Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of 2-{4-Hydroxy-3-[(2-hydroxyethylimino)methyl]phenylazo}benzoic Acid (H3L)
2.2.2. Preparation of Cyclic Dimeric Trimethyltin(IV) Complex of H3L, [Me3Sn(H2L)]2 (1)
2.2.3. Preparation of Tributyltin(IV) Complex of H3L, Bu3Sn(H2L) (2)
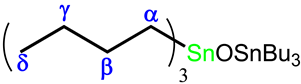 .
.2.2.4. Preparation of Dibutyltin (IV) Complex of H3L, [(Bu2Sn(H2L))2O]2 (3)
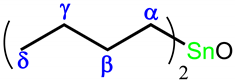 .
.2.3. Crystallographic Data Collection and Structure Refinement
2.4. Computational Details
2.5. Antibacterial Studies
3. Results and Discussion
3.1. Synthesis
3.2. IR-Spectroscopy
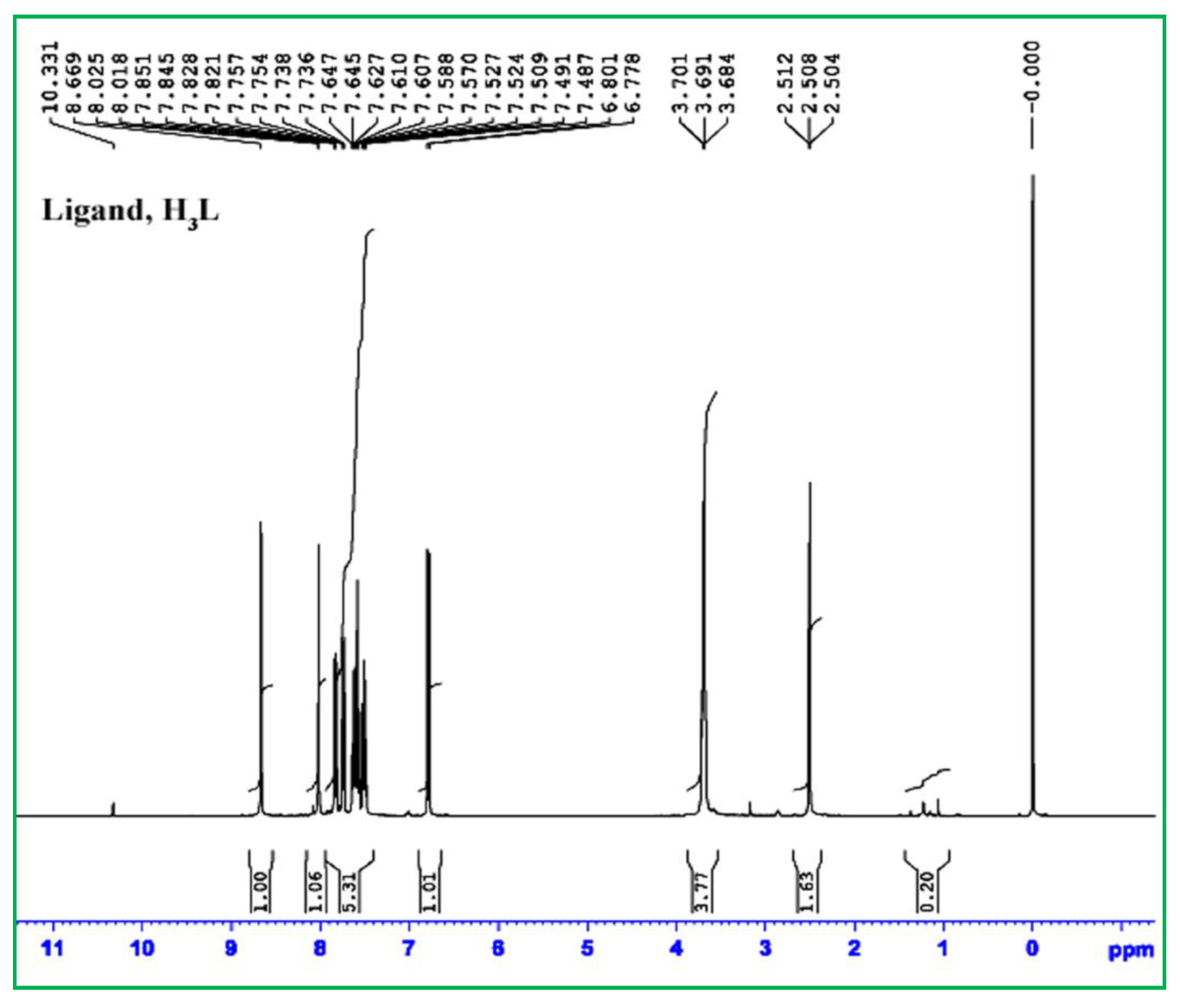
3.3. Multinuclear (1H, 13C and 119Sn) NMR-Spectroscopy
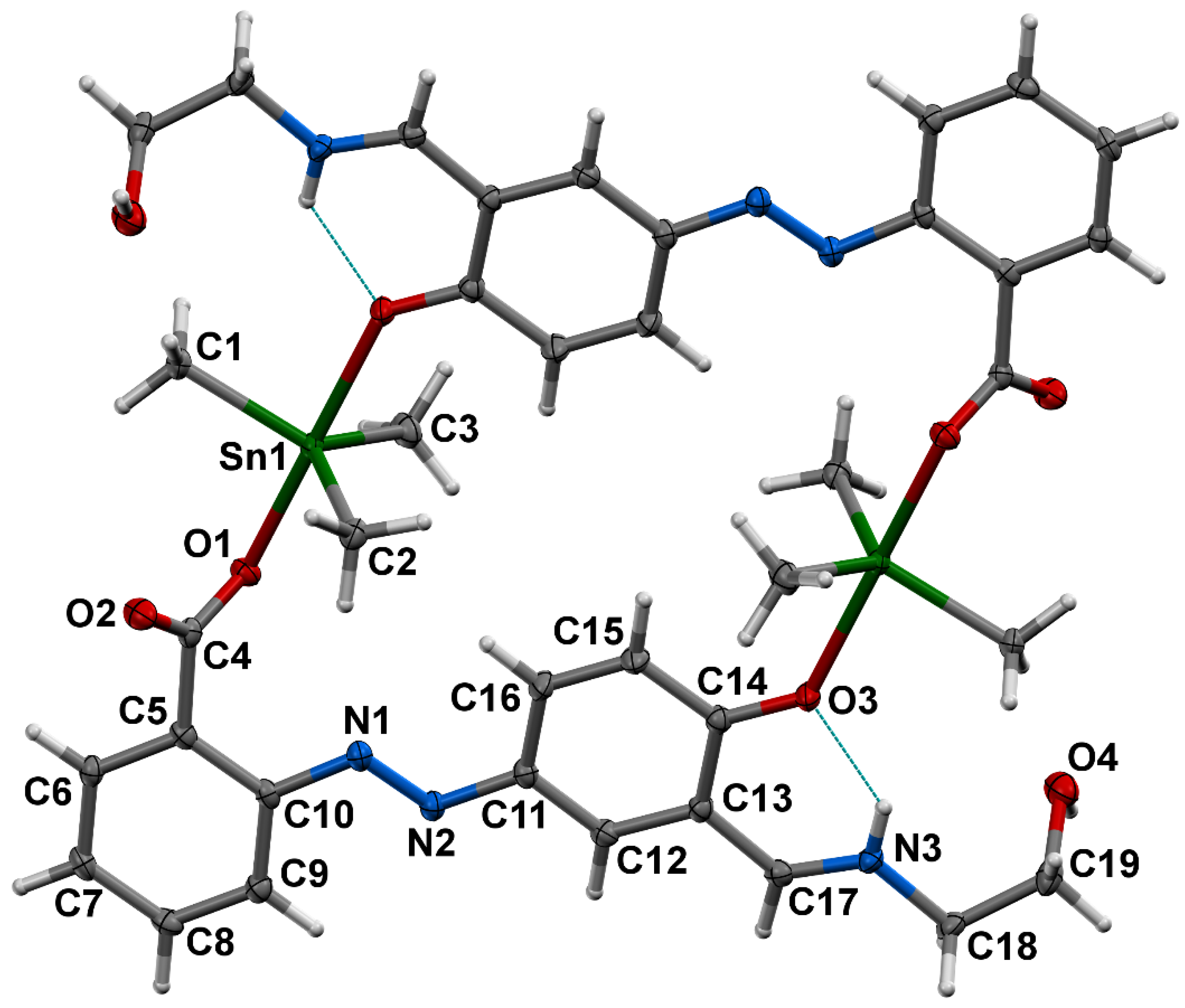
3.4. Crystal Structure of 1
3.5. Theoretical Study of Intermolecular Interactions
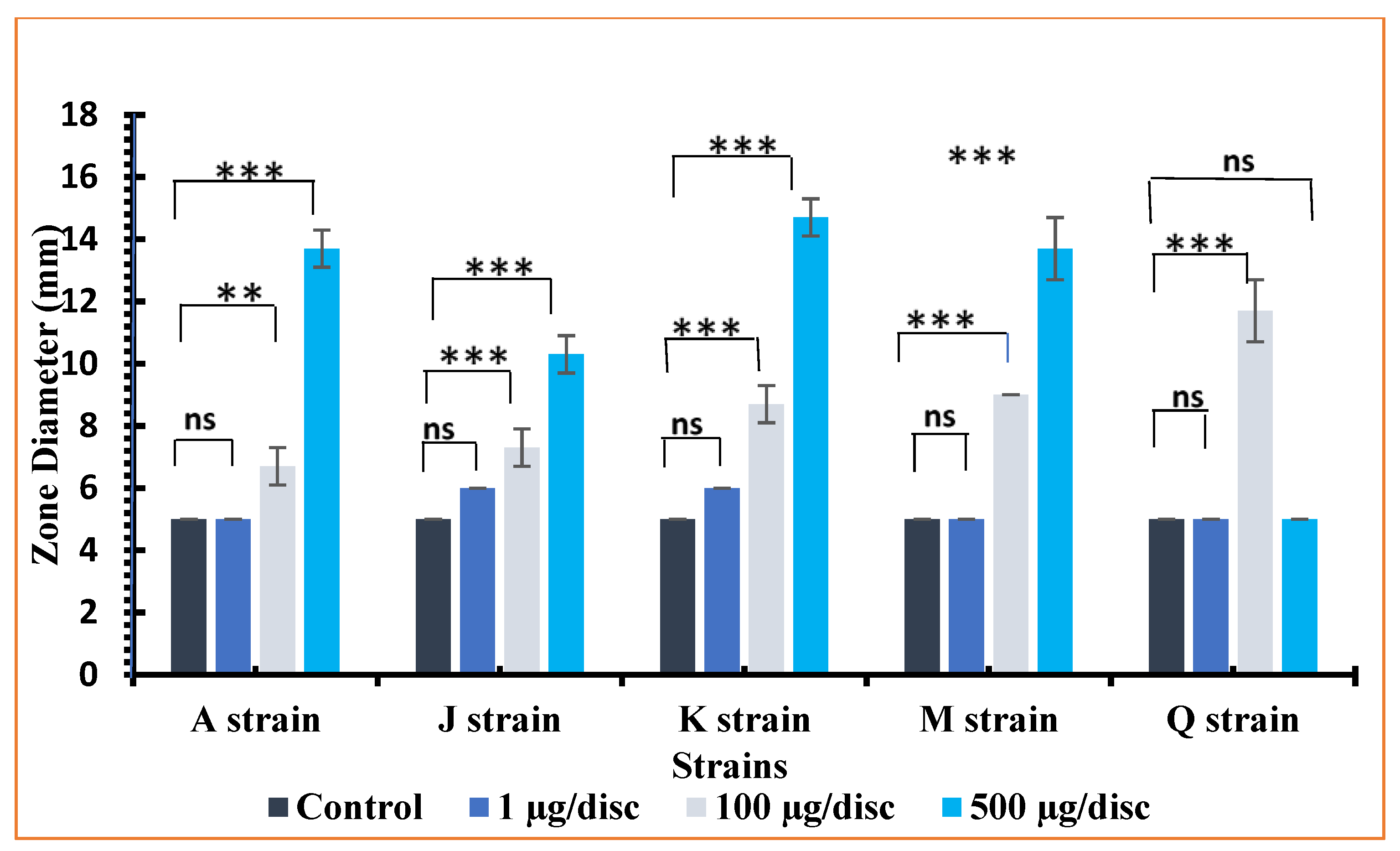
3.6. Antibacterial Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, A.K. Organotin compounds: Toxicology and biomedicinal applications. Appl. Organomet. Chem. 1987, 1, 39–56. [Google Scholar] [CrossRef]
- Ghazi, D.; Rasheed, Z.; Yousif, E. Review of organotin compounds: Chemistry and applications. Int. J. Res. Eng. Innov. 2018, 2, 340–348. [Google Scholar]
- Tabassum, S.; Khan, R.A.; Arjmand, F.; Sen, S.; Kayal, J.; Juvekar, A.S.; Zingde, S.M. Synthesis and characterization of glycoconjugate tin (IV) complexes: In vitro DNA binding studies, cytotoxicity, and cell death. J. Organomet. Chem. 2011, 696, 1600–1608. [Google Scholar] [CrossRef]
- Arjmand, F.; Jamsheera, A. Synthesis, characterization and in vitro DNA binding studies of tin (IV) complexes of tert-butyl 1-(2-hydroxy-1-phenylethylamino)-3-methyl-1-oxobutan-2-yl carbamate. J. Organomet. Chem. 2011, 696, 3572–3579. [Google Scholar] [CrossRef]
- Paul, A.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J. Biological Evaluation of Azo-and Imino-Based Carboxylate Triphenyltin (IV) Compounds. Eur. J. Inorg. Chem. 2020, 11–12, 930–941. [Google Scholar] [CrossRef]
