. Chymotrypsin-Like Main Protease (3CLpro or Mpro)
2.2. Papain-Like Protease (PLpro)
3. New X-ray Crystallographic Approaches to Studying Cys Chemistry
3.1. Serial Crystallography Is an Emerging Technology for Mechanistic Enzymology
3.2. Time-Resolved Serial Crystallography of Cys-Dependent Enzymes
3.3. Sulfur X-ray Spectroscopy to Monitor Electronic State of Cys
4. Targeting Cys Residues for Covalent Modification
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofer, F.; Kraml, J.; Kahler, U.; Kamenik, A.S.; Liedl, K.R. Catalytic Site pKa Values of Aspartic, Cysteine, and Serine Proteases: Constant pH MD Simulations. J. Chem. Inf. Model. 2020, 60, 3030–3042. [Google Scholar] [CrossRef] [PubMed]
- Houk, J.; Singh, R.; Whitesides, G.M. Measurement of thiol-disulfide interchange reactions and thiol pKa values. Methods Enzym. 1987, 143, 129–140. [Google Scholar] [CrossRef]
- Madzelan, P.; Labunska, T.; Wilson, M.A. Influence of peptide dipoles and hydrogen bonds on reactive cysteine pKa values in fission yeast DJ-1. FEBS J. 2012, 279, 4111–4120. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.J.; Parsonage, D.; Hall, A.; Karplus, P.A.; Poole, L.B. Cysteine pK(a) values for the bacterial peroxiredoxin AhpC. Biochemistry 2008, 47, 12860–12868. [Google Scholar] [CrossRef]
- Witt, A.C.; Lakshminarasimhan, M.; Remington, B.C.; Hasim, S.; Pozharski, E.; Wilson, M.A. Cysteine pKa depression by a protonated glutamic acid in human DJ-1. Biochemistry 2008, 47, 7430–7440. [Google Scholar] [CrossRef]
- Roos, G.; Foloppe, N.; Messens, J. Understanding the pK(a) of redox cysteines: The key role of hydrogen bonding. Antioxid Redox Signal. 2013, 18, 94–127. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-resolution protein structure determination by serial femtosecond crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Olmos, J.L., Jr.; Pandey, S.; Martin-Garcia, J.M.; Calvey, G.; Katz, A.; Knoska, J.; Kupitz, C.; Hunter, M.S.; Liang, M.; Oberthuer, D.; et al. Enzyme intermediates captured “on the fly” by mix-and-inject serial crystallography. BMC Biol. 2018, 16, 59. [Google Scholar] [CrossRef]
- Stagno, J.R.; Liu, Y.; Bhandari, Y.R.; Conrad, C.E.; Panja, S.; Swain, M.; Fan, L.; Nelson, G.; Li, C.; Wendel, D.R.; et al. Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography. Nature 2017, 541, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.; Budday, D.; de Oliveira, S.H.P.; Madzelan, P.; Marchany-Rivera, D.; Seravalli, J.; Hayes, B.; Sierra, R.G.; Boutet, S.; Hunter, M.S.; et al. Mix-and-inject XFEL crystallography reveals gated conformational dynamics during enzyme catalysis. Proc. Natl. Acad. Sci. USA 2019, 116, 25634–25640. [Google Scholar] [CrossRef] [PubMed]
- Sorigue, D.; Hadjidemetriou, K.; Blangy, S.; Gotthard, G.; Bonvalet, A.; Coquelle, N.; Samire, P.; Aleksandrov, A.; Antonucci, L.; Benachir, A.; et al. Mechanism and dynamics of fatty acid photodecarboxylase. Science 2021, 372, eabd5687. [Google Scholar] [CrossRef]
- Kern, J.; Chatterjee, R.; Young, I.D.; Fuller, F.D.; Lassalle, L.; Ibrahim, M.; Gul, S.; Fransson, T.; Brewster, A.S.; Alonso-Mori, R.; et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 2018, 563, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, P.; Schulz, E.C.; Dsouza, R.; Muller-Werkmeister, H.M.; Tellkamp, F.; Miller, R.J.D.; Pai, E.F. Time-resolved crystallography reveals allosteric communication aligned with molecular breathing. Science 2019, 365, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zhong, Q.; Gao, G.F. Overview of SARS-CoV-2 genome-encoded proteins. Sci. China Life Sci. 2022, 65, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Chandler, J.D.; Jones, D.P. The cysteine proteome. Free Radic. Biol. Med. 2015, 84, 227–245. [Google Scholar] [CrossRef]
