Abstract
There has been an urgent demand for novel neutron shielding materials (NSMs) with high mechanical strength and low acid corrosion rate to be used in compact shielding design. In this contribution, BPO4 ceramics (BPCs), one of the candidates for such materials, was successfully fabricated by a two-step method using H3BO3 and H3PO4 as raw materials. The evolution of the microstructures was then investigated, followed by testing of mechanical/thermal properties, acid corrosion resistance, and neutron shielding performance. The experimental results indicated that the calcination temperature as well as H3BO3 content in raw materials intuitively affected the densification process of the BPCs. The as-pared BPCs showed reliable mechanical properties with maximum CMOR, compressive strength and elastic modulus of 26.99 MPa, 86.89 MPa and 28.42 GPa, respectively, which also showed a high neutron shielding rate and low acid corrosion rate of 65.11% and 0.016%. The obtained results imply that the BPCs was a promising NSM for use in compact shielding design.
1. Introduction
In the past decades, compact shielding design has attracted widespread attention with the miniaturization and optimization of nuclear reactors, which were used to protect human health and improve equipment safety from potential neutron radiation. Owing to the limits of space, neutron shielding materials (NSMs) usually have to service in quite inclement environment with high temperature, corrosion, and so on. H3BO3 solution has been widely used in nuclear reactors as a moderator, and the concentration of H3BO3 became higher and approaches saturation due to the limitation of space. Considering the long-term service of NSMs in H3BO3 solution, the corrosion behavior had a great influence on the reliability of the materials. Thus, high performance NSMs with high mechanical strength and low acid corrosion rate were needed [1,2].
In general, concrete, alloy plate, polyethylene, as well as B4C ceramics have been widely used as NSMs. However, they fall short in some aspects for compact shielding design, such as space consumption, toxicity, poor acid corrosion resistance, poor thermal stability, and synthesis difficulties [3,4,5]. Therefore, developing novel NSMs with better properties has become urgent in the research of neutron radiation protection. Of particular note is that a compound with boron content can serve as an NSM, owing to its ability to capture thermal neutrons due to a huge thermal neutron absorbing cross section (3838.1 b) of isotope 10B [6]. BPO4 glass containing Li2O, Al2O3, ZnO2, PbO, and Bi2O3 had already been proved to posses excellent neutron shielding performance [7]. In addition, the ceramic materials usually have excellent characteristics such as high mechanical strength, good thermal stability, and superb corrosion resistance. The BPO4 ceramics (BPCs) had been prepared via SPS using H3BO3 and NH4H2PO4 as raw materials and had superb thermal stability at 1200 °C [8]. However, NH3 would be produced in this method, which causes pollution. As an acidic compound, neutralization reaction would not occur between BPO4 and acid solution. In this regard, BPCs should have enormous potential used in compact shielding design. Nevertheless, no related research focusing on using BPCs as NSMs have been reported. The mechanical/thermal properties and acid corrosion resistance have not been discussed. BPO4 has already been successfully synthesized by a variety of techniques including liquid-phase precipitation, sol-gel method, hydrothermal method, and so on [9,10]. Certainly, the cost of NSMs was also an important requirement to be considered.
In this work, BPCs was fabricated via a two-step route. First, BPO4 powders were synthesized by H3BO3 and H3PO4. The raw materials were mixed and pre-heated, ensuring that H3BO3 and H3PO4 were completely decomposed to minimize the porosity of the obtained final products. Then, these powders were used as precursor to be modeled, and the green body used a pressureless sintering method to prepare BPCs. Compared with the B4C ceramics that need to be sintered over 2000 °C and the BPCs prepared by SPS, the BPCs prepared in this work were low in sintering temperature, simple in process, and require less equipment, thereby effectively reducing the preparation cost. In the case of changing the calcination temperature and H3BO3 content, phase composition and microstructure, as well as the mechanical, thermal, neutron shielding, and anti-corrosion properties were investigated. This research aims to explore the application prospects of BPCs as NSMs in compact shielding design.
