Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of ZnO Nanoparticles
2.2. Synthesis of ZnO/BiOCl Nanocomposites
2.3. Synthesis of Ag Loaded ZnO/BiOCl Nanocomposites
2.4. Characterization
2.5. Photocatalytic Activity Measurements
3. Results and Discussion
3.1. XRD Analysis
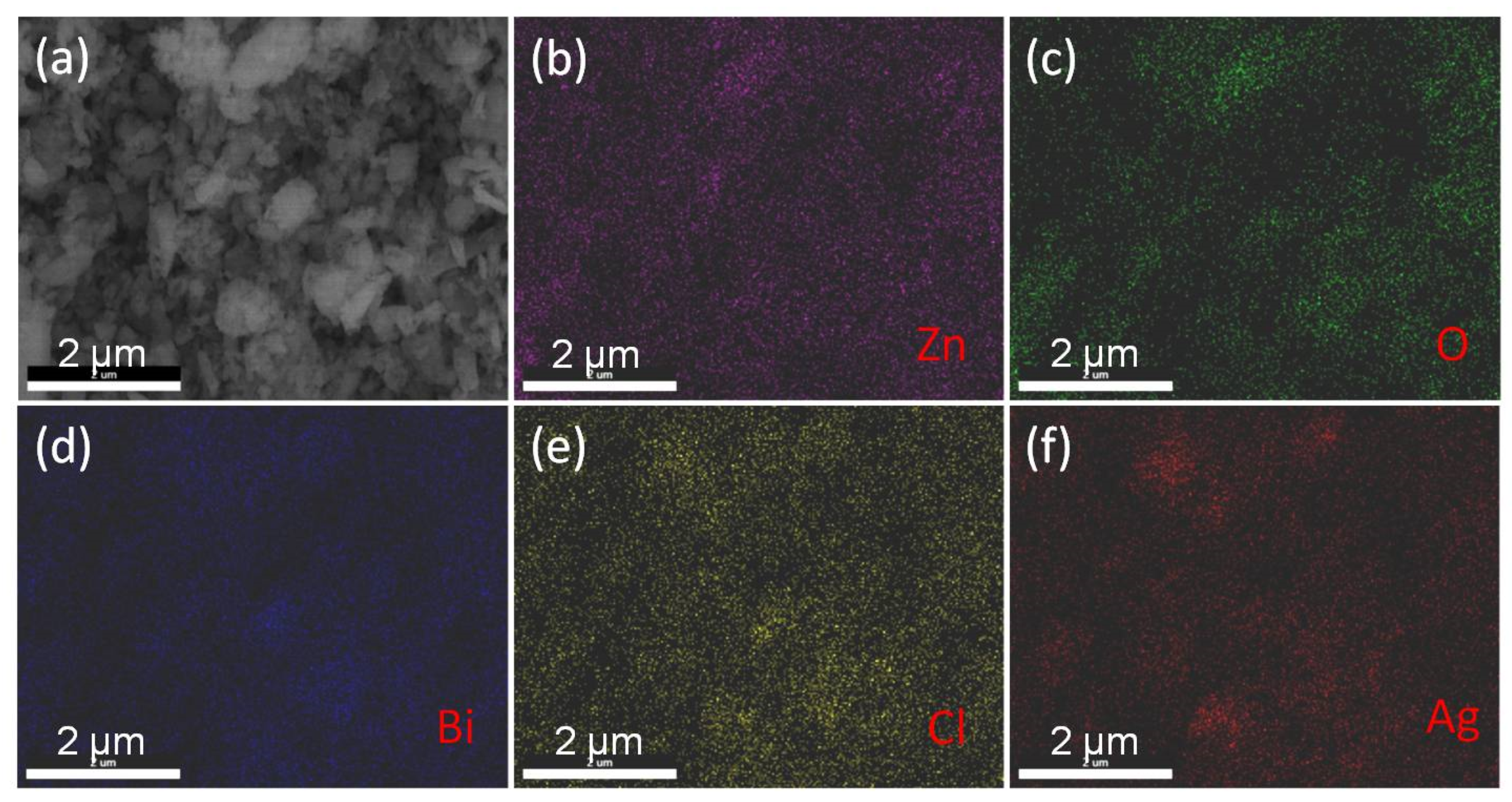
3.2. Morphology Analysis
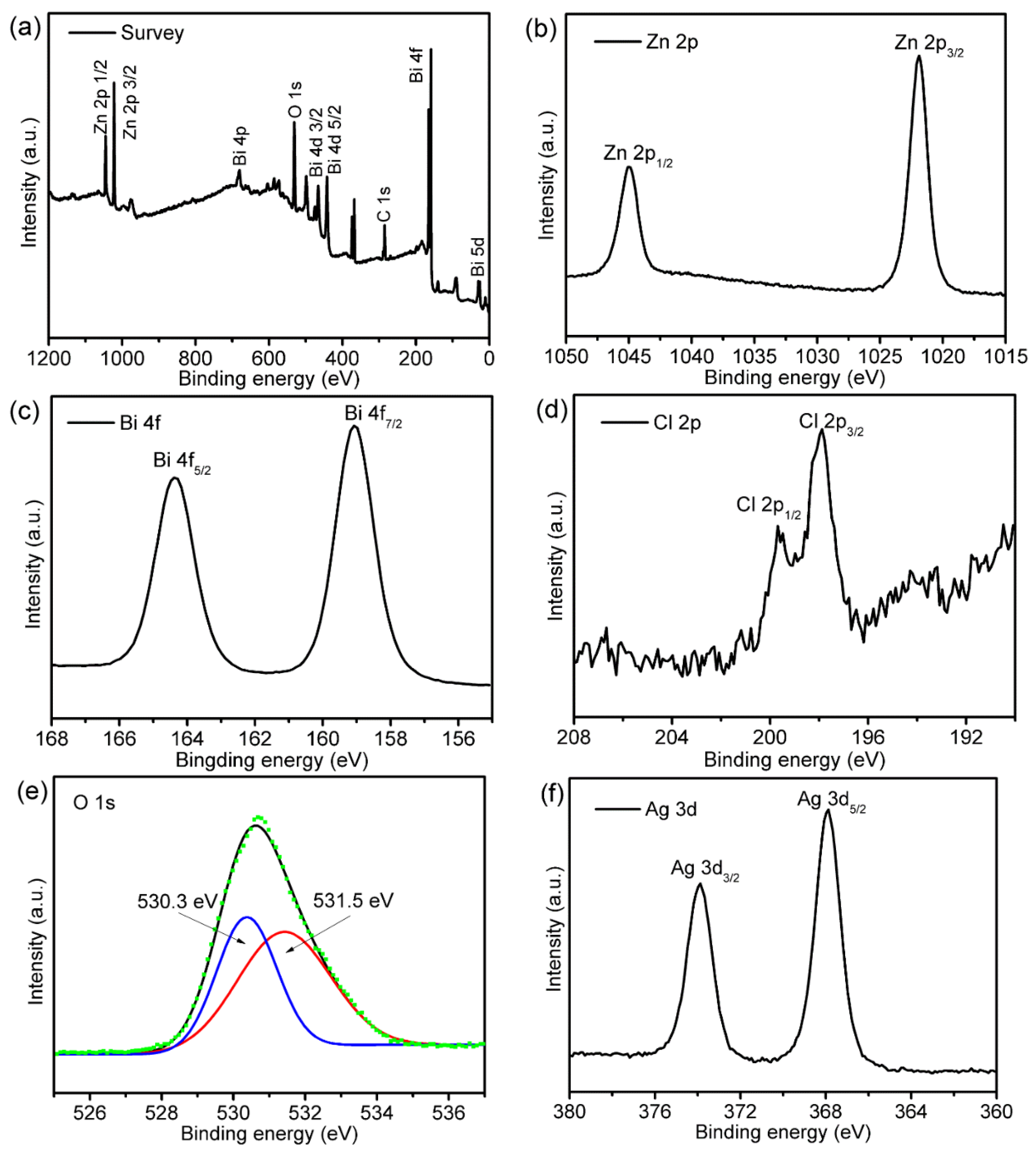
3.3. XPS Analysis
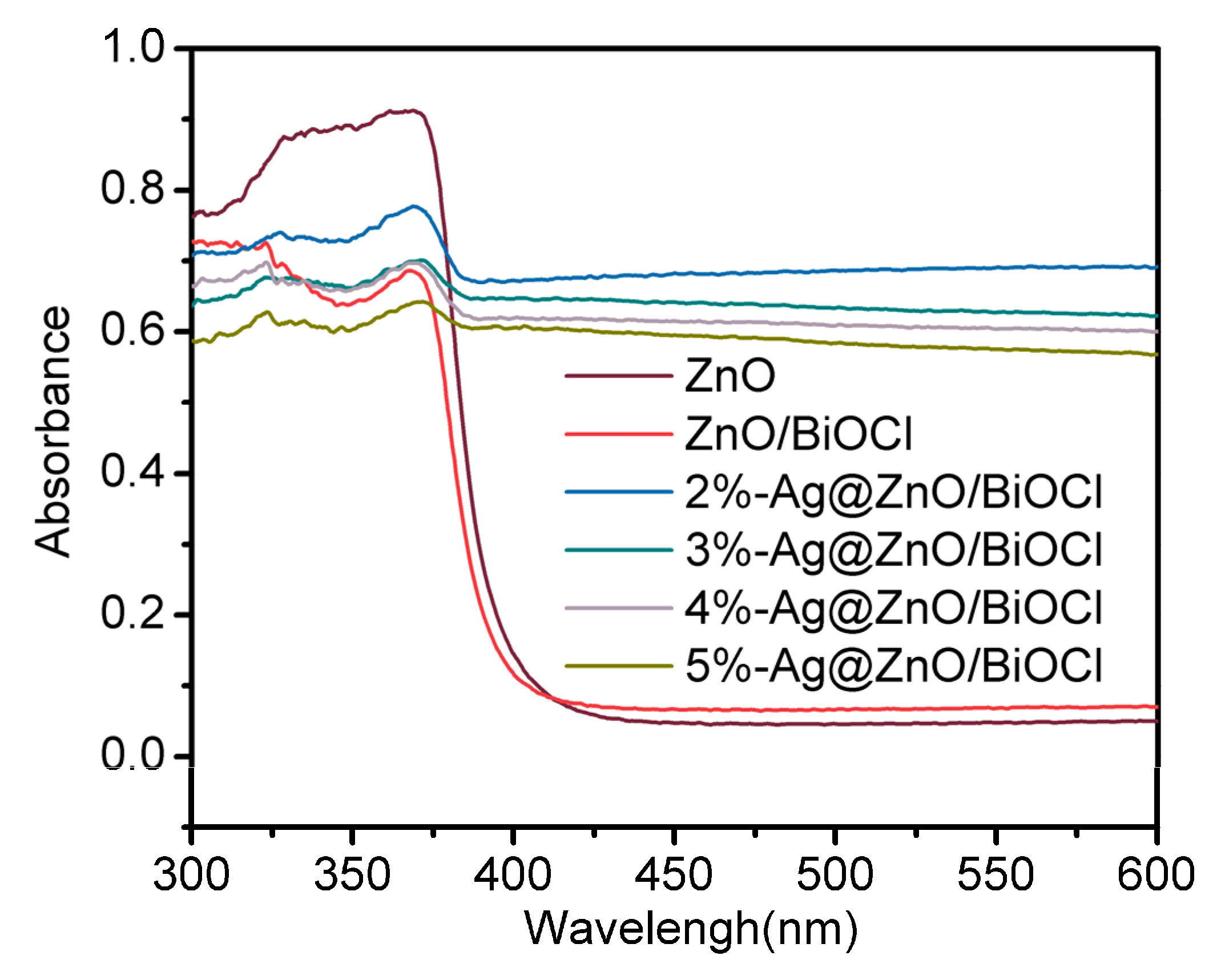
3.4. UV-Vis DRS Analysis
3.5. PL Analysis
4. Photocatalytic Activity
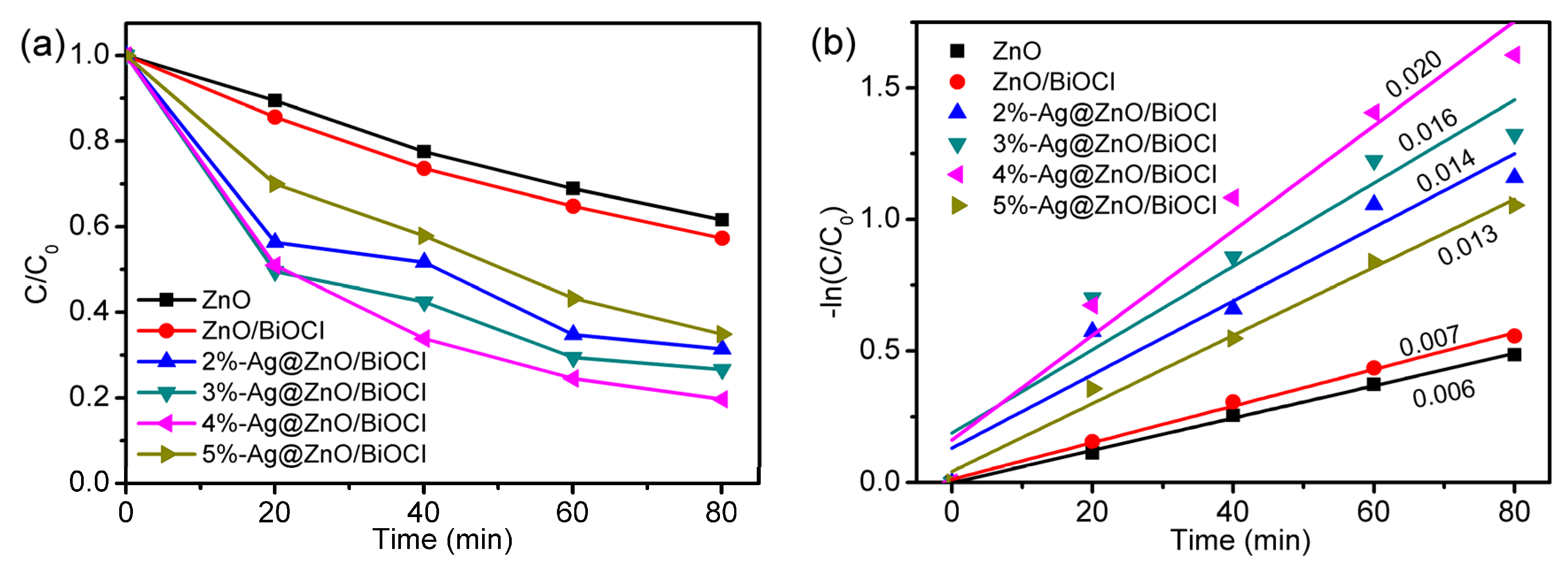
4.1. Photocatalytic Activity of Ag@ZnO/BiOCl
4.2. Effect of Catalyst Amount
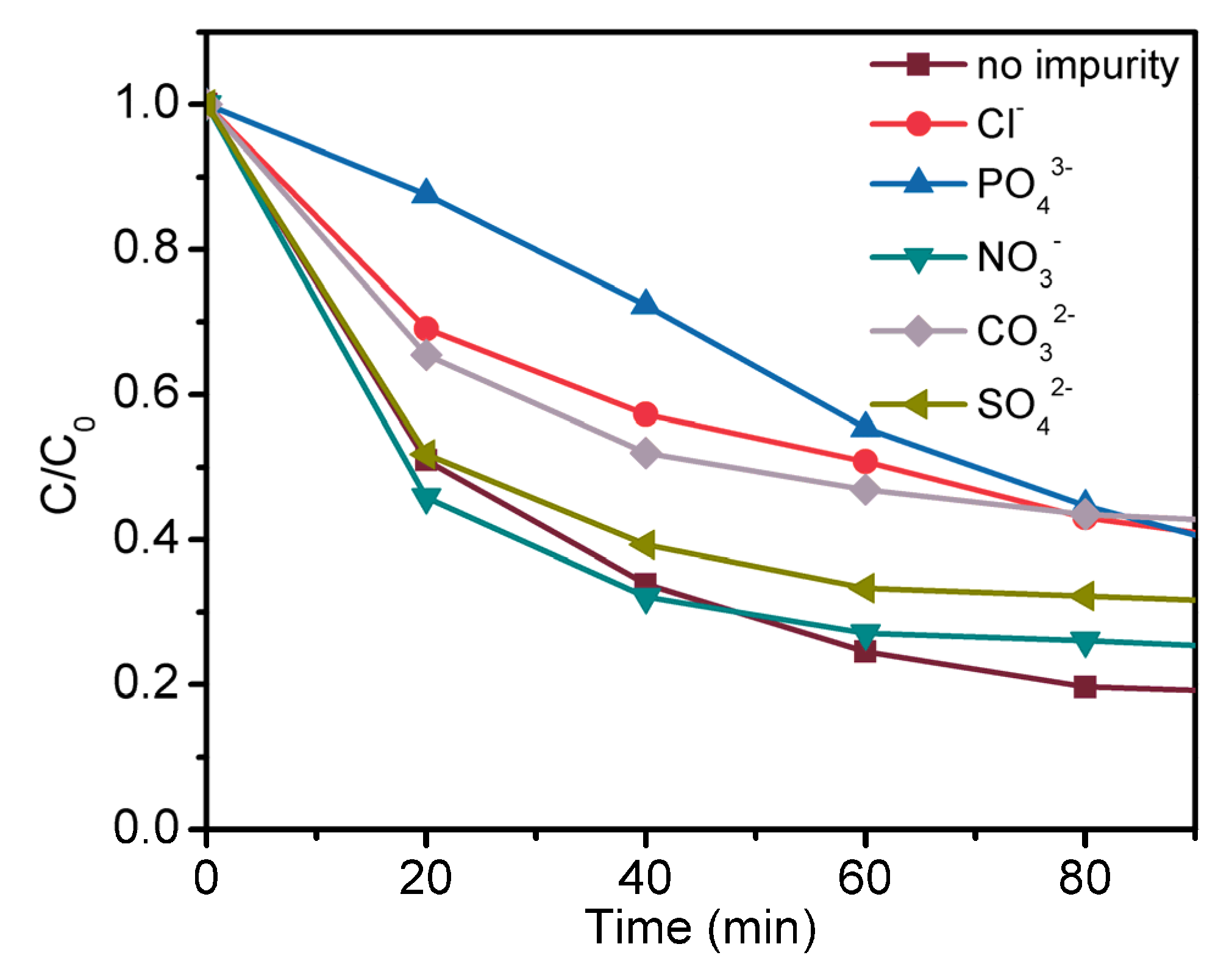
4.3. Effect of Impurity Ions
4.4. Active Species
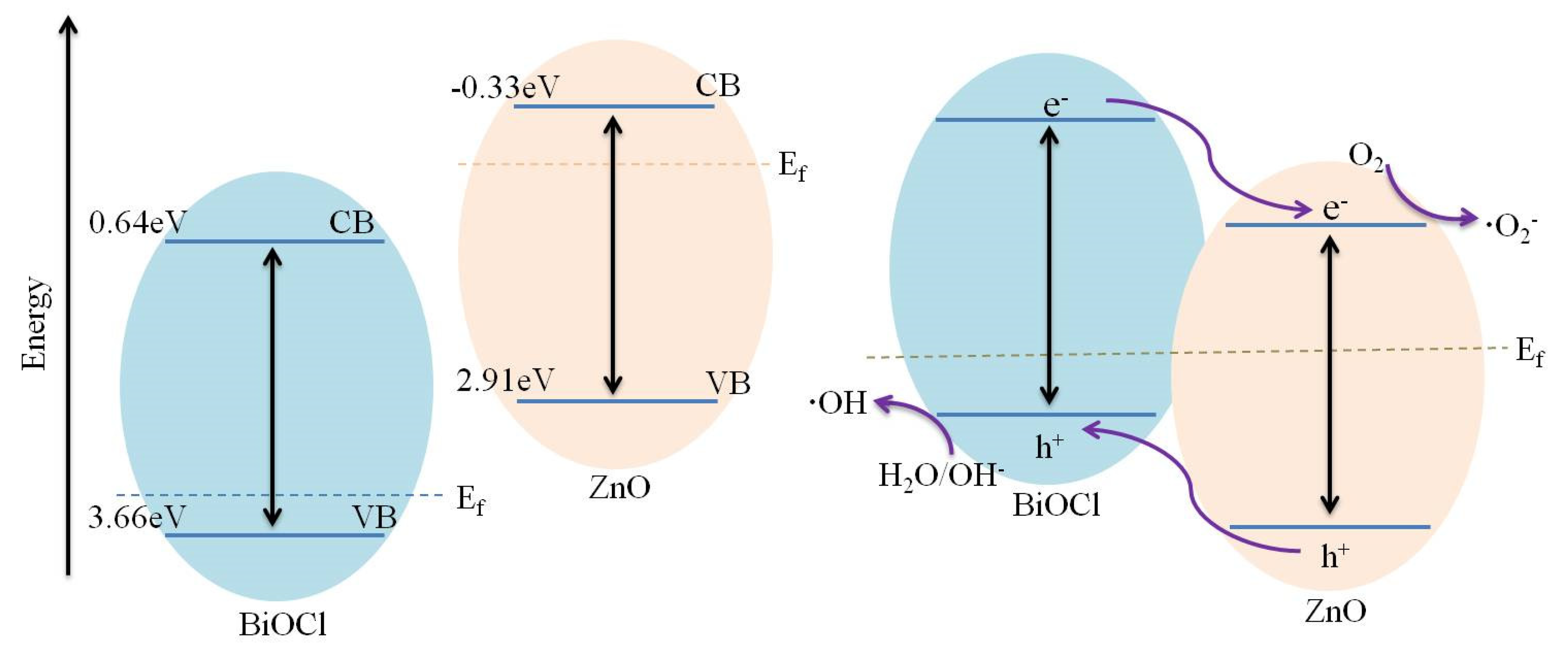