- Amir, M.K.; Khan, S.; Shah, A.; Butler, I.S. Anticancer activity of organotin (IV) carboxylates. Inorg. Chim. Acta 2014, 423, 14–25. [Google Scholar] [CrossRef]
- Devi, J. Pachwania, S. Recent advancements in DNA interaction studies of organotin (IV) complexes. Inorg. Chem. Commun. 2018, 91, 44–62. [Google Scholar] [CrossRef]
- Dahmani, M.; Harit, T.; Et-Touhami, A.; Yahyi, A.; Eddike, D.; Tillard, M.; Benabbes, R. Two novel macrocyclic organotin (IV) carboxylates based on bipyrazoledicarboxylic acid derivatives: Syntheses, crystal structures and antifungal activities. J. Organomet. Chem. 2021, 948, 121913. [Google Scholar] [CrossRef]
- Shoaib Ahmad Shah, S.; Ashfaq, M.; Waseem, A.; Mehboob Ahmed, M.; Najam, T.; Shaheen, S.; Rivera, G. Synthesis and biological activities of organotin (IV) complexes as antitumoral and antimicrobial agents. A Rev. Mini Rev. Med. Chem. 2015, 15, 406–426. [Google Scholar] [CrossRef]
- Debnath, P.; Singh, K.S.; Singh, K.K.; Singh, S.S.; Sieroń, L.; Maniukiewicz, W. Di-butyltin (IV) complexes with azo-carboxylates: Synthesis, characterization, crystal structures and their anti-diabetic assay. New J. Chem. 2020, 44, 5862–5872. [Google Scholar] [CrossRef]
- Tiekink, E.R. Structural chemistry of organotin carboxylates: A review of the crystallographic literature. Appl. Organomet. Chem. 1991, 5, 1–23. [Google Scholar] [CrossRef]
- Abbas, S.M.; Ali, S.; Hussain, S.T.; Shahzadi, S. structural diversity in organotin(IV) dithiocarboxylates and carboxylates. J. Coord. Chem. 2013, 66, 2217–2234. [Google Scholar] [CrossRef]
- Nath, M.; Saini, P.K. Chemistry and applications of organotin (IV) complexes of Schiff bases. Dalton Trans. 2011, 40, 7077–7121. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Masharing, C.; Das, B. Diorganotin (IV) complexes of polyaromatic azo-azomethine ligand derived from salicylaldehyde and ortho-aminophenol: Synthesis, characterization, and molecular structures. Heteroat. Chem. 2012, 23, 457–465. [Google Scholar] [CrossRef]