- Durdagi, S.; Dag, C.; Dogan, B.; Yigin, M.; Avsar, T.; Buyukdag, C.; Erol, I.; Ertem, F.B.; Calis, S.; Yildirim, G.; et al. Near-physiological-temperature serial crystallography reveals conformations of SARS-CoV-2 main protease active site for improved drug repurposing. Structure 2021, 29, 1382–1396.e1386. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.; Riley, B.T.; Kumaran, D.; Andi, B.; Fuchs, M.R.; McSweeney, S.; Keedy, D.A. The temperature-dependent conformational ensemble of SARS-CoV-2 main protease (M(pro)). IUCrJ 2022, 9, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Jedrzejczak, R.; Stols, L.; Langan, P.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Structural plasticity of SARS-CoV-2 3CL M(pro) active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 2020, 11, 3202. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Tan, K.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Room-temperature X-ray crystallography reveals the oxidation and reactivity of cysteine residues in SARS-CoV-2 3CL M(pro): Insights into enzyme mechanism and drug design. IUCrJ 2020, 7, 1028–1035. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; Weiss, K.L.; Zhang, Q.; Coates, L.; Kovalevsky, A. Direct Observation of Protonation State Modulation in SARS-CoV-2 Main Protease upon Inhibitor Binding with Neutron Crystallography. J. Med. Chem. 2021, 64, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Iketani, S.; Zask, A.; Khanizeman, N.; Bednarova, E.; Forouhar, F.; Fowler, B.; Hong, S.J.; Mohri, H.; Nair, M.S.; et al. Development of optimized drug-like small molecule inhibitors of the SARS-CoV-2 3CL protease for treatment of COVID-19. Nat. Commun. 2022, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kenward, C.; Worrall, L.J.; Vuckovic, M.; Gentile, F.; Ton, A.T.; Ng, M.; Cherkasov, A.; Strynadka, N.C.J.; Paetzel, M. X-ray crystallographic characterization of the SARS-CoV-2 main protease polyprotein cleavage sites essential for viral processing and maturation. Nat. Commun. 2022, 13, 5196. [Google Scholar] [CrossRef] [PubMed]
- Mengist, H.M.; Dilnessa, T.; Jin, T. Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Chem. 2021, 9, 622898. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, D.; Gehrtz, P.; Filep, M.; Fearon, D.; Gabizon, R.; Douangamath, A.; Prilusky, J.; Duberstein, S.; Cohen, G.; Owen, C.D.; et al. An automatic pipeline for the design of irreversible derivatives identifies a potent SARS-CoV-2 M(pro) inhibitor. Cell Chem. Biol. 2021, 28, 1795–1806.e1795. [Google Scholar] [CrossRef] [PubMed]
- Agost-Beltran, L.; de la Hoz-Rodriguez, S.; Bou-Iserte, L.; Rodriguez, S.; Fernandez-de-la-Pradilla, A.; Gonzalez, F.V. Advances in the Development of SARS-CoV-2 Mpro Inhibitors. Molecules 2022, 27, 2523. [Google Scholar] [CrossRef]
- Banerjee, R.; Perera, L.; Tillekeratne, L.M.V. Potential SARS-CoV-2 main protease inhibitors. Drug Discov. Today 2021, 26, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Bardaweel, S.K.; Zhong, H.A. An Updated Review on SARS-CoV-2 Main Proteinase (M(Pro)): Protein Structure and Small-Molecule Inhibitors. Curr. Top. Med. Chem. 2021, 21, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Scala, A.; Piperno, A.; Micale, N. SARS-CoV-2 M(pro): A Potential Target for Peptidomimetics and Small-Molecule Inhibitors. Biomolecules 2021, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Rawlings, N.D. Evolutionary lines of cysteine peptidases. Biol Chem. 2001, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Paasche, A.; Zipper, A.; Schafer, S.; Ziebuhr, J.; Schirmeister, T.; Engels, B. Evidence for substrate binding-induced zwitterion formation in the catalytic Cys-His dyad of the SARS-CoV main protease. Biochemistry 2014, 53, 5930–5946. [Google Scholar] [CrossRef]
