An N4-Tetradentate Hydrazone Ligand That Binds in a Neutral, Mono- and Bisdeprotonated Form to Iron(II) and Zinc(II) Metal Ions
Abstract
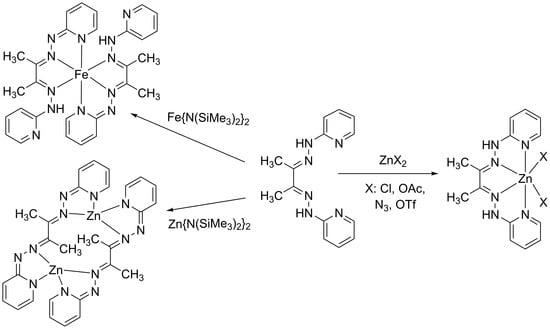
:1. Introduction
2. Materials and Methods
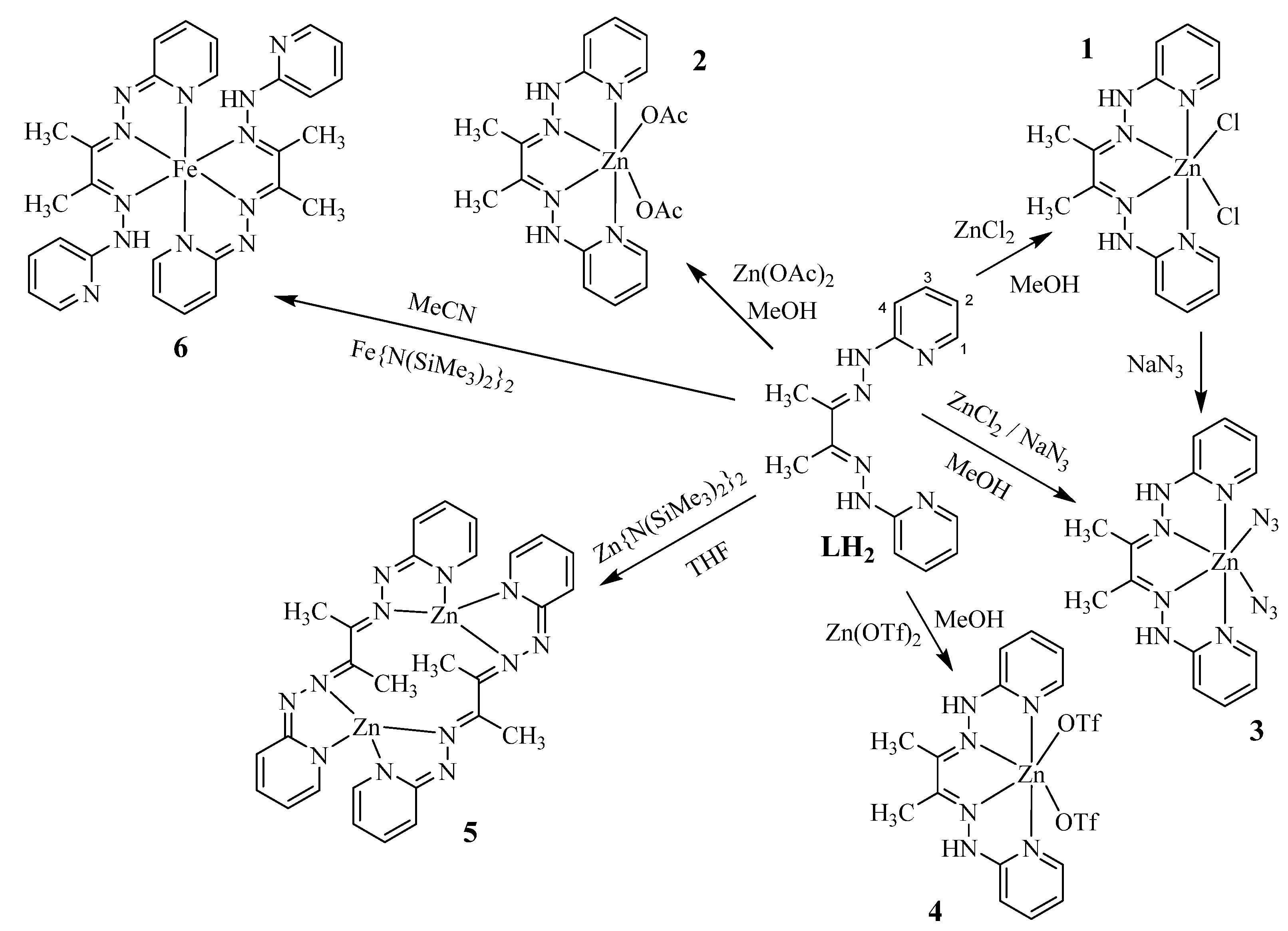
2.1. Synthesis of Neutral Zinc(II) Complexes
2.2. Synthesis of Deprotonated Complexes
3. Results and Discussions
3.1. Synthesis and Characterization
3.2. NMR Spectra of the Zinc Complexes 1–4
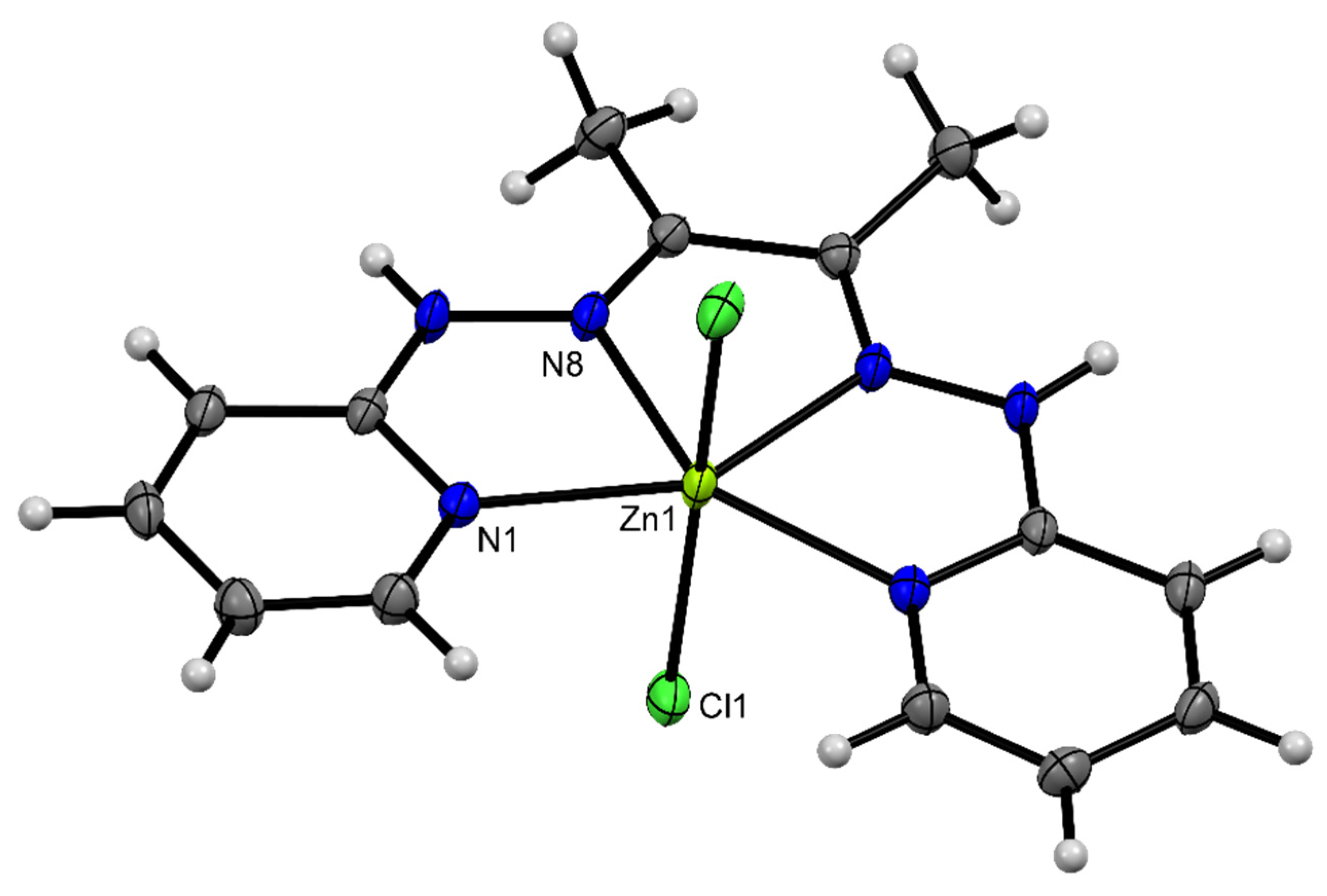
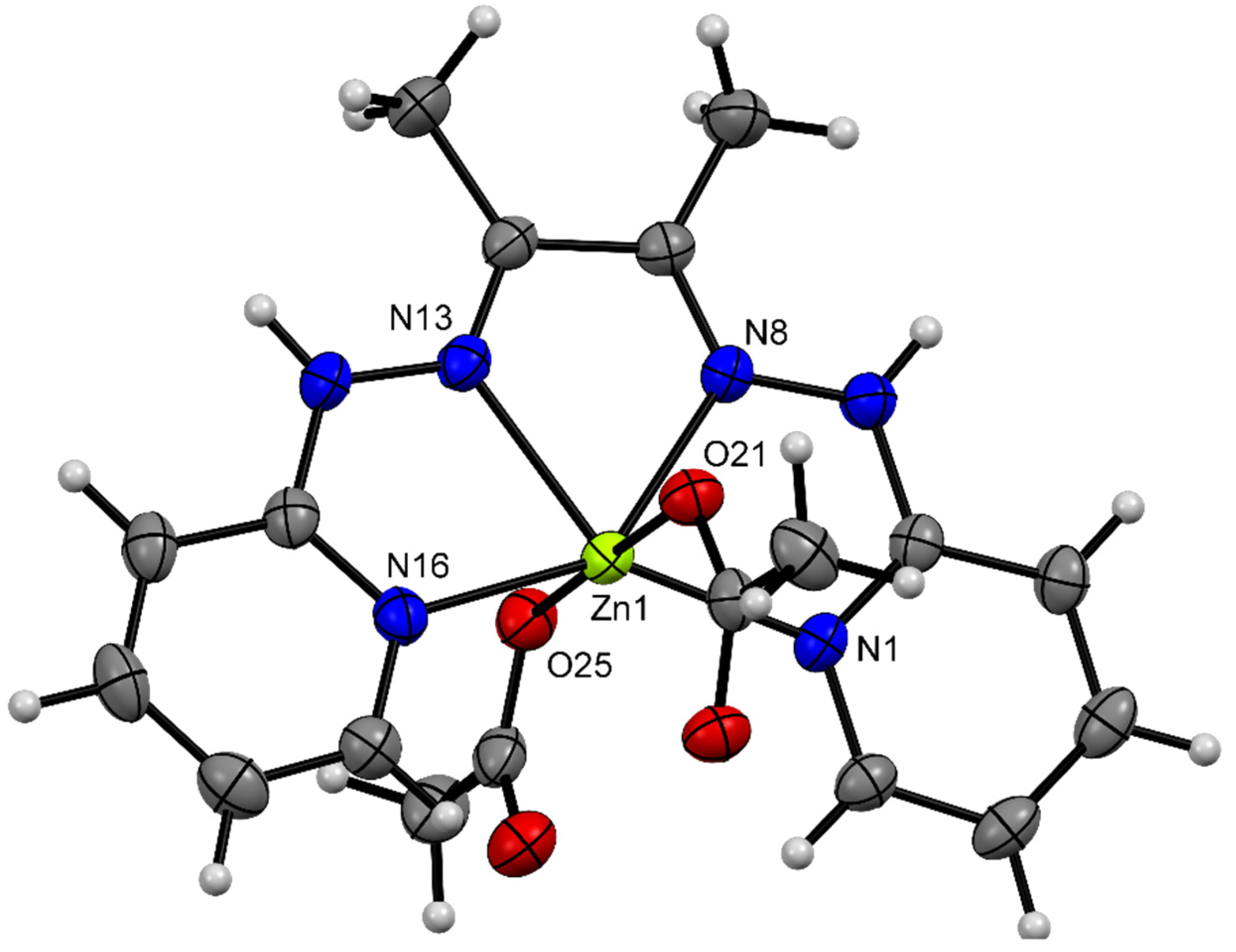
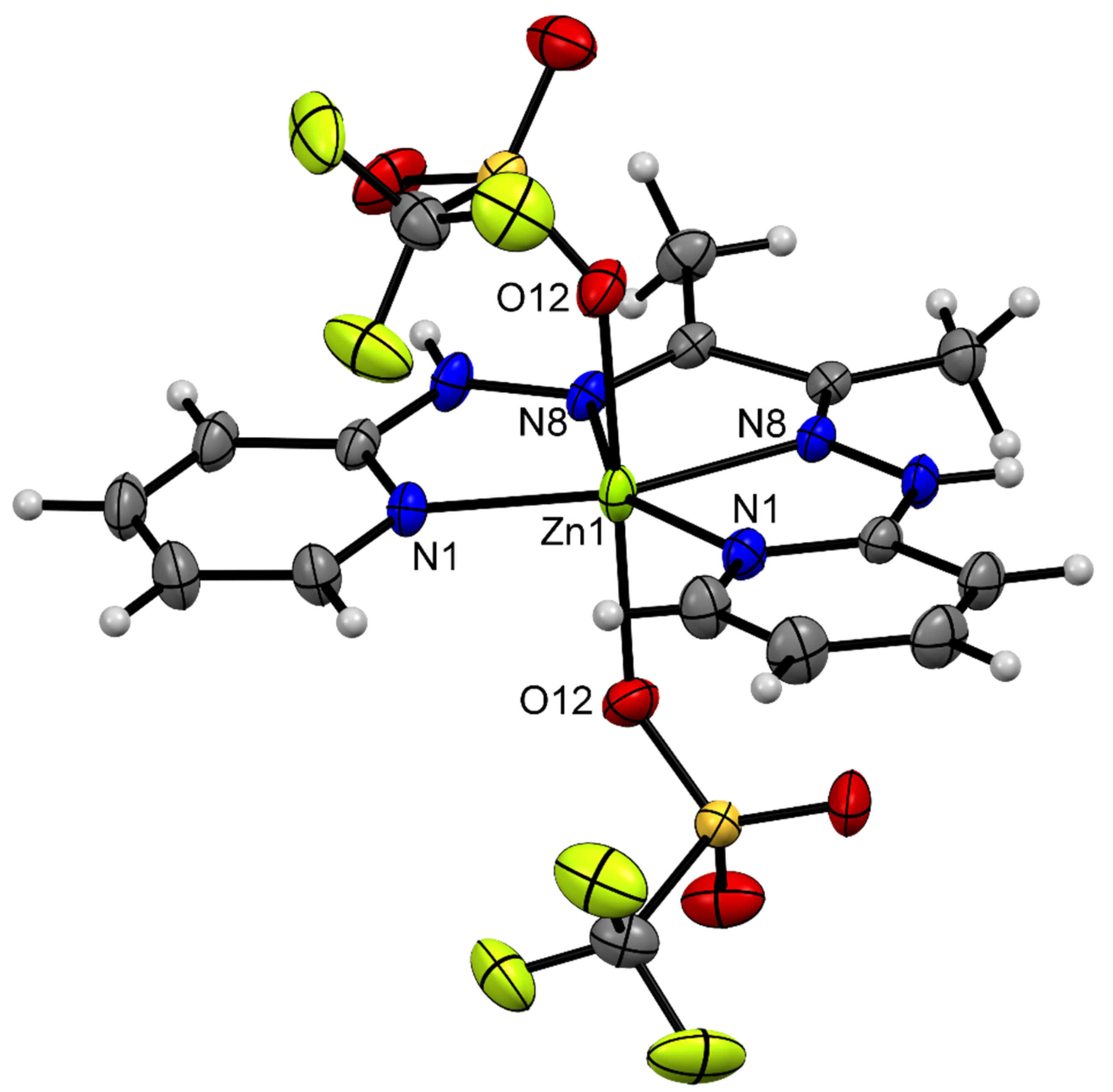
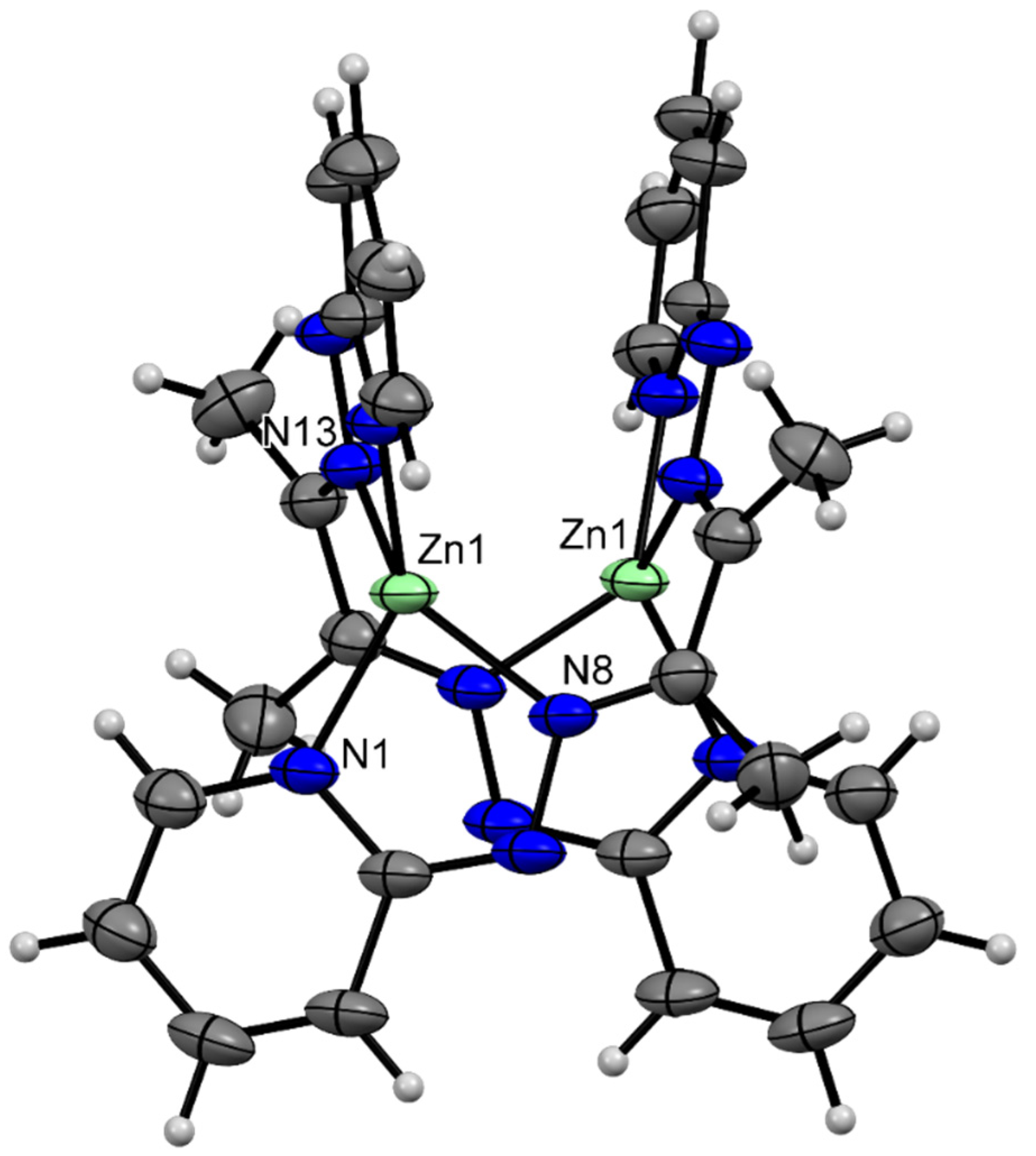
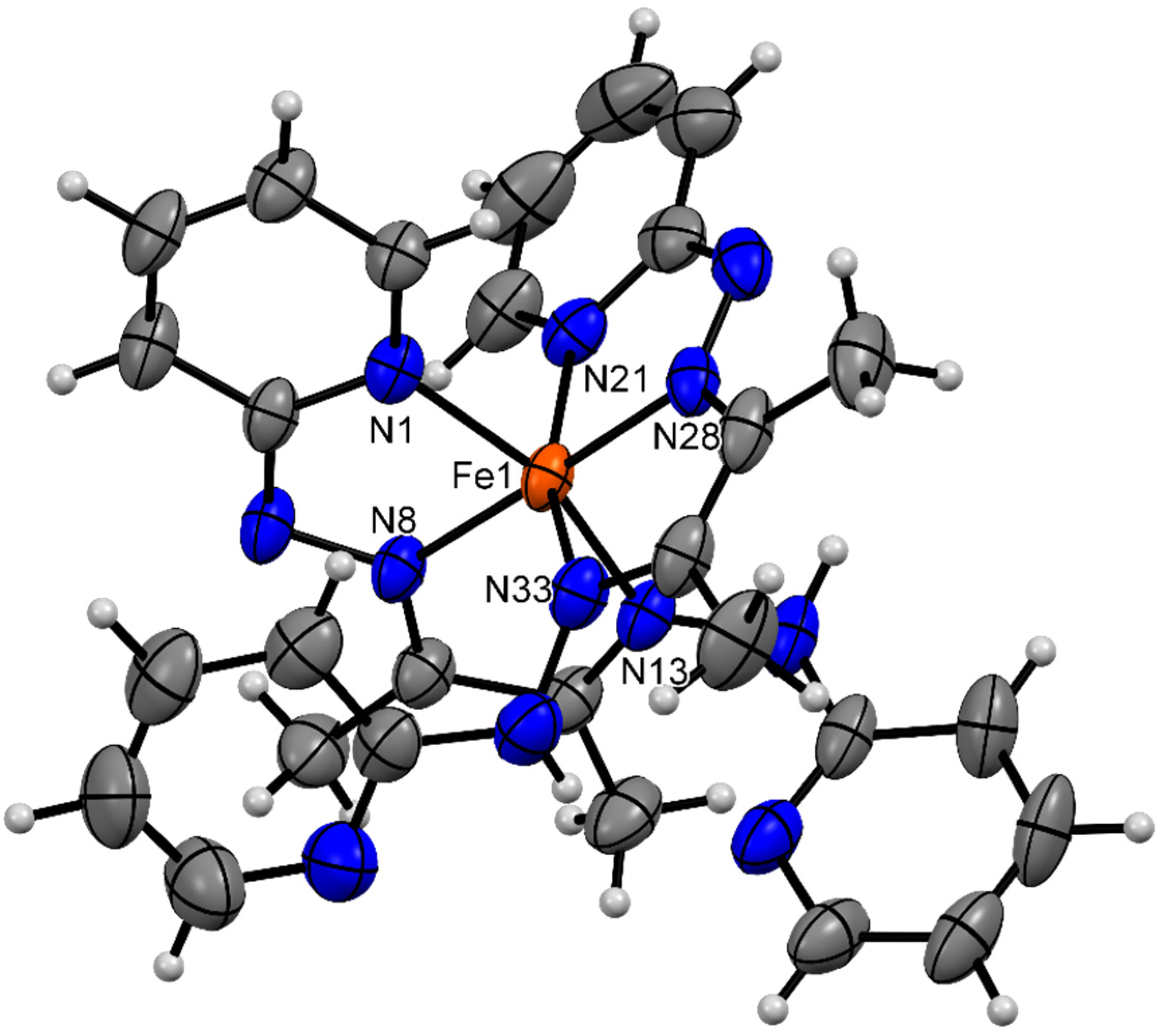
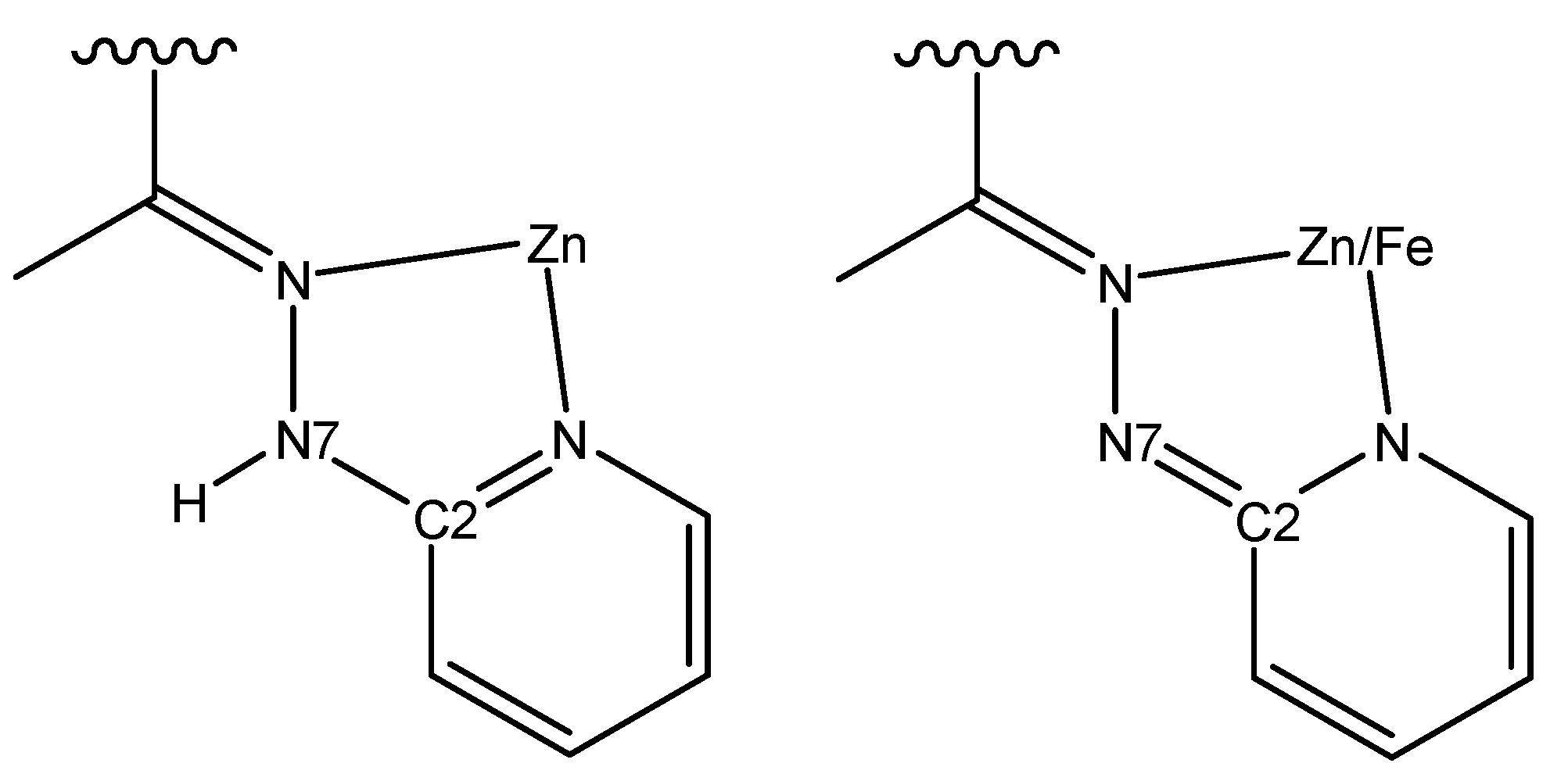
3.3. Description of Crystal Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Melville, NY, USA, 1994; p. 411. [Google Scholar]
- Haas, K.L.; Franz, K.J. Application of Metal Coordination Chemistry To Explore and Manipulate Cell Biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [Green Version]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life: An Introduction and Guide, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; p. 426. [Google Scholar]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef]
- Sekar, N.; Ramasamy, R.P. Recent advances in photosynthetic energy conversion. J. Photochem. Photobiol. C 2015, 22, 19–33. [Google Scholar] [CrossRef]
- Proppe, A.H.; Li, Y.C.; Aspuru-Guzik, A.; Berlinguette, C.P.; Chang, C.J.; Cogdell, R.; Doyle, A.G.; Flick, J.; Gabor, N.M.; van Grondelle, R.; et al. Bioinspiration in light harvesting and catalysis. Nat. Rev. Mat. 2020, 5, 828–846. [Google Scholar] [CrossRef]
- Kaminskaia, N.V.; Spingler, B.; Lippard, S.J. Hydrolysis of beta-Lactam Antibiotics Catalyzed by Dinuclear Zinc(II) Complexes—Functional Mimics of Metallo-beta-lactamases. J. Am. Chem. Soc. 2000, 122, 6411–6422. [Google Scholar] [CrossRef] [Green Version]