- Debnath, P.; Das, A.; Singh, K.S.; Yama, T.; Singh, S.S.; Butcher, R.J.; Maniukiewicz, W. Synthesis, structural characterization and antimicrobial activities of triorganotin (IV) azo-carboxylates derived from ortho/para-amino benzoic acids and β-naphthol. Inorg. Chim. Acta. 2019, 498, 119172. [Google Scholar] [CrossRef]
- Debnath, P.; Singh, K.S.; Devi, T.S.; Singh, S.S.; Butcher, R.J.; Sieroń, L.; Maniukiewicz, W. Synthesis, characterization, crystal structures and anti-diabetic activity of organotin (IV) complexes with 2-(4-hydroxynaphthylazo)-benzoic acid. Inorg. Chim. Acta. 2020, 510, 119736. [Google Scholar] [CrossRef]
- Debnath, P.; Singh, K.S.; Sharma, S.; Debnath, P.; Singh, S.S.; Sieroń, L.; Maniukiewicz, W. Synthesis, structural characterization, Hirshfeld surface analysis and in vitro-antimicrobial activities of triphenyltin (IV) compounds of azo-carboxylates derived from 2-or 4-amino benzoic acids and naphthalen-1 or 2-ol. J. Mol. Struct. 2021, 1223, 128971. [Google Scholar] [CrossRef]
- Yin, H.D.; Chen, S.W. Synthesis and characterization of di-and tri-organotin (IV) complexes with Schiff base ligand pyruvic acid 3-hydroxy-2-naphthoyl hydrazone. Inorg. Chim. Acta. 2006, 359, 3330–3338. [Google Scholar] [CrossRef]
- BasuBaul, T.S.; Basu, S.; de Vos, D.; Linden, A. Amino acetate functionalized Schiff base organotin (IV) complexes as anticancer drugs: Synthesis, structural characterization, and in vitro cytotoxicity studies. Investig. New Drugs. 2009, 27, 419–431. [Google Scholar] [CrossRef]
- Yin, H.; Liu, H.; Hong, M. Synthesis, structural characterization and DNA-binding properties of organotin (IV) complexes based on Schiff base ligands derived from 2-hydroxy-1-naphthaldy and 3-or 4-aminobenzoic acid. J. Organomet. Chem. 2012, 713, 11–19. [Google Scholar] [CrossRef]