- Solowiej, J.; Thomson, J.A.; Ryan, K.; Luo, C.; He, M.; Lou, J.; Murray, B.W. Steady-state and pre-steady-state kinetic evaluation of severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro cysteine protease: Development of an ion-pair model for catalysis. Biochemistry 2008, 47, 2617–2630. [Google Scholar] [CrossRef] [PubMed]
- van den Bedem, H.; Wilson, M.A. Shining light on cysteine modification: Connecting protein conformational dynamics to catalysis and regulation. J. Synchrotron Radiat. 2019, 26, 958–966. [Google Scholar] [CrossRef]
- Ravelli, R.B.; McSweeney, S.M. The ’fingerprint’ that X-rays can leave on structures. Structure 2000, 8, 315–328. [Google Scholar] [CrossRef]
- Carugo, O.; Djinovic Carugo, K. When X-rays modify the protein structure: Radiation damage at work. Trends Biochem. Sci. 2005, 30, 213–219. [Google Scholar] [CrossRef]
- Hough, M.A.; Owen, R.L. Serial synchrotron and XFEL crystallography for studies of metalloprotein catalysis. Curr. Opin. Struct. Biol. 2021, 71, 232–238. [Google Scholar] [CrossRef]
- Garman, E.F. Radiation damage in macromolecular crystallography: What is it and why should we care? Acta Cryst. D Biol. Cryst. 2010, 66, 339–351. [Google Scholar] [CrossRef]
- Yano, J.; Kern, J.; Irrgang, K.D.; Latimer, M.J.; Bergmann, U.; Glatzel, P.; Pushkar, Y.; Biesiadka, J.; Loll, B.; Sauer, K.; et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: A case study for metalloprotein crystallography. Proc. Natl. Acad. Sci. USA 2005, 102, 12047–12052. [Google Scholar] [CrossRef]
- Ullrich, S.; Nitsche, C. SARS-CoV-2 Papain-Like Protease: Structure, Function and Inhibition. ChemBioChem 2022, 23, e202200327. [Google Scholar] [CrossRef] [PubMed]
- Barretto, N.; Jukneliene, D.; Ratia, K.; Chen, Z.; Mesecar, A.D.; Baker, S.C. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005, 79, 15189–15198. [Google Scholar] [CrossRef] [PubMed]
- Mielech, A.M.; Kilianski, A.; Baez-Santos, Y.M.; Mesecar, A.D.; Baker, S.C. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 2014, 450–451, 64–70. [Google Scholar] [CrossRef]
- Ran, X.H.; Zhu, J.W.; Chen, Y.Y.; Ni, R.Z.; Mu, D. Papain-like protease of SARS-CoV-2 inhibits RLR signaling in a deubiquitination-dependent and deubiquitination-independent manner. Front. Immunol. 2022, 13, 947272. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Klemm, T.; Ebert, G.; Calleja, D.J.; Allison, C.C.; Richardson, L.W.; Bernardini, J.P.; Lu, B.G.; Kuchel, N.W.; Grohmann, C.; Shibata, Y.; et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020, 39, e106275. [Google Scholar] [CrossRef] [PubMed]
- Osipiuk, J.; Azizi, S.A.; Dvorkin, S.; Endres, M.; Jedrzejczak, R.; Jones, K.A.; Kang, S.; Kathayat, R.S.; Kim, Y.; Lisnyak, V.G.; et al. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat. Commun. 2021, 12, 743. [Google Scholar] [CrossRef]
- Kizer, D.E.; Clouse, J.A. Inhibited initial rates of poly-uridylic acid-directed phenylalanine incorporation by free ribosomes from the liver of rats fed hepatocarcinogens. Biochem. Pharm. 1975, 24, 1019–1023. [Google Scholar] [CrossRef]
- Menard, R.; Khouri, H.E.; Plouffe, C.; Laflamme, P.; Dupras, R.; Vernet, T.; Tessier, D.C.; Thomas, D.Y.; Storer, A.C. Importance of hydrogen-bonding interactions involving the side chain of Asp158 in the catalytic mechanism of papain. Biochemistry 1991, 30, 5531–5538. [Google Scholar] [CrossRef]
- Sargsyan, K.; Lin, C.C.; Chen, T.; Grauffel, C.; Chen, Y.P.; Yang, W.Z.; Yuan, H.S.; Lim, C. Multi-targeting of functional cysteines in multiple conserved SARS-CoV-2 domains by clinically safe Zn-ejectors. Chem. Sci. 2020, 11, 9904–9909. [Google Scholar] [CrossRef]