- Kaminskaia, N.V.; Spingler, B.; Lippard, S.J. Intermediate in beta-lactam hydrolysis catalyzed by a dinuclear zinc(II) complex: Relevance to the mechanism of metallo-beta-lactamase. J. Am. Chem. Soc. 2001, 123, 6555–6563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malina, J.; Hannon, M.J.; Brabec, V. Recognition of DNA bulges by dinuclear iron(II) metallosupramolecular helicates. FEBS J. 2014, 281, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Medina-Molner, A.; Rohner, M.; Pandiarajan, D.; Spingler, B. Mono- and dinuclear metal complexes containing the 1,5,9-triazacyclododecane ([12]aneN3) unit and their interaction with DNA. Dalton Trans. 2015, 44, 3664–3672. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V.; Singh, S.K. Perspectives of medicinally privileged chalcone based metal coordination compounds for biomedical applications. Eur. J. Med. Chem. 2019, 174, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Roopa; Bhalla, V.; Kumar, M. Beyond zinc coordination: Bioimaging applications of Zn(II)-complexes. Coord. Chem. Rev. 2021, 427, 213550. [Google Scholar] [CrossRef]
- Schiff, H. Eine neue Reihe organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Bermejo, E.; Castineiras, A.; Garcia, I.; West, D.X. Spectral and structural studies of mercury(II) complexes of 2-pyridineformamide N(4)-dimethylthiosemicarbazone. Polyhedron 2003, 22, 1147–1154. [Google Scholar] [CrossRef]
- Abou Melha, K.S. In-vitro antibacterial, antifungal activity of some transition metal complexes of thiosemicarbazone Schiff base (HL) derived from N4-(7′-chloroquinolin-4′-ylamino) thiosemicarbazide. J. Enzyme Inhib. Med. Chem. 2008, 23, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Zoubi, W.A. Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int. J. Org. Chem. 2013, 3, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Bas. Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalia, S.A.; Afsan, F.; Hossain, M.S.; Khan, M.N.; Zakaria, C.; Zahan, M.K.E.; Ali, M. A short review on chemistry of schiff base metal complexes and their catalytic application. Int. J. Chem. Stud. 2018, 6, 2859–2866. [Google Scholar]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Ziessel, R. Schiff-based bipyridine ligands. Unusual coordination features and mesomorphic behaviour. Coord. Chem. Rev. 2001, 216, 195–223. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Costes, J.-P.; Shova, S.; Wernsdorfer, W. Tetranuclear [Cu-Ln]2 single molecule magnets: Synthesis, structural and magnetic studies. Dalton Trans. 2008, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Kumar Sutar, A.; Lin, C.-C. Polymer-supported Schiff base complexes in oxidation reactions. Coord. Chem. Rev. 2009, 253, 1926–1946. [Google Scholar] [CrossRef]
- Orio, M.; Jarjayes, O.; Kanso, H.; Philouze, C.; Neese, F.; Thomas, F. X-Ray Structures of Copper(II) and Nickel(II) Radical Salen Complexes: The Preference of Galactose Oxidase for Copper(II). Angew. Chem. Int. Ed. 2010, 49, 4989–4992. [Google Scholar] [CrossRef]
- Adão, P.; Kuznetsov, M.L.; Barroso, S.; Martins, A.M.; Avecilla, F.; Pessoa, J.C. Amino Alcohol-Derived Reduced Schiff Base VIVO and VV Compounds as Catalysts for Asymmetric Sulfoxidation of Thioanisole with Hydrogen Peroxide. Inorg. Chem. 2012, 51, 11430–11449. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Wang, B.; Jia, X.; Zhang, X. The enhanced catalytic performance of cobalt catalysts towards butadiene polymerization by introducing a labile donor in a salen ligand. Dalton Trans. 2014, 43, 4169–4178. [Google Scholar] [CrossRef]