- Roy, M.; Roy, S.; Devi, N.M.; Singh, C.B.; Singh, K.S. Synthesis, structural characterization and antimicrobial activities of diorganotin (IV) complexes with azo-imino carboxylic acid ligand: Crystal structure and topological study of a doubly phenoxide-bridged dimeric dimethyltin (IV) complex appended with free carboxylic acid groups. J. Mol. Struct. 2016, 1119, 64–70. [Google Scholar]
- Baul, T.S.B.; Addepalli, M.R.; Lyčka, A.; van Terwingen, S.; da Silva, M.F.C.G. Synthesis and structural characterization of diorganotin (IV) complexes with heteroditopic pyridyl-ONO′-ligands. Inorg. Chim. Acta. 2020, 512, 119892. [Google Scholar] [CrossRef]
- BasuBaul, T.S.; Chaurasiya, A.; Rabha, M.; Khatua, S.; Lyčka, A.; Schollmeyer, D.; Jurkschat, K. Diorganotin Compounds Containing α-Aminoacidato Schiff Base Ligands Derived from Functionalized 2-Hydroxy-5-(aryldiazenyl) benzaldehyde. Syntheses, Structures and Sensing of Hydrogen Sulfide. Eur. J. Inorg. Chem. 2020, 18, 1803–1813. [Google Scholar] [CrossRef]
- BasuBaul, T.S.; Addepalli, M.R.; Duthie, A.; Singh, P.; Koch, B.; Gildenast, H.; Englert, U.; Rojas-León, I.; Höpfl, H. Triorganotin (IV) derivatives with semirigid heteroditopic hydroxo-carboxylato ligands: Synthesis, characterization, and cytotoxic properties. Appl. Organomet. Chem. 2021, 35, E608. [Google Scholar]
- Roy, M.; Roy, S.; Singh, K.S.; Kalita, J.; Singh, S.S. Synthesis, characterisation and anti-diabetic activities of triorganotin (IV) azo-carboxylates derived from amino benzoic acids and resorcinol: Crystal structure and topological study of a 48 membered macrocyclic-tetrameric trimethyltin (IV) complex. Inorg. Chim. Acta. 2016, 439, 164–172. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Qiu, T.; Sun, R.; Zhao, Z.; Tian, L.; Liu, X. Synthesis, structural characterization, and properties of triorganotin complexes of Schiff base derived from 3-aminobenzoic acid and salicylaldehyde or 2, 4-pentanedione. Appl. Organomet. Chem. 2020, 34, E5790. [Google Scholar] [CrossRef]
