Comparative Study of the Compressibility of M3V2O8 (M = Cd, Zn, Mg, Ni) Orthovanadates
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Crystal Structure
3.2. Pressure–Volume Equation of State and Compressibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Cui, Y. Microwave-assisted hydrothermal synthesis of hollow flower-like Zn2V2O7 with enhanced cycling stability as electrode for lithium ion batteries. Mater. Lett. 2018, 228, 369–371. [Google Scholar] [CrossRef]
- Sameie, H.; Sabbagh Alvani, A.A.; Naseri, N.; Du, S.; Rosei, F. First-principles study on ZnV2O6 and Zn2V2O7: Two new photoanode candidates for photoelectrochemical water oxidation. Ceram. Int. 2018, 44, 6607–6613. [Google Scholar] [CrossRef]
- Bayat, A.; Mahjoub, A.R.; Amini, M.M. Optical properties of hydrophilic surfaced self-assembled Cd2V2O7 hollow sphere shape architecture. Mater. Lett. 2016, 186, 252–255. [Google Scholar] [CrossRef]
- Zhi, J. Study of MgV2O6 as Cathode Material for Secondary Magnesium Batteries. Asian J. Chem. 2011, 23, 1399–1400. [Google Scholar]
- Ni, S.; Liu, J.; Chao, D.; Mai, L. Vanadate-Based Materials for Li-Ion Batteries: The Search for Anodes for Practical Applications. Adv. Energy Mater. 2019, 9, 1803324. [Google Scholar] [CrossRef]
- Hassan, A.; Iqbal, T.; Tahir, M.B.; Afsheen, S. A review on copper vanadate-based nanostructures for photocatalysis energy production. Int. J. Energy Res. 2019, 43, 9–28. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Lee, S.-H.; Ryu, K.-S. Synthesis of Zn3V2O8 nanoplatelets for lithium-ion battery and supercapacitor applications. RSC Adv. 2015, 5, 91822–91828. [Google Scholar] [CrossRef]
- Cabrera, I.; Kenzelmann, M.; Lawes, G.; Chen, Y.; Chen, W.; Erwin, R.; Gentile, T.R.; Leao, J.B.; Lynn, J.W.; Rogado, N.; et al. Coupled Magnetic and Ferroelectric Domains in Multiferroic Ni3V2O8. Phys. Rev. Lett. 2009, 103, 087201. [Google Scholar] [CrossRef]
- Bîrdeanu, M.-I.; Vaida, M.; Ursu, D.; Fagadar-Cosma, E. Obtaining and characterization of Zn3V2O8 and Mg3V2O8 pseudo binary oxide nanomaterials by hydrothermal method. In High Energy Gamma-Ray Astronomy, Proceedings of the 6th International Meeting on High-Energy Gamma-Ray Astronomy, Heidelberg, Germany, 11–15 July 2016; AIP Publishing LLC: Melville, NY, USA, 2017; p. 030006. [Google Scholar]
- Mazloom, F.; Masjedi-Arani, M.; Salavati-Niasari, M. Novel size-controlled fabrication of pure Zn3V2O8 nanostructures via a simple precipitation approach. J. Mater. Sci. Mater. Electron. 2015, 27, 1974–1982. [Google Scholar] [CrossRef]
- Luo, J.; Chen, R.; Zhang, X. The Effect in the Production and Luminescence Property of Zn3V2O8 with Eu-doping and the First Principle Calculation of α-Zn3V2O8. Mater. Sci. Eng. 2017, 274, 012087. [Google Scholar] [CrossRef]
- Qian, T.; Fan, B.; Wang, H.; Zhu, S. Structure and luminescence properties of Zn3V2O8 yellow phosphor for white light emitting diodes. Chem. Phys. Lett. 2019, 715, 34–39. [Google Scholar] [CrossRef]
- Matsushima, Y.; Koide, T.; Hiro-Oka, M.; Shida, M.; Sato, A.; Sugiyama, S.; Ito, M. Self-activated vanadate compounds toward realization of rare-earth-free full-color phosphors. J. Am. Ceram. Soc. 2015, 98, 1236–1244. [Google Scholar] [CrossRef]
- Díaz-Anichtchenko, D.; Santamaria-Perez, D.; Marqueño, T.; Pellicer-Porres, J.; Ruiz-Fuertes, J.; Ribes, R.; Errandonea, D. Comparative study of the high-pressure behavior of ZnV2O6, Zn2V2O7, and Zn3V2O8. J. Alloys Compd. 2020, 837, 155505. [Google Scholar] [CrossRef]