- Oberholzer, M.; Probst, B.; Bernasconi, D.; Spingler, B.; Alberto, R. Photosensitizing Properties of Alkynylrhenium(I) Complexes [Re(-C≡C-R)(CO)3(N∩N)] (N∩N = 2,2′-bipy, phen) for H2 Production. Eur. J. Inorg. Chem. 2014, 3002–3009. [Google Scholar] [CrossRef]
- Das, P.; Linert, W. Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord. Chem. Rev. 2016, 311, 1–23. [Google Scholar] [CrossRef]
- Beigi, Z.; Kianfar, A.H.; Farrokhpour, H.; Roushani, M.; Azarian, M.H.; Mahmood, W.A.K. Synthesis, characterization and spectroscopic studies of nickel (II) complexes with some tridentate ONN donor Schiff bases and their electrocatalytic application for oxidation of methanol. J. Mol. Liq. 2018, 249, 117–125. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Mosberger, M.; Probst, B.; Spingler, B.; Alberto, R. Influence of Hetero-Biaryl Ligands on the Photo-Electrochemical Properties of [ReINCS(N∩N)(CO)3]-Type Photosensitizers. Eur. J. Inorg. Chem. 2019, 2019, 3518–3525. [Google Scholar] [CrossRef]
- Wesley Jeevadason, A.; Kalidasa Murugavel, K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Ganguly, A.; Basu, S.; Banerjee, K.; Chakraborty, P.; Sarkar, A.; Chatterjee, M.; Chaudhuri, S.K. Redox active copper chelate overcomes multidrug resistance in T-lymphoblastic leukemia cell by triggering apoptosis. Mol. BioSyst. 2011, 7, 1701–1712. [Google Scholar] [CrossRef]
- Ghosh, R.D.; Chakraborty, P.; Banerjee, K.; Adhikary, A.; Sarkar, A.; Chatterjee, M.; Das, T.; Choudhuri, S.K. The molecular interaction of a copper chelate with human P-glycoprotein. Mol. Cell. Biochem. 2012, 364, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Chakraborty, P.; Banerjee, K.; Choudhuri, S.K. The role of a Schiff base scaffold, N-(2-hydroxy acetophenone) glycinate-in overcoming multidrug resistance in cancer. Eur. J. Pharm. Sci. 2014, 51, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Geldard, J.F.; Lions, F. Tridentate Chelate Compounds. III. Inorg. Chem. 1963, 2, 270–282. [Google Scholar] [CrossRef]