2. Materials and Methods
2.1. Synthesis of BPO4 Powder
To synthesize BPO4 powder, H3BO3 (Macklin Biochemical Co, Ltd., Shanghai, China, ≥99.5 wt% pure) and H3PO4 (Macklin Biochemical Co, Ltd., Shanghai, China, ≥85 wt% pure) were used as raw materials. After being accurately weighed according to different molar ratio from 0.9/1 to 1.3/1(coded as B0.9-B1.3 below, according to Table 1), the reagents were mixed until a viscous milky-white mass was obtained. Then the sufficient uniformed mixture was heated to 700 °C at a rate of 5 °C/min and a holding time of 3 h. After cooling, the sintered bulk material was ground into powder, which served as BPO4 precursor. The X-ray diffraction (XRD) graph of the BPO4 precursor with different formulations were shown in Figure 1. Significant diffraction peaks of tetragonal BPO4 (BPO4, PDF#00-006-0297) could be observed after thermal treatment, and little surplus B2O3 phase (B2O3, PDF#00-014-0696) was detected in B1.2 and B1.3 simultaneously due to the decomposition of H3BO3. The scanning electron microscopy (SEM) graph and transmission electron microscopy (TEM) pattern of B1 precursor were shown in Figure 2, indicating that BPO4 powder consisted of particles with even grain size within 0.5 μm. The reactions between H3BO3 and H3PO4 were shown in Equations (1)–(3);
2H3BO3(s) = B2O3(l) + 3H2O(g)
2H3PO4(l) = P2O5(l) + 3H2O(g)
B2O3(l) + P2O5(l) = 2BPO4(s)

Table 1.
Batch composition of the fabricated BPCs.

Figure 1.
(a)XRD pattern of BPO4 precursor and (b)partially enlarged image of the area enclosed by the red rectangle in (a).
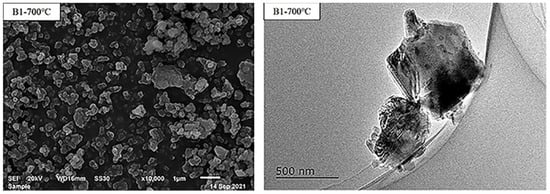
Figure 2.
SEM graph and TEM graph of B1 precursor.
2.2. Preparation of BPCs
In order to enhance the bonding performance, 0.5 wt% CMC (CMC, Macklin Biochemical Co, Ltd., Shanghai, China) and 2.5 wt% deionized water were added to the precursor, and the mixture was blended in a rotor drum for 3 h at a revolution rate about 30 rpm utilizing ZrO2 balls as processing medium, to guarantee the homogeneity of the final products.
Rectangular bars (50 × 10 × 10 mm) and cylindrical samples (Φ20 × 20 mm and Φ50 × 5, 10, 15 mm) were prepared through uniaxial pressing at 150 MPa. After being dried at 110 °C for 24 h, the obtained products were heated to 900~1100 °C at a rate of 3 °C/min and held for 3 h in an air atmosphere to produce the desired BPCs.
2.3. Evaluation Methods
The phase compositions of as-pared BPCs were analyzed by X-ray diffraction (Philips, X’Pert PRO, Cu Kα, Netherlands) in the 2θ range of 10~90°, while the microstructures were observed by scanning electron microscopy (JSM-6610, JEOL, Japan) and transmission electron microscopy (TEM, Tecnai G2 F20, S-TWIN, Tokyo, Japan). The linear shrinkage (LS) was given by Equation (4):
where L0 was the diameter of green body and L1 was the diameter of samples after calcination. Archimedes’ method was used to calculate the apparent porosity and bulk density while using a kerosene solution (0.79 g/cm3) as the medium. A three-point bending experiment was performed to test the cold modulus of rupture (CMOR) at room temperature with a span of 30 mm and a cross head speed of 0.5 MPa/s (GB/T 3001-2017). Compressive strength was measured on a hydraulic universal testing machine (ETM, Wance, China) using a load speed of 0.5 mm/min. The elastic modulus of the samples was analyzed using RFDA professional, IMCE, Belgium. Thermal conductivities at room temperature were tested with a thermal constants analyzer (Hot Disk TPS 2500S, Hot Disk AB, Sweden).
Linear shrinkage = [(L0 − L1)/L0] × 100%
Samples with dimensions of Φ50 × 5, 10 and 15 mm were used to test neutron shielding properties with the 241Am-Be neutron source/3He proportional counter (induction volume: Φ2 cm × 12.7 cm, Beijing Belipff Electronic Technology Co., Ltd., Beijing, China). At the same time, the weight of specimens was measured before and after immersion in H3BO3 solution (12.5 wt%) for 24 h at 80 °C to characterize the acid corrosion rate. In order to avoid accidental error, all the data was calculated from 3 groups of parallel experiments.