- Chou, C.Y.; Lai, H.Y.; Chen, H.Y.; Cheng, S.C.; Cheng, K.W.; Chou, Y.W. Structural basis for catalysis and ubiquitin recognition by the severe acute respiratory syndrome coronavirus papain-like protease. Acta Cryst. D Biol. Cryst. 2014, 70, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M.; et al. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design. Sci. Adv. 2020, 6, eabd4596. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Liu, J.; Shen, J.; Dai, J.; Xu, G.; Lu, K.; Han, C.; Wang, Y.; Xu, X.; Tong, Y.; et al. Development of potent and selective inhibitors targeting the papain-like protease of SARS-CoV-2. Cell Chem. Biol. 2021, 28, 855–865.e859. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sacco, M.D.; Xia, Z.; Lambrinidis, G.; Townsend, J.A.; Hu, Y.; Meng, X.; Szeto, T.; Ba, M.; Zhang, X.; et al. Discovery of SARS-CoV-2 Papain-like Protease Inhibitors through a Combination of High-Throughput Screening and a FlipGFP-Based Reporter Assay. ACS Cent. Sci. 2021, 7, 1245–1260. [Google Scholar] [CrossRef]
- Calleja, D.J.; Kuchel, N.; Lu, B.G.C.; Birkinshaw, R.W.; Klemm, T.; Doerflinger, M.; Cooney, J.P.; Mackiewicz, L.; Au, A.E.; Yap, Y.Q.; et al. Insights Into Drug Repurposing, as Well as Specificity and Compound Properties of Piperidine-Based SARS-CoV-2 PLpro Inhibitors. Front. Chem. 2022, 10, 861209. [Google Scholar] [CrossRef]
- Srinivasan, V.; Brognaro, H.; Prabhu, P.R.; de Souza, E.E.; Gunther, S.; Reinke, P.Y.A.; Lane, T.J.; Ginn, H.; Han, H.; Ewert, W.; et al. Antiviral activity of natural phenolic compounds in complex at an allosteric site of SARS-CoV-2 papain-like protease. Commun. Biol 2022, 5, 805. [Google Scholar] [CrossRef]
- Narayanan, A.; Narwal, M.; Majowicz, S.A.; Varricchio, C.; Toner, S.A.; Ballatore, C.; Brancale, A.; Murakami, K.S.; Jose, J. Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Commun. Biol. 2022, 5, 169. [Google Scholar] [CrossRef]
- Lewis, S.D.; Johnson, F.A.; Shafer, J.A. Potentiometric determination of ionizations at the active site of papain. Biochemistry 1976, 15, 5009–5017. [Google Scholar] [CrossRef]
- Polgar, L. Catalytic Mechanisms of Cysteine Peptidases. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 2, pp. 1773–1784. [Google Scholar]
- Shokhen, M.; Khazanov, N.; Albeck, A. Challenging a paradigm: Theoretical calculations of the protonation state of the Cys25-His159 catalytic diad in free papain. Proteins 2009, 77, 916–926. [Google Scholar] [CrossRef]
- Zhai, X.; Meek, T.D. Catalytic Mechanism of Cruzain from Trypanosoma cruzi As Determined from Solvent Kinetic Isotope Effects of Steady-State and Pre-Steady-State Kinetics. Biochemistry 2018, 57, 3176–3190. [Google Scholar] [CrossRef]
- Bai, X.C.; McMullan, G.; Scheres, S.H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 2015, 40, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, I.; Lewis-Ballester, A.; Echelmeier, A.; Brehm, G.; Zatsepin, N.A.; Grant, T.D.; Coe, J.D.; Lisova, S.; Nelson, G.; Zhang, S.; et al. Snapshot of an oxygen intermediate in the catalytic reaction of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2019, 116, 3572–3577. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.N. X-Ray Free-Electron Lasers for the Structure and Dynamics of Macromolecules. Annu. Rev. Biochem. 2019, 88, 35–58. [Google Scholar] [CrossRef]
- Orville, A.M. Recent results in time resolved serial femtosecond crystallography at XFELs. Curr. Opin. Struct. Biol. 2020, 65, 193–208. [Google Scholar] [CrossRef]
- Kupitz, C.; Basu, S.; Grotjohann, I.; Fromme, R.; Zatsepin, N.A.; Rendek, K.N.; Hunter, M.S.; Shoeman, R.L.; White, T.A.; Wang, D.; et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 2014, 513, 261–265. [Google Scholar] [CrossRef]