- BasuBaul, T.S.; Chaurasiya, A.; Duthie, A.; Montes-Tolentino, P.; Höpfl, H. Coordination-driven self-assembly of macrocycles and 1D or 2D coordination polymers using heteroditopic pyridyl-carboxylate ligands: The case study of 5-[(E)-2-(3-pyridyl)-1-diazenyl]-2-hydroxybenzoate in combination with {RnSn}(n= 2 and 3). Cryst. Growth Des. 2019, 19, 6656–6671. [Google Scholar] [CrossRef]
- BasuBaul, T.S.; Das, P.; Eng, G.; Linden, A. Synthesis and Characterization of Some Triphenyltin (IV) Complexes from Sterically Crowded [((E)-1-{2-Hydroxy-5-[(E)-2-(aryl)-1-diazenyl] phenyl} methylidene) amino] acetate Ligands and Crystal Structure Analysis of a Tetrameric Triphenyltin (IV) Compound. J. Inorg. Organomet. Polym. Mater. 2010, 20, 134–141. [Google Scholar] [CrossRef][Green Version]
- Baul, T.S.B.; Singh, K.S.; Holčapek, M.; Jirásko, R.; Rivarola, E.; Linden, A. Synthesis, characterization and crystal structures of polymeric and dimeric triphenyltin (IV) complexes of 4-[((E)-1-{2-hydroxy-5-[(E)-2-(2-carboxyphenyl)-1-diazenyl] phenyl} methylidene) amino] aryls. J. Organomet. Chem. 2005, 690, 4232–4242. [Google Scholar] [CrossRef]
- Singh, K.S.; Roy, M.; Roy, S.; Ghosh, B.; Devi, N.M.; Singh, C.B.; Mun, L.K. Synthesis, characterization and antimicrobial activities of triorganotin (IV) complexes with azo-azomethine carboxylate ligands: Crystal structure of a tributyltin (IV) and a trimethyltin (IV) complex. J. Coord. Chem. 2017, 70, 361–380. [Google Scholar] [CrossRef]
- Shang, X.; Cui, J.; Wu, J.; Pombeiro, A.J.L.; Li, Q. Polynuclear diorganotin (IV) complexes with arylhydroxamates: Syntheses, structures and in vitro cytotoxic activities. J. Inorg. Biochem. 2008, 102, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Rigaku, O.D. CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C 71, 3–8. [Google Scholar]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer (Version 3.1); University of Western Australia: Nedland, Australia, 2012. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van der Waals volumes and radii of metals in covalent compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Fox, in Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Barros, C.L.; De Oliveira, P.J.P.; Jorge, F.E.; Canal Neto, A.; Campos, M. Gaussian basis set of double zeta quality for atoms Rb through Xe: Application in non-relativistic and relativistic calculations of atomic and molecular properties. Mol. Phys. 2010, 108, 1965–1972. [Google Scholar] [CrossRef]
- Jorge, F.E.; Canal Neto, A.; Camiletti, G.G.; Machado, S.F. Contracted Gaussian basis sets for Douglas–Kroll–Hess calculations: Estimating scalar relativistic effects of some atomic and molecular properties. J. Chem. Phys. 2009, 130, 064108. [Google Scholar] [CrossRef]
- Neto, A.C.; Jorge, F.E. All-electron double zeta basis sets for the most fifth-row atoms: Application in DFT spectroscopic constant calculations. Chem. Phys. Lett. 2013, 582, 158–162. [Google Scholar] [CrossRef]
- De Berrêdo, R.C.; Jorge, F.E. All-electron double zeta basis sets for platinum: Estimating scalar relativistic effects on platinum (II) anticancer drugs. J. Mol. Struc-THEOCHEM. 2010, 961, 107–112. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalt. Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯ F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting noncovalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]






| Parameters | 1 |
|---|---|
| Empirical formula | C38H46N6O8Sn2 |
| Formula weight | 952.23 |
| Temperature (K) | 100 (3) |
| Wavelength (Å) | 0.71073 |
| Crystal system | triclinic |
| Space group | P-1 |
| a (Å) | 7.1138 (1) |
| b (Å) | 10.4253 (2) |
| c (Å) | 14.0461 (2) |
| α (°) | 94.753 (1) |
| β (°) | 93.448 (1) |
| γ (°) | 108.603 (1) |
| Volume (Å3) | 979.74 (3) |
| Z | 1 |
| Density (Mg/m3) | 1.614 |
| Absorp. coeff. (mm−1) | 1.333 |
| F (000) | 480 |
| Crystal size (mm3) | 0.04 × 0.05 × 0.33 |
| Theta range for data collection | 2.9, 25.0 |