- Diaz-Anichtchenko, D.; Turnbull, R.; Bandiello, E.; Anzellini, S.; Errandonea, D. High-Pressure Structural Behavior and Equation of State of Kagome Staircase Compound, Ni3V2O8. Crystals 2020, 10, 910. [Google Scholar] [CrossRef]
- Kesari, S.; Garg, A.B.; Clemens, O.; Joseph, B.; Rao, R. Pressure-Induced Structural Behavior of Orthorhombic Mn3(VO4)2: Raman Spectroscopic and X-ray Diffraction Investigations. ACS Omega 2022, 7, 3099–3108. [Google Scholar] [CrossRef]
- Beltrán, A.; Gracia, L.; Andrés, J. Polymorphs of ZnV2O6 under pressure: A first-principle investigation. J. Phys. Chem. C 2019, 123, 3239–3253. [Google Scholar] [CrossRef]
- Lopez-Moreno, S.; Errandonea, D.; Rodriguez-Hernandez, P.; Munoz, A. Polymorphs of CaSeO4 under pressure: A first-principles study of structural, electronic, and vibrational properties. Inorg. Chem. 2015, 54, 1765–1777. [Google Scholar] [CrossRef]
- Benmakhlouf, A.; Errandonea, D.; Bouchenafa, M.; Maabed, S.; Bouhemadou, A.; Bentabet, A. New pressure-induced polymorphic transitions of anhydrous magnesium sulfate. Dalton Trans. 2017, 46, 5058–5068. [Google Scholar] [CrossRef]
- Errandonea, D.; Gracia, L.; Lacomba-Perales, R.; Polian, A.; Chervin, J.C. Compression of scheelite-type SrMoO4 under quasi-hydrostatic conditions: Redefining the high-pressure structural sequence. J. Appl. Phys. 2013, 113, 123510. [Google Scholar] [CrossRef]
- Dovesi, R.; Saunders, V.R.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.J.; et al. CRYSTAL14 User’s Manual; University of Torino: Torino, Italy, 2014. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of theelectron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M.E. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analyticrepresentation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.crystal.unito.it/basis-sets.php/ (accessed on 14 October 2022).
- Freysoldt, C.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Kresse, G.; Janotti, A.; van de Walle, C.G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 253–305. [Google Scholar] [CrossRef]
- Diaz-Anichtchenko, D.; Gracia, L.; Errandonea, D. Density-functional study of pressure-induced phase transitions and electronic properties of Zn2V2O7. RSC Adv. 2021, 11, 10401–10415. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Anichtchenko, D.; Errandonea, D. Pressure-induced phase transitions and electronic properties of Cd2V2O7. RSC Adv. 2022, 12, 14827–14837. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Gopal, R.; Calvo, C. Crystal structure of α-Zn3V2O8. Can. J. Chem. 1971, 49, 3056–3059. [Google Scholar] [CrossRef]
- Krishnamachari, N.; Calvo, C. Refinement of the Structure of Mg3V2O8. Can. J. Chem. 1971, 49, 1629–1637. [Google Scholar] [CrossRef]
- Saurbrei, E.E.; Faggiani, R.; Calvo, C. Refinement of the Crystal structures of Cd3V2O8 and Ni3V2O8. Acta Crist. 1973, B29, 2304–2306. [Google Scholar] [CrossRef]
- Yahia, H.B.; Gaudin, E.; Feral-Martin, C.; Darriet, J. Structural study of the NaCdVO4–Cd3V2O8 and CdO–V2O5 sections of the ternary system Na2O–CdO–V2O5. J. Solid State Chem. 2010, 183, 776–783. [Google Scholar] [CrossRef]
- Burke, K. Perspective on density functional theory. J. Chem. Phys. 2012, 136, 150901. [Google Scholar] [CrossRef]
- Available online: https://materialsproject.org/ (accessed on 14 October 2022).