- Barty, A.; Caleman, C.; Aquila, A.; Timneanu, N.; Lomb, L.; White, T.A.; Andreasson, J.; Arnlund, D.; Bajt, S.; Barends, T.R.; et al. Self-terminating diffraction gates femtosecond X-ray nanocrystallography measurements. Nat. Photonics 2012, 6, 35–40. [Google Scholar] [CrossRef]
- Chapman, H.N. Structure Determination Using X-ray Free-Electron Laser Pulses. Methods Mol. Biol 2017, 1607, 295–324. [Google Scholar] [CrossRef]
- Chapman, H.N.; Caleman, C.; Timneanu, N. Diffraction before destruction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130313. [Google Scholar] [CrossRef]
- Lakshminarasimhan, M.; Madzelan, P.; Nan, R.; Milkovic, N.M.; Wilson, M.A. Evolution of new enzymatic function by structural modulation of cysteine reactivity in Pseudomonas fluorescens isocyanide hydratase. J. Biol. Chem. 2010, 285, 29651–29661. [Google Scholar] [CrossRef]
- Pearson, A.R.; Mehrabi, P. Serial synchrotron crystallography for time-resolved structural biology. Curr. Opin. Struct. Biol. 2020, 65, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, J.M.; Conrad, C.E.; Nelson, G.; Stander, N.; Zatsepin, N.A.; Zook, J.; Zhu, L.; Geiger, J.; Chun, E.; Kissick, D.; et al. Serial millisecond crystallography of membrane and soluble protein microcrystals using synchrotron radiation. IUCrJ 2017, 4, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.C.; Yorke, B.A.; Pearson, A.R.; Mehrabi, P. Best practices for time-resolved serial synchrotron crystallography. Acta Cryst. D Struct. Biol. 2022, 78, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.C.; Stauch, B.; Ishchenko, A.; Cherezov, V. A Bright Future for Serial Femtosecond Crystallography with XFELs. Trends Biochem. Sci. 2017, 42, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Akita, F.; Sugahara, M.; Kubo, M.; Nakajima, Y.; Nakane, T.; Yamashita, K.; Umena, Y.; Nakabayashi, M.; Yamane, T.; et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017, 543, 131–135. [Google Scholar] [CrossRef]
- Maestre-Reyna, M.; Yang, C.H.; Nango, E.; Huang, W.C.; Ngurah Putu, E.P.G.; Wu, W.J.; Wang, P.H.; Franz-Badur, S.; Saft, M.; Emmerich, H.J.; et al. Serial crystallography captures dynamic control of sequential electron and proton transfer events in a flavoenzyme. Nat. Chem. 2022, 14, 677–685. [Google Scholar] [CrossRef]
- Poddar, H.; Heyes, D.J.; Schiro, G.; Weik, M.; Leys, D.; Scrutton, N.S. A guide to time-resolved structural analysis of light-activated proteins. FEBS J. 2022, 289, 576–595. [Google Scholar] [CrossRef]
- Klan, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Monteiro, D.C.F.; Amoah, E.; Rogers, C.; Pearson, A.R. Using photocaging for fast time-resolved structural biology studies. Acta Cryst. D Struct. Biol. 2021, 77, 1218–1232. [Google Scholar] [CrossRef]
- Tosha, T.; Nomura, T.; Nishida, T.; Saeki, N.; Okubayashi, K.; Yamagiwa, R.; Sugahara, M.; Nakane, T.; Yamashita, K.; Hirata, K.; et al. Capturing an initial intermediate during the P450nor enzymatic reaction using time-resolved XFEL crystallography and caged-substrate. Nat. Commun. 2017, 8, 1585. [Google Scholar] [CrossRef]
- Respondek, T.; Garner, R.N.; Herroon, M.K.; Podgorski, I.; Turro, C.; Kodanko, J.J. Light activation of a cysteine protease inhibitor: Caging of a peptidomimetic nitrile with Ru(II)(bpy)2. J. Am. Chem. Soc. 2011, 133, 17164–17167. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Deiters, A.; Cropp, T.A.; King, D.; Schultz, P.G. A genetically encoded photocaged amino acid. J. Am. Chem. Soc. 2004, 126, 14306–14307. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Mahesh, M.; Elsasser, S.J.; Hancock, S.M.; Uttamapinant, C.; Chin, J.W. Genetic encoding of photocaged cysteine allows photoactivation of TEV protease in live mammalian cells. J. Am. Chem. Soc. 2014, 136, 2240–2243. [Google Scholar] [CrossRef] [PubMed]