3. Results and Discussion
3.1. Phase Composition
The results obtained for the variation in phase composition of samples B1.3 prepared at 900~1100 °C were shown in Figure 3. Compared to Figure 1, BPO4 was still the main crystalline phase and B2O3 diffraction peaks grew weaker. On the one hand, the lattice constant of B2O3 (10.06 × 10.06 × 16.00 Å) has a huge difference from that of BPO4 (4.34 × 4.34 × 2.00 Å), which resulted in B2O3 being difficult to dissolve in BPO4. Thus, B2O3 was speculated to mainly participate in the formation of the glass phase. On the other hand, part of B2O3 would volatilized considering the high processing temperature. After contrasting the main peak near 24.5°, it can be found that the relative strength showed significant growth when the temperature increased from 900 °C to 1100 °C [11]. The results indicated that higher temperature was advantageous for the development of BPO4 grains.

Figure 3.
XRD patterns of B1.3 treated at 900~1100 °C.
3.2. Microstructures
Figure 4 displayed the microstructures of samples B0.9 treated at 900~1100 °C. It could be clearly seen that the grain size improved significantly as the temperature increased. In contrast, the porosity of the ceramics decreased and the micropores formed by the accumulation of grains gradually evolved into larger pores. Moreover, while it showed irregular tortuous shape at 900 °C, the observed pore morphology exhibited a more regular smooth round at higher temperature. The phenomenon could be explained as follows: the rate of mass transfer between particles became faster when temperature increased, which led to an increase of grain size. At the same time, P2O5 melted to form a liquid phase and the viscosity of the liquid-phase gradually decreased, which provided conditions for liquid-phase sintering and greatly accelerated the densification [12]. However, the evaporation of P2O5 was completed when the temperature was higher than 1000 °C and the liquid phase disappeared, resulting in abnormal growth of some grains. Finally, adjacent grains were sintered together due to “neck reaction” and formed a grain clusters structure, which led to changes in pore shape.
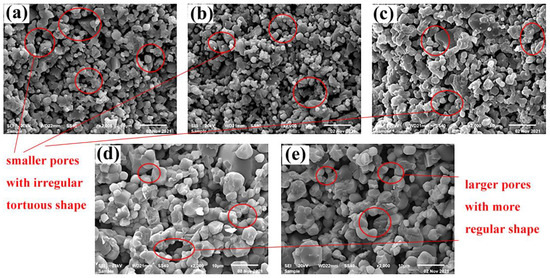
Figure 4.
SEM images of the B0.9 samples treated at (a) 900 °C, (b) 950 °C, (c) 1000 °C, (d) 1050 °C, and (e) 1100 °C.
The microstructures of samples with different H3BO3 content calcinated at 1100 °C were shown in Figure 5. Part of the grains in Figure 5b–e grew abnormally and the grain size in Figure 5a behaved much more uniformly. In addition, glass phase between the grains could be detected in Figure 5d,e, which filled the grain gaps. Table 2 showed the content of H3BO3 and H3PO4 that theoretically unreacted. It could be calculated that the remaining B2O3 content in the sample increased as H3BO3 increased while H3BO3 would decomposed into B2O3 according to Equation (1). There would be a larger amount of B2O3 in the samples from B1 to B1.3, which led to more lavish liquid phase due to the melting of B2O3 at higher temperature. Under the action of liquid phase sintering, particles faded away and regrew, leading to grain recrystallization and reorganization. As a result, the number of abnormally grown grains was reduced from B1 to B1.3. Part of the pores between the grains in B1.2 and B1.3 were filled by excessive B2O3 amorphous phase, which led to a further decrease of porosity. Liquid phase sintering also occurred in B0.9, the grain size was finer, and no obvious abnormal growth could be observed.

Figure 5.
SEM images of the samples treated at 1100 °C (a) B0.9, (b) B1, (c) B1.1, (d) B1.2, and (e) B1.3.

Table 2.
The content of H3BO3 and H3PO4 that theoretically unreacted.