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Laverock, J.; Piper, L.F.J.; Preston, A.R.H.; Chen, B.; McNulty, J.; Smith, K.E.; Guo, J.H. Strain dependence of bonding and hybridization across the metal-insulator transition of VO2. Phys. Rev. B 2012, 85, 081104. [Google Scholar] [CrossRef]
- Errandonea, D. High pressure crystal structures of orthovanadates and their properties. J. Appl. Phys. 2020, 128, 040903. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R.J. EosFit7-GUI: A new GUI tool for equation of state calculations, analyses and teaching. J. Appl. Cryst. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Anzellini, S.; Burakovsky, L.; Turnbull, R.; Bandiello, E.; Errandonea, D. P–V–T Equation of State of Iridium Up to 80 GPa and 3100 K. Crystals 2021, 11, 452. [Google Scholar] [CrossRef]
- Angel, R.J. Equations of state. Rev. Mineral. Geochem. 2000, 41, 35–59. [Google Scholar] [CrossRef]
- Piskunov, S.; Heifets, E.; Eglitis, R.I.; Borstel, G. Bulk properties and electronic structure of SrTiO3, BaTiO3, PbTiO3 perovskites: An ab initio HF/DFT study. Comput. Mater. Sci. 2004, 29, 165–178. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjon, F.J. Pressure effects on the structural and electronic properties of ABX4 scintillating crystals. Prog. Mater. Sci. 1997, 53, 711–773. [Google Scholar] [CrossRef]
- Díaz-Anichtchenko, D.; Turnbull, R.; Bandiello, E.; Anzellini, S.; Achary, S.N.; Errandonea, D. Pressure-induced chemical decomposition of copper orthovanadate (α-Cu3V2O8). J. Mater. Chem. C 2021, 9, 13402–13409. [Google Scholar] [CrossRef]