4.5. Photocatalytic Mechanism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malesic-Eleftheriadou, N.; Evgenidou, E.N.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gao, J.; Li, W.; Huang, J.; Yu, G. Determination of 27 pharmaceuticals and personal care products (PPCPs) in water: The benefit of isotope dilution. Front. Environ. Sci. Eng. 2019, 14, 8. [Google Scholar] [CrossRef]
- Dehghan, A.; Zarei, A.; Jaafari, J.; Shams, M.; Mousavi Khaneghah, A. Tetracycline removal from aqueous solutions using zeolitic imidazolate frameworks with different morphologies: A mathematical modeling. Chemosphere 2019, 217, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhuang, C.; Li, Y.; Gao, C.; Jiang, W.; Sun, Z.; Qi, K. Anchoring ultra-small TiO2 quantum dots onto ultra-thin and large-sized Mxene nanosheets for highly efficient photocatalytic water splitting. Ceram. Int. 2021, 47, 21769–21776. [Google Scholar] [CrossRef]
- Yang, X.; Tian, J.; Guo, Y.; Teng, M.; Liu, H.; Li, T.; Lv, P.; Wang, X. ZnO Nano-Rod Arrays Synthesized with Exposed {0001} Facets and the Investigation of Photocatalytic Activity. Crystals 2021, 11, 522. [Google Scholar] [CrossRef]
- Qi, K.; Zada, A.; Yang, Y.; Chen, Q.; Khataee, A. Design of 2D–2D NiO/g-C3N4 heterojunction photocatalysts for degradation of an emerging pollutant. Res. Chem. Intermed. 2020, 46, 5281–5295. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Li, W.; Zhuang, C.; Gao, C.; Jiang, W.; Sun, W.; Qi, K.; Sun, Z.; Han, X. Designing