- Hankore, E.D.; Zhang, L.; Chen, Y.; Liu, K.; Niu, W.; Guo, J. Genetic Incorporation of Noncanonical Amino Acids Using Two Mutually Orthogonal Quadruplet Codons. ACS Synth. Biol. 2019, 8, 1168–1174. [Google Scholar] [CrossRef]
- Schmidt, M. Mix and Inject: Reaction Initiation by Diffusion for Time-Resolved Macromolecular Crystallography. Adv. Cond. Matter Phys. 2013, 2013, 167276. [Google Scholar] [CrossRef]
- Monteiro, D.C.F.; von Stetten, D.; Stohrer, C.; Sans, M.; Pearson, A.R.; Santoni, G.; van der Linden, P.; Trebbin, M. 3D-MiXD: 3D-printed X-ray-compatible microfluidic devices for rapid, low-consumption serial synchrotron crystallography data collection in flow. IUCrJ 2020, 7, 207–219. [Google Scholar] [CrossRef]
- Sierra, R.G.; Gati, C.; Laksmono, H.; Dao, E.H.; Gul, S.; Fuller, F.; Kern, J.; Chatterjee, R.; Ibrahim, M.; Brewster, A.S.; et al. Concentric-flow electrokinetic injector enables serial crystallography of ribosome and photosystem II. Nat. Methods 2016, 13, 59–62. [Google Scholar] [CrossRef]
- Butryn, A.; Simon, P.S.; Aller, P.; Hinchliffe, P.; Massad, R.N.; Leen, G.; Tooke, C.L.; Bogacz, I.; Kim, I.S.; Bhowmick, A.; et al. An on-demand, drop-on-drop method for studying enzyme catalysis by serial crystallography. Nat. Commun. 2021, 12, 4461. [Google Scholar] [CrossRef]
- Sui, S.; Wang, Y.; Kolewe, K.W.; Srajer, V.; Henning, R.; Schiffman, J.D.; Dimitrakopoulos, C.; Perry, S.L. Graphene-based microfluidics for serial crystallography. Lab. Chip 2016, 16, 3082–3096. [Google Scholar] [CrossRef]
- Mehrabi, P.; Schulz, E.C.; Agthe, M.; Horrell, S.; Bourenkov, G.; von Stetten, D.; Leimkohl, J.P.; Schikora, H.; Schneider, T.R.; Pearson, A.R.; et al. Liquid application method for time-resolved analyses by serial synchrotron crystallography. Nat. Methods 2019, 16, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Goda, M.; Hashimoto, Y.; Takase, M.; Herai, S.; Iwahara, Y.; Higashibata, H.; Kobayashi, M. Isonitrile hydratase from Pseudomonas putida N19-2. Cloning, sequencing, gene expression, and identification of its active acid residue. J. Biol. Chem. 2002, 277, 45860–45865. [Google Scholar] [CrossRef]
- Wilson, M.A. Mapping Enzyme Landscapes by Time-Resolved Crystallography with Synchrotron and X-ray Free Electron Laser Light. Annu. Rev. Biophys. 2022, 51, 79–98. [Google Scholar] [CrossRef]
- Scott, W.G.; Murray, J.B.; Arnold, J.R.; Stoddard, B.L.; Klug, A. Capturing the structure of a catalytic RNA intermediate: The hammerhead ribozyme. Science 1996, 274, 2065–2069. [Google Scholar] [CrossRef]
- Ridder, I.S.; Rozeboom, H.J.; Kalk, K.H.; Dijkstra, B.W. Crystal structures of intermediates in the dehalogenation of haloalkanoates by L-2-haloacid dehalogenase. J. Biol. Chem. 1999, 274, 30672–30678. [Google Scholar] [CrossRef] [PubMed]
- Petsko, G.A.; Ringe, D. Observation of unstable species in enzyme-catalyzed transformations using protein crystallography. Curr. Opin. Chem. Biol. 2000, 4, 89–94. [Google Scholar] [CrossRef]
- Garman, E.F.; Weik, M. Radiation damage to biological samples: Still a pertinent issue. J. Synchrotron. Radiat. 2021, 28, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Fehnel, E.A.; Carmack, M. The ultraviolet absorption spectra of organic sulfur compounds; compounds containing the sulfide function. J. Am. Chem. Soc. 1949, 71, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Polgar, L. Spectrophotometric determination of mercaptide ion, an activated form of SH-group in thiol enzymes. FEBS Lett. 1974, 38, 187–190. [Google Scholar] [CrossRef]
- Noda, L.H.; Kuby, S.A.; Lardy, H.A. Properties of thiolesters: Kinetics of hydrolysis in dilute aqueous media. J. Am. Chem. Soc. 1953, 75, 913–917. [Google Scholar] [CrossRef]