- She, J.; Sawamura, S.; Wondraczek, L. Scratch hardness of rare-earth substituted calcium aluminosilicate glasses. J. Non-Cryst. Solids 2019, 1, 100010. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, J.; Pan, W. Theoretical investigations of the physical properties of zircon-type YVO4. J. Solid State Chem. 2012, 185, 42–48. [Google Scholar] [CrossRef]
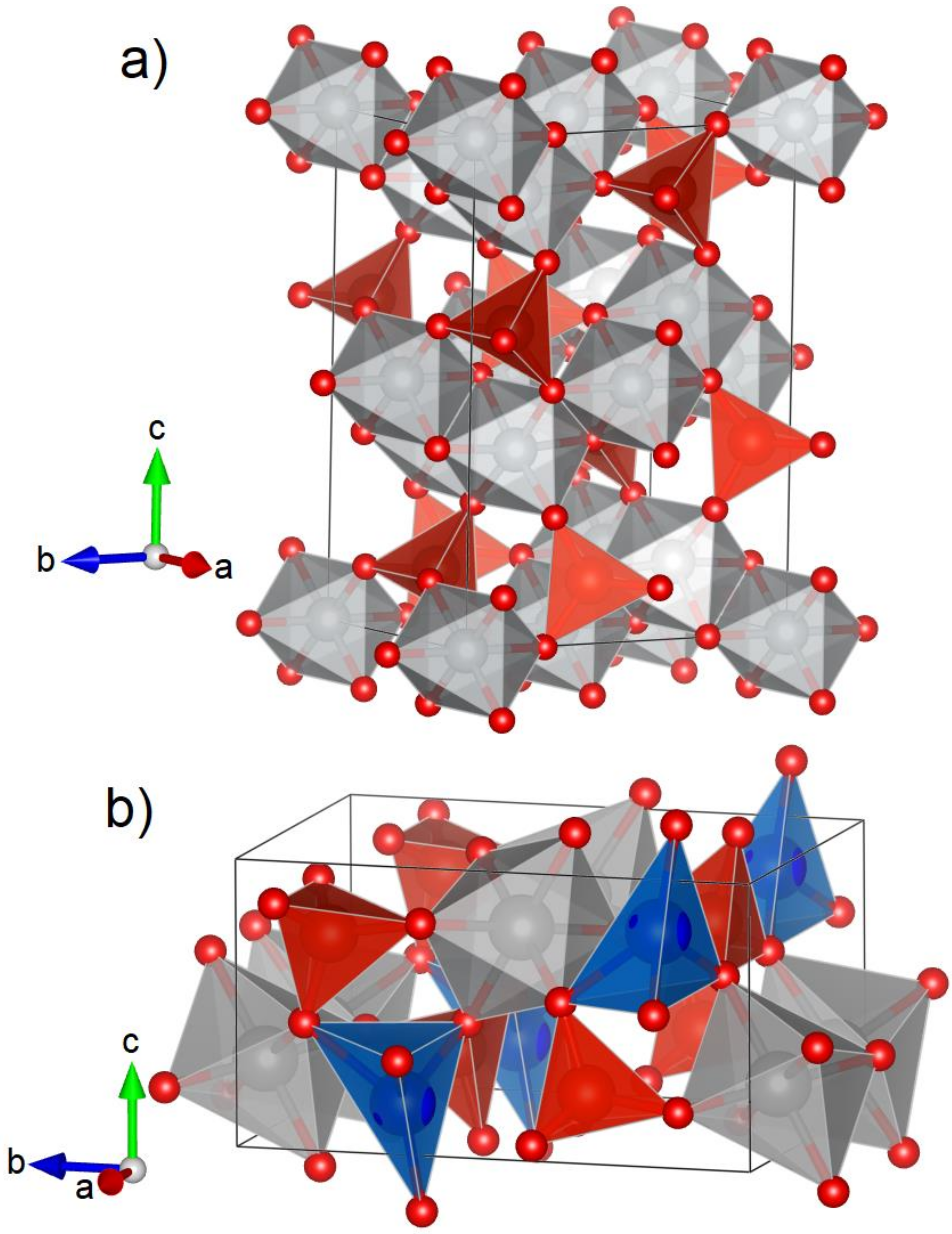
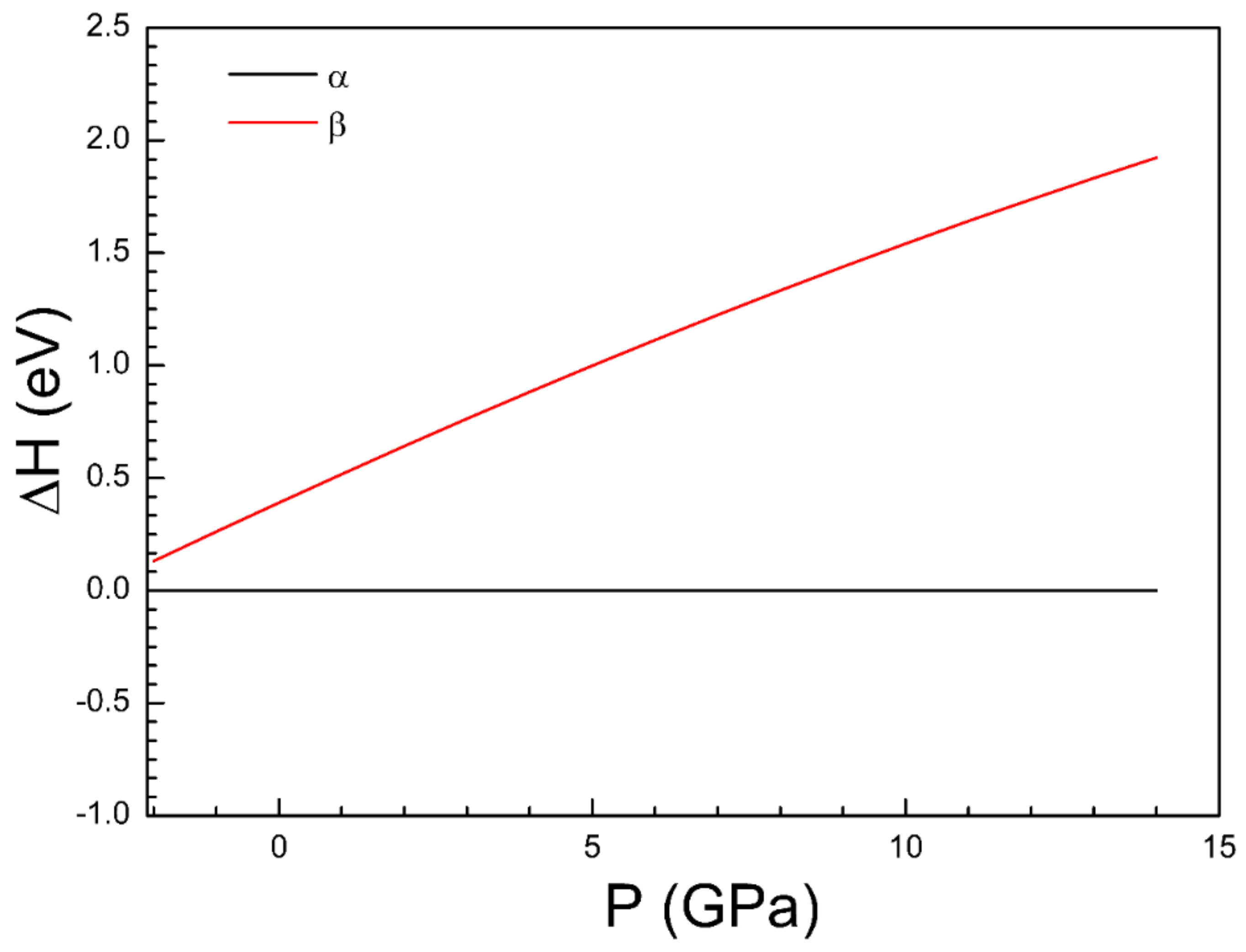

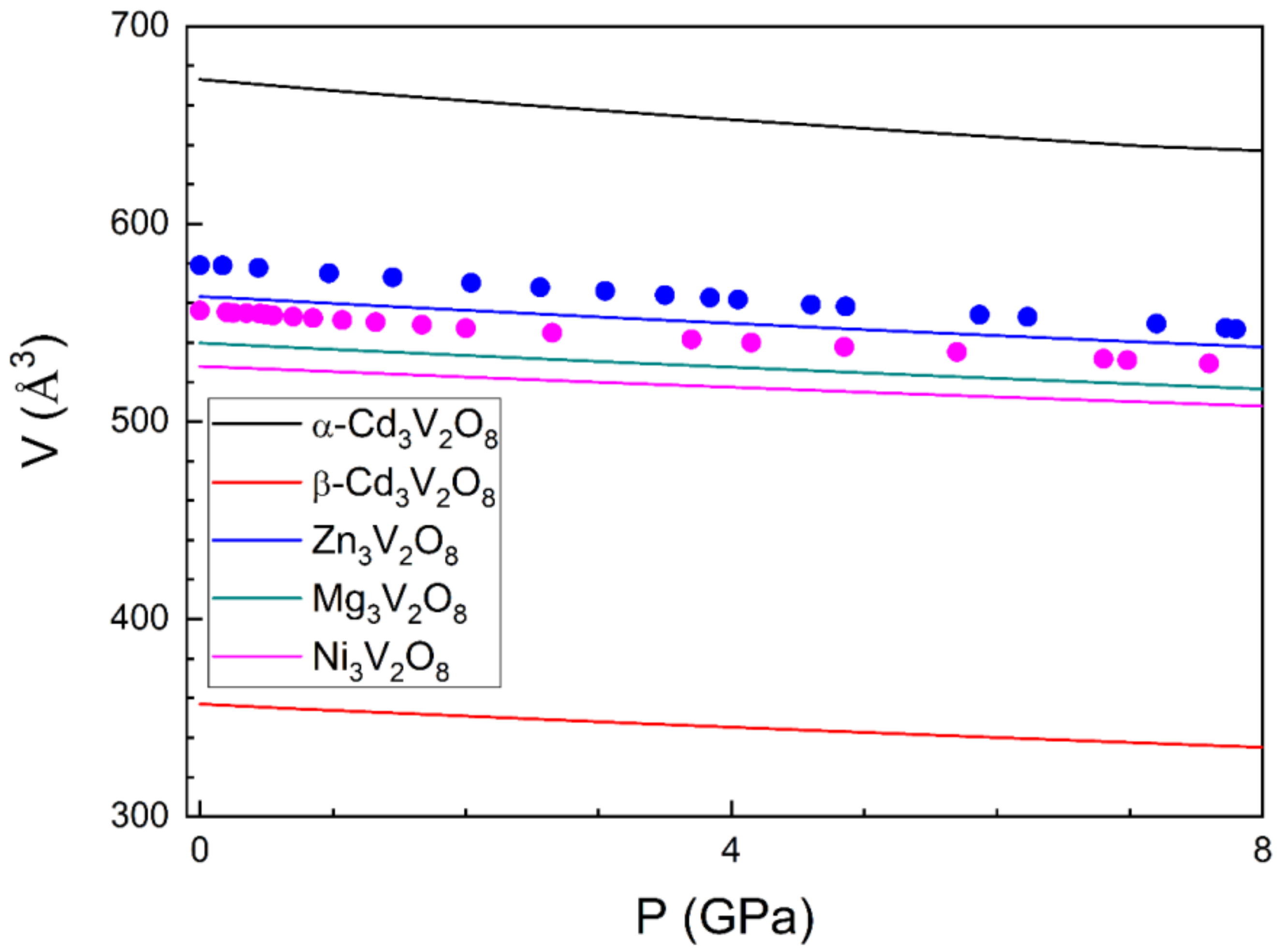
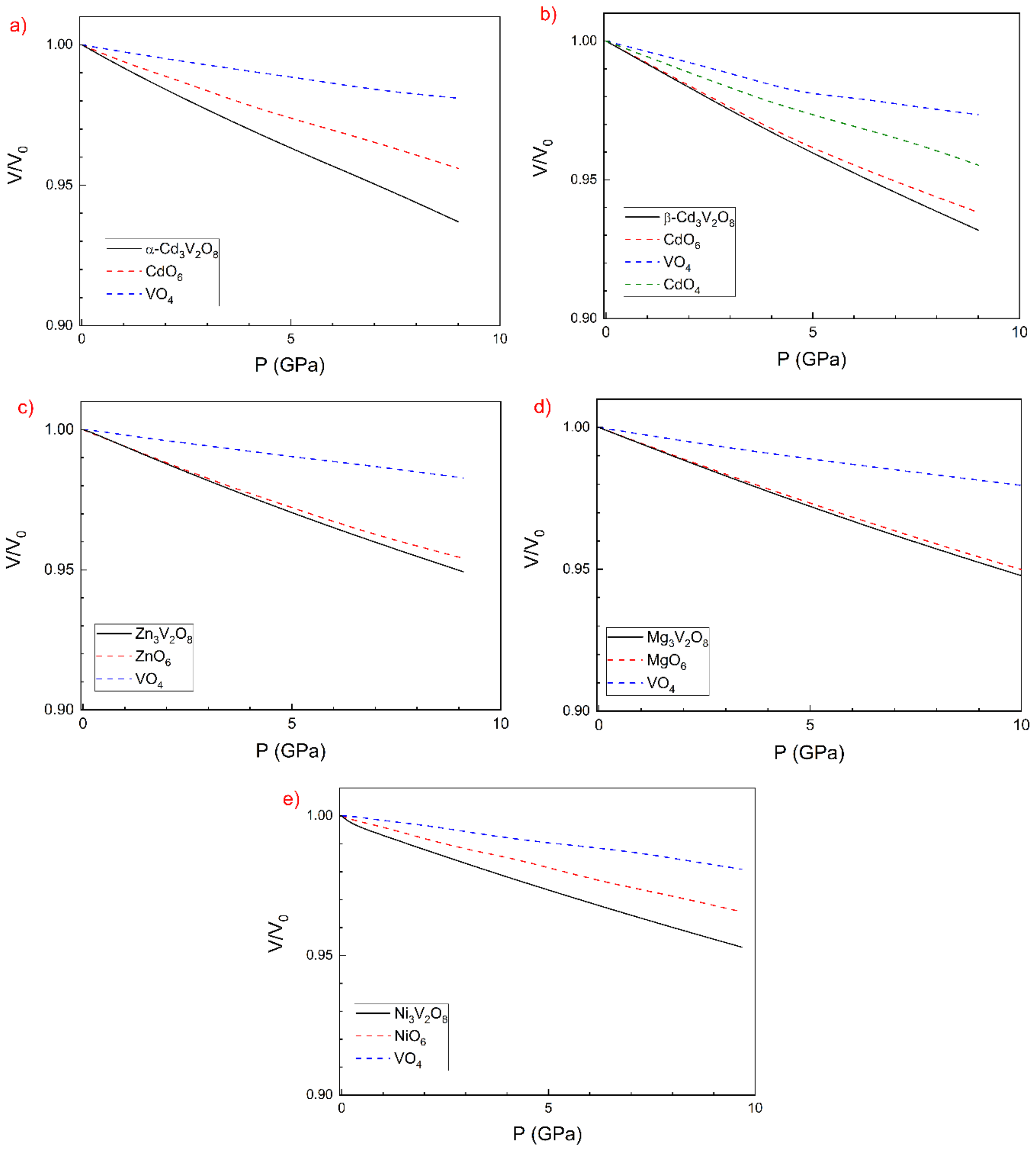

| Atom | Site | x | y | z |
|---|---|---|---|---|
| Cd1 | 4a | 0 | 0 | 0 |
| Cd2 | 8e | 0.25 | 0.1554 | 0.25 |
| V1 | 8f | 0 | 0.3902 | 0.1247 |
| O1 | 8f | 0 | 0.2784 | 0.2491 |
| O2 | 8g | 0 | 0.0121 | 0.247 |
| O3 | 16g | 0.2134 | 0.3815 | 0.9847 |
| α-Cd3V2O8 | B3LYP | ||
| a (Å) | 6.5837 | ||
| b (Å) | 11.9604 | ||
| c (Å) | 8.5473 | ||
| V (Å3) | 673.046 | ||