| Index ranges | −8<= h <=8; −12<= k <=12; −16<= l <=16 |
| Reflection collected | 26,309 |
| Data completeness | 99.9% |
| Independent reflections | 3559 [R(int) = 0.022] |
| Goodness of fit on F2 | 1.11 |
| Final R indices [I > 2sigma(I)] | 0.0152 |
| R indices (all data) | 0.0159 |
| Largest diff. peak and hole (e Å−3) | 0.58 and −0.22 |
| Atoms | Bond Length (Å) |
| Sn1-O1 | 2.2126 (11) |
| Sn1-C1 | 2.1270 (18) |
| Sn1-C2 | 2.1306 (15) |
| Sn1-C3 | 2.1303 (19) |
| Sn1-O3 i N1-N2 | 2.3091 (11) 1.261 (2) |
| Atoms | Bond Angle (°) |
| O1-Sn1-C1 | 95.52 (6) |
| O1-Sn1-C2 | 93.02 (6) |
| O1-Sn1-C3 | 86.09 (6) |
| O1-Sn1-O3 i | 177.68 (5) |
| C1-Sn1-C2 | 117.20 (7) |
| C1-Sn1-C3 | 121.47 (8) |
| O3 i-Sn1-C1 | 84.61 (5) |
| C2-Sn1-C3 | 121.13 (7) |
| O3 i-Sn1-C2 | 88.97 (6) |
| O3 i-Sn1-C3 | 91.86 (6) |
| Compound | D–H…A | d (D–H) | d (H…A) | d (D…A) | <(DHA) |
|---|---|---|---|---|---|
| 1 | N3-H3…O3 O4-H4…O2 ii | 0.83 (2) 0.85 (3) | 1.97 (2) 1.96 (3) | 2.6316 (18) 2.7829 (18) | 136 (2) 159 (3) |
| C18-H18B…O2 iii | 0.99 | 2.56 | 3.529(2) | 167 |
| Contact | ρ(r) | ∇2ρ(r) | λ2 | Hb | V(r) | G(r) | ELF | Eint * |
|---|---|---|---|---|---|---|---|---|
| O–H···O 1.958 Å | 0.024 | 0.094 | −0.024 | 0.003 | −0.018 | 0.021 | 0.067 | 5.6 |
| C···C 3.310 Å | 0.006 | 0.019 | −0.006 | 0.001 | −0.003 | 0.004 | 0.022 | 0.9 |
| Compounds | Conc. | Zone of Inhibition at Different Concentration (mm) | ||||
|---|---|---|---|---|---|---|
| Gram (−) ve Bacteria | Gram (+) ve Bacteria | |||||
| K. pneumoniae (A) | V. cholerae (M) | Shigella boydii (Q) | S. Aureus (J) | S. Pneumoniae (K) | ||
| Control | 5.0 ± 0.00 | 5.0 ± 0.00 | 5.0 ± 0.00 | 5.0 ± 0.00 | 5.0 ± 0.00 | |
| Polymyxin B | 10 μg | 5.0 ± 0.0 | - | - | - | - |
| 100 μg | 7.0 ± 0.0 | - | - | - | - | |
| 500 μg | 12.0 ± 0.6 | - | - | - | - | |
| Gentamicin | 10 μg | - | 15 ± 0.0 | 17 ± 0 | - | - |
| 100 μg | - | 22 ± 0.0 | 23.3 ± 0.6 | - | - | |
| 500 μg | - | 25 ± 0.0 | 36.7 ± 0.6 | - | - | |
| Vancomycin | 30 μg | - | - | - | 14 ± 0 | 12 ± 0 |