- Holden, W.M.; Jahrman, E.P.; Govind, N.; Seidler, G.T. Probing Sulfur Chemical and Electronic Structure with Experimental Observation and Quantitative Theoretical Prediction of Kalpha and Valence-to-Core Kbeta X-ray Emission Spectroscopy. J. Phys. Chem. A 2020, 124, 5415–5434. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.A.; Paris, E.; Giuli, G.; Eeckhout, S.G.; Kavcic, M.; Zitnik, M.; Bucar, K.; Pettersson, L.G.M.; Glatzel, P. Electronic Structure of Sulfur Studied by X-ray Absorption and Emission Spectroscopy. Anal. Chem. 2009, 81, 6516–6525. [Google Scholar] [CrossRef]
- George, G.N.; Pickering, I.J.; Cotelesage, J.J.H.; Vogt, L.I.; Dolgova, N.V.; Regnier, N.; Sokaras, D.; Kroll, T.; Sneeden, E.Y.; Hackett, M.J.; et al. Visualizing sulfur with X-rays: From molecules to tissues. Phosphorus Sulfur. 2019, 194, 618–623. [Google Scholar] [CrossRef]
- Abraham, B.; Nowak, S.; Weninger, C.; Armenta, R.; Defever, J.; Day, D.; Carini, G.; Nakahara, K.; Gallo, A.; Nelson, S.; et al. A high-throughput energy-dispersive tender X-ray spectrometer for shot-to-shot sulfur measurements. J. Synchrotron. Radiat. 2019, 26, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Van Kuiken, B.E.; Ross, M.R.; Strader, M.L.; Cordones, A.A.; Cho, H.; Lee, J.H.; Schoenlein, R.W.; Khalil, M. Picosecond sulfur K-edge X-ray absorption spectroscopy with applications to excited state proton transfer. Struct. Dyn. 2017, 4, 044021. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.; Ward, R.A. Structure-based design of targeted covalent inhibitors. Chem. Soc. Rev. 2018, 47, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.A. Covalent inhibitors in drug discovery: From accidental discoveries to avoided liabilities and designed therapies. Drug Discov. Today 2015, 20, 1061–1073. [Google Scholar] [CrossRef]
- Johnson, D.S.; Weerapana, E.; Cravatt, B.F. Strategies for discovering and derisking covalent, irreversible enzyme inhibitors. Future Med. Chem. 2010, 2, 949–964. [Google Scholar] [CrossRef]
- Maurais, A.J.; Weerapana, E. Reactive-cysteine profiling for drug discovery. Curr. Opin. Chem. Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Liu, Q.; Sabnis, Y.; Zhao, Z.; Zhang, T.; Buhrlage, S.J.; Jones, L.H.; Gray, N.S. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. 2013, 20, 146–159. [Google Scholar] [CrossRef]
- Hodge, C.D.; Edwards, R.A.; Markin, C.J.; McDonald, D.; Pulvino, M.; Huen, M.S.; Zhao, J.; Spyracopoulos, L.; Hendzel, M.J.; Glover, J.N. Covalent Inhibition of Ubc13 Affects Ubiquitin Signaling and Reveals Active Site Elements Important for Targeting. ACS Chem. Biol. 2015, 10, 1718–1728. [Google Scholar] [CrossRef]
- Bashore, C.; Jaishankar, P.; Skelton, N.J.; Fuhrmann, J.; Hearn, B.R.; Liu, P.S.; Renslo, A.R.; Dueber, E.C. Cyanopyrrolidine Inhibitors of Ubiquitin Specific Protease 7 Mediate Desulfhydration of the Active-Site Cysteine. ACS Chem. Biol. 2020, 15, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Shin, H.R.; Berdan, C.A.; Ford, B.; Ward, C.C.; Olzmann, J.A.; Zoncu, R.; Nomura, D.K. Covalent targeting of the vacuolar H(+)-ATPase activates autophagy via mTORC1 inhibition. Nat. Chem. Biol. 2019, 15, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Weikart, N.D.; Linne, U.; Mootz, H.D. Covalent inhibition of SUMO and ubiquitin-specific cysteine proteases by an in situ thiol-alkyne addition. Bioorg. Med. Chem. 2013, 21, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Kathman, S.G.; Xu, Z.; Statsyuk, A.V. A fragment-based method to discover irreversible covalent inhibitors of cysteine proteases. J. Med. Chem. 2014, 57, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.K.; Cancilla, M.T.; Shiau, T.P.; Kung, J.; Chen, T.; Erlanson, D.A. Allosteric inhibition of PTP1B activity by selective modification of a non-active site cysteine residue. Biochemistry 2005, 44, 7704–7712. [Google Scholar] [CrossRef] [PubMed]