- Chiswell, B.; Lions, F. Quadridentate Chelate Compounds. 4. Metal Complexes from Butane-2,3-Dione Bis(2′-Pyridylhydrazone). Inorg. Chem. 1964, 3, 490–493. [Google Scholar] [CrossRef]
- Bailey, N.A.; James, T.A.; McCleverty, J.A.; McKenzie, E.D.; Moore, R.D.; Worthington, J.M. Novel Class of Dimeric Nickel(II) Compounds with Quadridentate Nitrogen Ligands—Crystal-Structure of [Ni2(C16H16N6)2]. J. Chem. Soc. Chem. Commun. 1972, 11, 681–682. [Google Scholar] [CrossRef]
- Bailey, N.A.; McKenzie, E.D.; Worthington, J.M. Structure and Redox Properties of Some Planar [M11N4] Chelate Compounds of Cobalt and Nickel. 3. Crystal and Molecular-Structure of Bis[Cyclohexane-1,2-Bis-2′-Pyridyl-Hydrazonato]-Dinickel(II), 2-Benzene. Inorg. Chim. Acta 1980, 43, 145–153. [Google Scholar] [CrossRef]
- Guhathakurta, B.; Pradhan, A.B.; Das, S.; Bandyopadhyay, N.; Lu, L.; Zhu, M.; Naskar, J.P. Spectroscopic and molecular docking studies on the interaction of human serum albumin with copper(II) complexes. Spectrochim. Acta A 2017, 173, 740–748. [Google Scholar] [CrossRef]
- Guhathakurta, B.; Biswas, C.; Naskar, J.P.; Lu, L.P.; Zhu, M.L. Synthesis and Crystal Structure of [Cu(BDBPH)(ClO4)2] (BDBPH = Butane-2,3-dione bis(2′-pyridylhydrazone). J. Chem. Crystallogr. 2011, 41, 1694–1699. [Google Scholar] [CrossRef]
- Ohki, Y.; Ohta, S.; Tatsumi, K.; Davis, L.M.; Girolami, G.S.; Royer, A.M.; Rauchfuss, T.B. Monomeric iron(II) complexes having two sterically hindered arylthiolates. Inorg. Synth. 2010, 35, 137–143. [Google Scholar]