| β-Cd3V2O8 | B3LYP | Exp. [34] | ε(%) |
| a (Å) | 6.8744 | 6.9882 | −1.6 |
| b (Å) | 5.3107 | 5.3251 | −0.3 |
| c (Å) | 9.7796 | 9.8133 | −0.3 |
| V (Å3) | 357.032 | 365.18 | −2.2 |
| Zn3V2O8 | B3LYP | Exp. [32] | ε(%) |
| a (Å) | 6.0332 | 6.088 | −0.9 |
| b (Å) | 11.4247 | 11.489 | −0.6 |
| c (Å) | 8.1709 | 8.280 | −1.3 |
| V (Å3) | 563.200 | 579.145 | −2.8 |
| Mg3V2O8 | B3LYP | Exp. [33] | ε(%) |
| a (Å) | 5.9061 | 6.053 | −2.4 |
| b (Å) | 11.2516 | 11.442 | −1.7 |
| c (Å) | 8.1216 | 8.33 | −2.5 |
| V (Å3) | 539.705 | 576.923 | −6.4 |
| Ni3V2O8 | B3LYP | Exp. [34] | ε(%) |
| a (Å) | 5.685 | 5.936 | −4.2 |
| b (Å) | 11.4508 | 11.42 | −0.3 |
| c (Å) | 8.1093 | 8.24 | −1.5 |
| V (Å3) | 527.903 | 558.582 | −5.5 |
| Compound | VHSE06 (Å3) | VPBE (Å3) | εHSE06(%) | εPBE(%) | VMP (Å3) | εMP(%) |
|---|---|---|---|---|---|---|