- Bum-Erdene, K.; Zhou, D.; Gonzalez-Gutierrez, G.; Ghozayel, M.K.; Si, Y.; Xu, D.; Shannon, H.E.; Bailey, B.J.; Corson, T.W.; Pollok, K.E.; et al. Small-Molecule Covalent Modification of Conserved Cysteine Leads to Allosteric Inhibition of the TEADYap Protein-Protein Interaction. Cell Chem. Biol. 2019, 26, 378–389.e313. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.C.; Merlini, E.; Guijas, C.; DeMeester, K.E.; Njomen, E.; Kozina, E.M.; Yokoyama, M.; Vinogradova, E.; Reardon, H.T.; Melillo, B.; et al. Selective inhibitors of SARM1 targeting an allosteric cysteine in the autoregulatory ARM domain. Proc. Natl. Acad. Sci. USA 2022, 119, e2208457119. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Lam, J.; Nguyen, J.T.; O’Brien, T.; Wells, J.A. Discovery of an allosteric site in the caspases. Proc. Natl. Acad. Sci. USA 2004, 101, 12461–12466. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, M.E.; Horning, B.D.; Khattri, R.; Roy, N.; Lu, J.P.; Whitby, L.R.; Ye, E.; Brannon, J.C.; Parker, A.; Chick, J.M.; et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. Nat. Chem. Biol. 2022, 18, 1288. [Google Scholar] [CrossRef] [PubMed]



| Cys State | Description |
|---|---|
| R-SH | Thiol/thiolate (unmodified; pKa~8.5) |
| R-SNO | S-nitrosation |
| R-S-OH | Sulfenic acid/sulfenate (pKa~6) |
| R-S-OOH | Sulfinic acid/sulfinate (pKa~2) |
| R-S-OOOH | Sulfonic acid/sulfonate (pKa~−2) |
| R-S-S-G | Glutathionylation |
| R-S-S-R′ | Disulfide |
| R-S-Ub | Ubiquitination |
| R-S-M | Metal binding/coordination |
| R-S-C-R′ | Alkylation |
| R-S-CO-CH3 | Acetylation |
| Protein | Number of Cys Residues; % | Cys of Known Functional Importance 1 | Role of Functional Cys |
|---|---|---|---|
| nsp1 | 1; 0.6 | NA | NA |
| nsp2 | 27; 4.2 | NA | NA |
| nsp3 (PLPro) | 10; 3.2 | C111 *, C189+, C192+, C224+, C226+ | Nucleophile *, Zn2+ binding+ |
| nsp4 | 16; 3.2 | NA | NA |
| nsp5 (MPro) | 12; 3.9 | C145 | Nucleophile |
| nsp6 | 10; 3.4 | NA | NA |
| nsp7 | 3; 3.6 | NA | NA |
| nsp8 | 2; 1.0 | NA | NA |
| nsp9 | 3; 2.7 | NA | NA |
| nsp10 | 13; 9.4 | C74, C77, C90, C117, C120, C128, C130 | Zn2+ binding |
| nsp11 | 1; 7.7 | NA | NA |
| nsp12 | 29; 3.1 | C301, C306, C310, C487, C645, C646 | Zn2+ binding |
| nsp13 | 26; 4.3 | NA | NA |
| nsp14 | 23; 4.4 | NA | NA |
| nsp15 | 5; 1.4 | NA | NA |
| nsp16 | 5; 1.7 | C115 | adenosine stabilization |
| E Protein | 3; 4.0 | NA | NA |
| M protein | 4; 1.8 | NA | NA |
| S protein | 40; 3.1 | NA | NA |
| N protein | 0; 0.0 | NA | NA |
| orf3a | 7; 2.5 | NA | NA |
| orf3b | 0; 0.0 | NA | NA |
| orf6 | 0; 0.0 | NA | NA |
| orf7a | 6; 5.0 | NA | NA |
| orf7b | 2; 4.7 | NA | NA |
| orf8b | 7; 5.8 | NA | NA |
| orf9b | 0; 0.0 | NA | NA |
| orf9c | 5; 7.4 | NA | NA |
| orf10 | 1; 2.6 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, N.; Wilson, M.A. Understanding Cysteine Chemistry Using Conventional and Serial X-ray Protein Crystallography. Crystals 2022, 12, 1671. https://doi.org/10.3390/cryst12111671
Smith N, Wilson MA. Understanding Cysteine Chemistry Using Conventional and Serial X-ray Protein Crystallography. Crystals. 2022; 12(11):1671. https://doi.org/10.3390/cryst12111671
Chicago/Turabian StyleSmith, Nathan, and Mark A. Wilson. 2022. "Understanding Cysteine Chemistry Using Conventional and Serial X-ray Protein Crystallography" Crystals 12, no. 11: 1671. https://doi.org/10.3390/cryst12111671
APA StyleSmith, N., & Wilson, M. A. (2022). Understanding Cysteine Chemistry Using Conventional and Serial X-ray Protein Crystallography. Crystals, 12(11), 1671. https://doi.org/10.3390/cryst12111671