3.3. Mechanical and Thermal Properties
It could be obviously seen in Table 3 that most of the samples followed a shrinkage trend after calcination. The shrinkage rate of the sample showed a step-by-step increase along with the increase of heat temperature and H3BO3 content. B0.9 held the minimum shrinkage rate, and exhibited small amount of expansion at 900 °C and 950 °C. Figure 6 showed the mechanical and thermal properties of the as-pared BPCs. It was displayed in the Figure 6a,b that the apparent porosity and bulk density showed completely opposite trends of change with the increase of heating temperature. When the temperature increased, the grains slipped and rearranged because of wetting and subsequent dissolution of small crystal grains in the liquid phase. This promoted the growth of grain and densification of the BPCs, resulting in the decrease of porosity and increase of bulk density [13]. From B1 to B1.3, the apparent porosity decreased, and bulk density increased with the increase of H3BO3 content at same temperature. The test results were consistent with the transformation of the microstructure. Intuitively, there would be more B2O3 amorphous phase to fill the grain gaps due to a greater amount of H3BO3. Surplus B2O3 would form a liquid phase, which distributed between the particles, led to capillary force and promoted the densification of ceramics [14]. The lowest apparent porosity and highest bulk density were 23.3% and 1.95 g/cm3, respectively, which were obtained in B0.9 treated at 1000 °C. When there were excessive H3PO4, the grain size grew much more uniformly, resulting in fewer and smaller pores. However, the grain grew larger when temperature exceeded 1000 °C, and the accumulation of larger grains caused the increase in porosity.

Table 3.
LS of the samples with different formulations treated at 900~1100 °C for 3 h.

Figure 6.
Effect of the H3BO3 content and calcinating temperature on the mechanical and thermal properties of the BPCs (a) apparent porosity, (b) bulk density, (c) CMOR, (d) compressive strength, (e) elastic modulus and (f) thermal conductivity.
As illustrated in Figure 6c–e, as the calcination temperature improved, the CMOR, compressive strength and elastic modulus of the samples were generally enhanced. In addition, the same phenomenon occurred when H3BO3/H3PO4 proportion deviated from 1:1. B0.9 treated at 1100 °C possessed the maximum CMOR, compressive strength and elastic modulus of 26.99 MPa, 86.89 MPa and 28.42 GPa, respectively. Referring to the theoretical sintering principle, the higher heat temperature as well as the additional B2O3/P2O5 would both accelerate the densification of BPCs, which was showed in improved mechanical properties. Furthermore, when there was too much H3BO3, high calcinating temperature would cause structural destruction which can be found in B1.3 at 1100 °C, thereby damaging the properties of samples. The mechanical properties of ceramics were closely related to the porosity. A simple relationship based on the minimum solid area was as follows:
where σ and σ0 along with E and E0 were the mechanical strength and elastic modulus of the porous samples and completely densified samples, respectively; n was a constant; and P was the porosity of the specimens. This relationship stated that the mechanical strength increased as the porosity decreases. The results of this study were found to be consistent with this relationship.
σ = σ0 exp (−nP)
E = E0 exp (−nP)
The thermal conductivity of BPCs obtained at room temperature were presented in Figure 6f. The thermal conductivity of the samples was mildly impacted by the heating temperature. For instance, increasing the heating temperature from 900 to 1100 °C leading to a change in the thermal conductivity of B1 from 1.63 to 2.28 W/(m·K). On the other hand, B0.9 holds significantly higher thermal conductivity than the other groups which range from 10.33 to 11.25 W/(m·K). This could be attributed to the influences of the heating-induced decrease in the porosity. The heat conduction process of porous ceramics primarily contained heat radiation and heat conduction, and heat conduction dominated at low temperatures. The thermal conduction process included solid conduction and gas conduction, and the gas conductivity played a central role in the total thermal conductivity while thermal conductivity of air was much lower than that of solid framework. B0.9 held much lower porosity, so the thermal conductivity of this group surged intuitively as well. Furthermore, B2O3 would spontaneously transform into a molten flowing-liquid at high temperatures. With increasing temperature, the viscosity of the molten liquid phases became lower, and the diffusion rate became faster; finally, a network structure with high thermal conductivity could be formed inside the material, which explained the phenomenon that the thermal conductivity gradually increased from B1 to B1.3.
3.4. Acid-Corrosion Resistance
Table 4 showed the acid corrosion rate of different samples. The corrosion rate was defined as the rate of mass loss of the specimens after corrosion. The specimens were weighted with an electronic balance whose accuracy was 0.0001 g before and after being immersed in 12.5 wt% H3BO3 solution at 80 °C for 24 h. According to the standard (GB/T 17897-2016), the amount of solution required per square centimeter of sample surface area was not less than 20 mL. Obviously, the more H3BO3 content in the prescription, the more likely the corresponding samples suffered structurally damage in the acid solution. On the contrary, raising calcinating temperature had the opposite effect. The effect of H3BO3 content on the performance was more significant than that of the heating temperature. B0.9 treated at 1000 °C held the lowest corrosion rate of 0.016%, which showed ultra-low acid corrosion rate compared to polyethylene and PE-B4C composites (0.1%). The corrosion process of samples includes overall corrosion and local corrosion, while the local corrosion played a decisive role in destruction. Porosity was one of the main factors affecting the corrosion rate. B0.9 treated at 1000 °C held the lowest apparent porosities, which prevented the acid solution from eroding inside of the samples. In the meantime, the absence of glass phase eliminated inter-grain corrosion, allowing the samples to remain in acid solution for a longer period of time. The SEM graph of B0.9 × 1000 °C before and after corrosion was shown in Figure 7. We could clearly find that corrosion on grain surface was more serious, but the grain morphology was nonetheless preserved and relatively complete, and the binding between the grain was still tight. Partial BPO4 was dissolved in the solution, resulting in the weight loss of materials. In summary, BPCs showed excellent acid corrosion resistance.