- Darensbourg, D.J.; Holtcamp, M.W.; Struck, G.E.; Zimmer, M.S.; Niezgoda, S.A.; Rainey, P.; Robertson, J.B.; Draper, J.D.; Reibenspies, J.H. Catalytic activity of a series of Zn(II) phenoxides for the copolymerization of epoxides and carbon dioxide. J. Am. Chem. Soc. 1999, 121, 107–116. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro Software System, 171.36; Agilent Technologies: Oxford, UK, 2013. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Sovago, L.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Spingler, B.; Schnidrig, S.; Todorova, T.; Wild, F. Some thoughts about the single crystal growth of small molecules. CrystEngComm 2012, 14, 751–757. [Google Scholar] [CrossRef]
- Alcock, N.W.; Berry, A.; Moore, P. Structure of a complex of 1,4,8,11-tetraazacyclotetradecane (cyclam) with zinc(II) chloride. Acta Cryst. 1992, C48, 16–19. [Google Scholar] [CrossRef]
- Liang, X.-Q.; Xiao, H.-P.; Liu, B.-L.; Li, Y.-Z.; Zuo, J.-L.; You, X.-Z. Syntheses, structures and luminescent properties of four d10-metal coordination polymers based on the 2,4,5-tri(4-pyridyl)-imidazole ligand. Polyhedron 2008, 27, 2494–2500. [Google Scholar] [CrossRef]
- Wen, Y.; Sheng, T.; Hu, S.; Wang, Y.; Tan, C.; Ma, X.; Xue, Z.; Wang, Y.; Wu, X. Effect of anions on the self-assembly of Zn(II) with a hydrogenated Schiff base ligand: Structural diversity and photoluminescent properties. CrystEngComm 2013, 15, 2714–2721. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.K.; Lee, H.; Nayab, S.; Shin, J.W. Zinc (II), palladium (II) and cadmium (II) complexes containing 4-methoxy-N-(pyridin-2-ylmethylene) aniline derivatives: Synthesis, characterization and methyl methacrylate polymerization. Appl. Organomet. Chem. 2019, 33, e4797. [Google Scholar] [CrossRef]