| α-Cd3V2O8 | 656.413 | 669.242 | ||||
| β-Cd3V2O8 | 348.866 | 355.061 | −4.5 | −2.8 | 384.85 | 5.4 |
| Zn3V2O8 | 549.367 | 559.630 | −5.1 | −3.4 | 609.12 | 5.2 |
| Mg3V2O8 | 530.461 | 541.487 | −8.1 | −6.1 | 599.51 | 3.9 |
| Ni3V2O8 | 503.762 | 504.320 | −9.8 | −8.7 | 579.38 | 3.7 |
| GPa−1 | ||
| α-Cd3V2O8 | GPa−1 | |
| GPa−1 | ||
| GPa−1 | ||
| β-Cd3V2O8 | GPa−1 | |
| GPa−1 | ||
| GPa−1 | GPa−1 [14] | |
| Zn3V2O8 | GPa−1 | GPa−1 |
| GPa−1 | GPa−1 | |
| GPa−1 | ||
| Mg3V2O8 | GPa−1 | |
| GPa−1 | ||
| GPa−1 | GPa−1 [15] | |
| Ni3V2O8 | GPa−1 | GPa−1 |
| GPa−1 | GPa−1 |
| Phase | |||
|---|---|---|---|
| α-Cd3V2O8 | 673.0 | 112 | 5.0 |
| β-Cd3V2O8 | 357.0 | 92 | 4.0 |
| Zn3V2O8 | 563.2 | 136 | 5.4 |
| Mg3V2O8 | 539.7 | 146 | 4.4 |
| Ni3V2O8 | 527.9 | 171 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Anichtchenko, D.; Errandonea, D. Comparative Study of the Compressibility of M3V2O8 (M = Cd, Zn, Mg, Ni) Orthovanadates. Crystals 2022, 12, 1544. https://doi.org/10.3390/cryst12111544
Díaz-Anichtchenko D, Errandonea D. Comparative Study of the Compressibility of M3V2O8 (M = Cd, Zn, Mg, Ni) Orthovanadates. Crystals. 2022; 12(11):1544. https://doi.org/10.3390/cryst12111544
Chicago/Turabian StyleDíaz-Anichtchenko, Daniel, and Daniel Errandonea. 2022. "Comparative Study of the Compressibility of M3V2O8 (M = Cd, Zn, Mg, Ni) Orthovanadates" Crystals 12, no. 11: 1544. https://doi.org/10.3390/cryst12111544
APA StyleDíaz-Anichtchenko, D., & Errandonea, D. (2022). Comparative Study of the Compressibility of M3V2O8 (M = Cd, Zn, Mg, Ni) Orthovanadates. Crystals, 12(11), 1544. https://doi.org/10.3390/cryst12111544