Table 4.
Acid corrosion rate of the samples with different formulations treated at 900~1100 °C for 3 h.
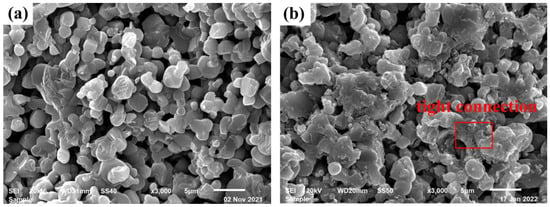
Figure 7.
SEM images of B0.9 × 1000 °C (a) before corrosion and (b) after corrosion.
3.5. Neutron Shielding Performance
To explore the potential of BPCs applications as NSMs, samples with thicknesses of 5, 10, and 15 mm were placed in the path between the radiation and high-dose particle detector. The neutron attenuation was evaluated using a 241Am-Be source that has 300 mCi activity. The neutrons in this source had an average energy of 4.5 MeV. For each specimen, the test time was set as 5 min. The neutrons passing through the samples (I) were counted by a He-3 probe neutron detector. Then the same procedure was conducted without using the samples to measure the collimated neutrons (I0). The neutron shielding rate (NSR) was calculated using Equation (7):
NSR = (I0 − I)/I0
Table 5 showed the results of the neutron shielding tests. Based on the neutron shielding theory, the attenuation of neutrons can be separated into two processes. Fast neutrons are slowed down through elastic and inelastic scattering, and then moderated into the thermal neutron energy level. Subsequently, thermal neutrons are absorbed by absorption elements, such as B. Notably, increasing the thickness contributed toward enhancing the neutron shielding performance. The greater thickness could increase the collision probability of neutrons with the material and make the neutrons more likely to be absorbed by the B element, thereby greatly improving the shielding efficiency. Under the condition of the same thickness, it showed the relatively weak effect of H3BO3 content on the neutron shielding rate. For shielding materials with the same thickness, samples with lower porosity have higher neutron absorption element content, which can lead to more neutrons being absorbed, thereby improving the neutron shielding performance. For this reason, B0.9 had a fairly good shielding efficiency of 64.78%. After comparison, the B1.3 sample treated at 1100 °C (Φ50 × 15 mm) showed the best neutron shielding performance of 65.11%. Table 6 summarizes the previously reported acid corrosion rate, neutron shielding rate, compressive strength and refractoriness of some other neutron shielding materials as well as the BPCs in our work.

Table 5.
Neutron shielding rate of the samples with different formulations treated at 1100 °C for 3 h, whose thicknesses were 5, 10, and 15 mm, respectively.

Table 6.
Comparison of acid corrosion rate of different neutron shielding materials.
4. Conclusions
In this work, BPCs were prepared via a two-step method at a temperature range of 900~1100 °C using H3BO3 and H3PO4 as raw materials. The experimental results showed that BPO4 was able to be fully produced at 700 °C. Based on the SEM observation, physicochemical tests and neutron shielding characterization, it was demonstrated that BPCs prepared from pre-fired BPO4 powders possess superior mechanical properties, excellent acid corrosion resistance, and outstanding neutron shielding performance. The calcination temperature as well as H3BO3 content intuitively affected the densification process of the BPCs. It is worth noting that, when there was excess H3PO4 in raw materials, the grain size grew more uniform and the sample performance improved much more obviously. The B0.9 samples showed the lowest apparent porosity of 23.3%, maximum CMOR, compressive strength and elastic modulus of 26.99 MPa, 86.89 MPa and 28.42 GPa, and ultra-low acid corrosion rate of 0.016%. In the meantime, BPCs owned controllable thermal conductivity from 1.63 to 11.25 W/(m·K) at room temperature. When they served as NSMs, BPCs exhibited an outstanding neutron shielding rate of 64.78% (B0.9, 1100 °C × 3 h, 15 mm). Therefore, BPCs have promising applications in compact nuclear reactors. The findings of the work can be beneficial to further research on the potential applications of BPCs.