- Conradie, J.; Conradie, M.M.; Tawfiq, K.M.; Al-Jeboori, M.J.; Coles, S.J.; Wilson, C.; Potgieter, J.H. Novel dichloro(bis{2-[1-(4-methylphenyl)-1H-1,2,3-triazol-4-yl-κN3]pyridine-κN})metal(II) coordination compounds of seven transition metals (Mn, Fe, Co, Ni, Cu, Zn and Cd). Polyhedron 2018, 151, 243–254. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Call, A.; Cibian, M.; Yamauchi, K.; Sakai, K. An earth-abundant system for light-driven CO2 reduction to CO using a pyridinophane iron catalyst. Chem. Commun. 2019, 55, 8552–8555. [Google Scholar] [CrossRef] [PubMed]
- Saghatforoush, L.; Moeini, K.; Golsanamlou, V.; Amani, V.; Bakhtiari, A.; Mardani, Z. Structural, spectral and theoretical study of the coordination of 3,6-bis(2-pyridyl)tetrazine ligand with zinc(II) and mercury(II). Inorg. Chim. Acta 2018, 483, 392–401. [Google Scholar] [CrossRef]
- Neels, A.; Stoeckli-Evans, H. Trinuclear zinc(II) complexes and polymeric cadmium(II) complexes with the ligand 2,5-bis(2-pyridyl)pyrazine: Synthesis, spectral analysis, and single-crystal and powder X-ray analyses. Inorg. Chem. 1999, 38, 6164–6170. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Suzuki, T.; Kaizaki, S. Crystal structures, magnetic and spectroscopic properties of manganese(II), cobalt(II), nickel(II) and zinc(II) dichloro complexes bearing two 2-pyridyl-substituted imino nitroxides. J. Chem. Soc. Dalton Trans. 2001, 1566–1572. [Google Scholar] [CrossRef]
- Dori, Z.; Ziolo, R.F. Chemistry of coordinated azides. Chem. Rev. 1973, 73, 247–254. [Google Scholar] [CrossRef]
- Ray, S.; Konar, S.; Jana, A.; Jana, S.; Patra, A.; Chatterjee, S.; Golen, J.A.; Rheingold, A.L.; Mandal, S.S.; Kar, S.K. Three new pseudohalide bridged dinuclear Zn(II), Cd(II) complexes of pyrimidine derived Schiff base ligands: Synthesis, crystal structures and fluorescence studies. Polyhedron 2012, 33, 82–89. [Google Scholar] [CrossRef]