| H3L | 1 μg | 6.0 ± 1.0 | 5.0 ± 0.0 | 5.7 ± 1.0 | 6.7 ± 0.6 | 5.3 ± 1.0 |
| 100 μg | 7.7 ± 0.6 | 7.7 ± 1.0 | 10.3 ± 1.0 | 7.7 ± 0.6 | 7.7 ± 1.0 | |
| 500 μg | 14.0 ± 1.0 | 13.7 ± 1.0 | 15.3 ± 1.0 | 9.3 ± 0.6 | 8.7 ± 1.0 | |
| 1 | 1 μg | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 |
| 100 μg | 6.7 ± 0.6 | 9.0 ± 0.0 | 11.7 ± 1.0 | 7.3 ± 0.6 | 8.7 ± 0.6 | |
| 500 μg | 13.7 ± 0.6 | 13.7 ± 1.0 | 5.0 ± 0.0 | 10.3 ± 0.6 | 14.7 ± 0.6 | |
| 2 | 1 μg | 5.0 ± 0.0 | 8.3 ± 1.0 | 6.3 ± 1.0 | 6.3 ± 0.6 | 6.0 ± 0.0 |
| 100 μg | 5.0 ± 0.0 | 17.7 ± 1.0 | 14.7 ± 1.0 | 7.0 ± 0.0 | 7.3 ± 0.6 | |
| 500 μg | 6.3 ± 0.6 | 18.7 ± 1.0 | 18.0 ± 0.0 | 8.7 ± 0.6 | 8.0 ± 1.0 | |
| 3 | 1 μg | 5.0 ± 0.0 | 5.0 ± 0.0 | 6.0 ± 0.0 | 6.3 ± 0.5 | 10.7 ± 0.5 |
| 100 μg | 5.0 ± 0.0 | 11.7 ± 0.5 | 14.7 ± 0.5 | 7.0 ± 0.0 | 6.0 ± 0.0 | |
| 500 μg | 5.0 ± 0.0 | 8.3 ± 0.5 | 19.7 ± 0.5 | 8.7 ± 0.5 | 9.3 ± 0.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, P.; Debnath, P.; Roy, M.; Sieroń, L.; Maniukiewicz, W.; Aktar, T.; Maiti, D.; Novikov, A.S.; Misra, T.K. Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity. Crystals 2022, 12, 1582. https://doi.org/10.3390/cryst12111582
Debnath P, Debnath P, Roy M, Sieroń L, Maniukiewicz W, Aktar T, Maiti D, Novikov AS, Misra TK. Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity. Crystals. 2022; 12(11):1582. https://doi.org/10.3390/cryst12111582
Chicago/Turabian StyleDebnath, Pratima, Paresh Debnath, Manojit Roy, Lesław Sieroń, Waldemar Maniukiewicz, Tamanna Aktar, Debasish Maiti, Alexander S. Novikov, and Tarun Kumar Misra. 2022. "Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity" Crystals 12, no. 11: 1582. https://doi.org/10.3390/cryst12111582
APA StyleDebnath, P., Debnath, P., Roy, M., Sieroń, L., Maniukiewicz, W., Aktar, T., Maiti, D., Novikov, A. S., & Misra, T. K. (2022). Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity. Crystals, 12(11), 1582. https://doi.org/10.3390/cryst12111582