Author Contributions
Conceptualization, Y.Z.; Investigation, Y.Z.; Project administration, S.L., W.J., R.C. and D.L.; Resources, Y.L.; Supervision, Y.L.; Validation, Y.Z.; Writing—original draft, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51772221) and the National Natural Science Foundation of China (No. 51902229).
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 51772221) and the National Natural Science Foundation of China (No. 51902229).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hei, D.; Chen, R.; Liu, F.; Lao, D.; Jia, W. A novel design of neutron shielding composite materials with three-dimensionally interwoven structure and excellent properties. J. Alloys Compd. 2020, 845, 156328. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Q.; Qin, J.; Wu, Y.; Zhang, T.; Xie, Z.; Jiang, X.; Zhang, G.; Xu, H.; Zheng, X.; et al. Study on Composite Material for Shielding Mixed Neutron and γ-Rays. IEEE Trans. Nucl. Sci. 2008, 55, 2376–2384. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Zhang, X.; Wu, H.; Guo, S.; Wang, Y. Enhancing the neutron shielding ability of polyethylene composites with an alternating multi-layered structure. Compos. Sci. Technol. 2017, 150, 16–23. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.; Thabet, A.A. Recycled high-density polyethylene plastics added with lead oxide nanoparticles as sustainable radiation shielding materials. J. Clean. Prod. 2018, 176, 276–287. [Google Scholar] [CrossRef]
- Soltani, Z.; Beigzadeh, A.; Ziaie, F.; Asadi, E. Effect of particle size and percentages of Boron carbide on the thermal neutron radiation shielding properties of HDPE/B4C composite: Experimental and simulation studies. Radiat. Phys. Chem. 2016, 127, 182–187. [Google Scholar] [CrossRef]
- Feng, Y.C.; Geng, L.; Fan, G.H.; Li, A.B.; Zheng, Z.Z. The properties and microstructure of hybrid composites reinforced with WO3 particles and Al18B4O33 whiskers by squeeze casting. Mater. Des. 2009, 30, 3632–3635. [Google Scholar] [CrossRef]
- Tekin, H.; Altunsoy, E.; Kavaz, E.; Sayyed, M.; Agar, O.; Kamislioglu, M. Photon and neutron shielding performance of boron phosphate glasses for diagnostic radiology facilities. Results Phys. 2019, 12, 1457–1464. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Bin Hong, W.; Yan, H.; Wu, S.Y.; Chen, X.M. Preparation and microwave dielectric properties of BPO4 ceramics with ultra-low dielectric constant. J. Mater. Sci. Mater. Electron. 2021, 32, 6660–6667. [Google Scholar] [CrossRef]
- Shan, Z.C. A new method for preparation of BPO4. Chem. World 2003, 6, 289–290. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.; Jiang, H.; Gong, H. Synthesis of BPO4 powder by solvent azeotropic dehydration method. Sci. Technol. Chem. Indus. 2012, 20, 37–39. [Google Scholar] [CrossRef]
- Rashad, M.; Balasubramanian, M. Characteristics of porous mullite developed from clay and AlF3·3H2O. J. Eur. Ceram. Soc. 2018, 38, 3673–3680. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; He, Z.; Xiang, R.; Jia, W.; Li, S.; Lao, D.; Zhou, Z.; Dong, C. Structural stability and neutron-shielding capacity of GdBO3-Al18B4O33 composite ceramics: Experimental investigation and numerical simulation. Ceram. Int. 2021, 47, 20935–20947. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Li, S.; Xu, N.; Wang, H.; Luo, H.; Chen, P. Novel two-step sintering and in situ bonding method for fabrication of ZrP2O7 ceramics. Ceram. Int. 2021, 47, 23875–23879. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, J.; Hu, P.; Ding, F.; Yang, J.; Yuan, F. A novel low-temperature strategy for synthesis of alumina ceramics with uniform and interconnected pores by silica coating. J. Mater. Sci. 2016, 52, 1603–1616. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, Z.; Chen, J.; Yao, M.; Chen, Z.; Ejaz, A. The effects of temperature and aeration on the corrosion of A508III low alloy steel in boric acid solutions at 25–95 °C. J. Nucl. Mater. 2016, 480, 88–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).