- Sunkari, S.S.; Kharediya, B.; Saha, S.; Elrez, B.; Sutter, J.-P. Chain of dimers to assembly of trimers: Temperature and ligand influenced formation of novel supramolecular assemblies of Cu(II) with isomeric (aminomethyl) pyridines and azide. New J. Chem. 2014, 38, 3529–3539. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Stockheim, C.; Wieghardt, K.; Deck, W.; Gregorzik, R.; Vahrenkamp, H.; Nuber, B.; Weiss, J. Mononuclear and Dinuclear Zinc(II) Complexes of Biological Relevance—Crystal-Structures of [L2Zn](PF6)2, [L’Zn(O2CPh)2(H2O)], [L’2Zn2(μ-OH)2](ClO4)2, and [L’2Zn2(μ-OH)(μ-CH3CO2)2](ClO4)·H2O (L = 1,4,7-Triazacyclononane, L’ = 1,4,7-Trimethyl-1,4,7-Triazacyclononane). Inorg. Chem. 1992, 31, 1451–1457. [Google Scholar] [CrossRef]
- González, D.M.; Cisterna, J.; Brito, I.; Roisnel, T.; Hamon, J.-R.; Manzur, C. Binuclear Schiff-base zinc(II) complexes: Synthesis, crystal structures and reactivity toward ring opening polymerization of rac-lactide. Polyhedron 2019, 162, 91–99. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandiarajan, D.; Fox, T.; Spingler, B. An N4-Tetradentate Hydrazone Ligand That Binds in a Neutral, Mono- and Bisdeprotonated Form to Iron(II) and Zinc(II) Metal Ions. Crystals 2021, 11, 982. https://doi.org/10.3390/cryst11080982
Pandiarajan D, Fox T, Spingler B. An N4-Tetradentate Hydrazone Ligand That Binds in a Neutral, Mono- and Bisdeprotonated Form to Iron(II) and Zinc(II) Metal Ions. Crystals. 2021; 11(8):982. https://doi.org/10.3390/cryst11080982
Chicago/Turabian StylePandiarajan, Devaraj, Thomas Fox, and Bernhard Spingler. 2021. "An N4-Tetradentate Hydrazone Ligand That Binds in a Neutral, Mono- and Bisdeprotonated Form to Iron(II) and Zinc(II) Metal Ions" Crystals 11, no. 8: 982. https://doi.org/10.3390/cryst11080982
APA StylePandiarajan, D., Fox, T., & Spingler, B. (2021). An N4-Tetradentate Hydrazone Ligand That Binds in a Neutral, Mono- and Bisdeprotonated Form to Iron(II) and Zinc(II) Metal Ions. Crystals, 11(8), 982. https://doi.org/10.3390/cryst11080982