large-sized cocatalysts for fast charge separation towards highly efficient visible-light-driven hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 28545–28553. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Qureshi, M.N.; Liu, S.-y.; Wang, R. Accelerating Photocatalytic Hydrogen Production and Pollutant Degradation by Functionalizing g-C3N4 With SnO2. Front. Chem. 2020, 7, 941. [Google Scholar] [CrossRef] [Green Version]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Liu, S.-y.; Ru, J.; Liu, F. NiP/CuO composites: Electroless plating synthesis, antibiotic photodegradation and antibacterial properties. Chemosphere 2021, 267, 129220. [Google Scholar] [CrossRef]
- Al Suliman, N.; Awada, C.; Alshoaibi, A.; Shaalan, N.M. Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals 2020, 10, 1024. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Shan, J.; Liu, J.; Huang, X. Preparation, Characterization of Graphitic Carbon Nitride Photo-Catalytic Nanocomposites and Their Application in Wastewater Remediation: A Review. Crystals 2021, 11, 723. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, S.; Cao, Y.; Jiang, J. Synthesis of a Novel Photocatalyst MVO4/g-C3N4 (M = La, Gd) with Better Photocatalytic Activity for Tetracycline Hydrochloride Degradation under Visible-Light Irradiation. Crystals 2021, 11, 756. [Google Scholar] [CrossRef]
- Jin, Y.; Xing, Z.; Li, Y.; Han, J.; Lorenz, H.; Chen, J. Synthetic BiOBr/Bi2S3/CdS Crystalline Material and Its Degradation of Dye under Visible Light. Crystals 2021, 11, 899. [Google Scholar] [CrossRef]
- Qi, K.; Lv, W.; Khan, I.; Liu, S.-y. Photocatalytic H2 generation via CoP quantum-dot-modified g-C3N4 synthesized by electroless plating. Chin. J. Catal. 2020, 41, 114–121. [Google Scholar] [CrossRef]
- Ruqaishy, M.A.; Marzouqi, F.A.; Qi, K.; Liu, S.-Y.; Karthikeyan, S.; Kim, Y.; Al-Kindy, S.M.Z.; Kuvarega, A.T.; Selvaraj, R. Template-free preparation of TiO2 microspheres for the photocatalytic degradation of organic dyes. Korean J. Chem. Eng. 2018, 35, 2283–2289. [Google Scholar] [CrossRef]
- He, W.; Wu, H.; Wamer, W.G.; Kim, H.-K.; Zheng, J.; Jia, H.; Zheng, Z.; Yin, J.-J. Unraveling the Enhanced Photocatalytic Activity and Phototoxicity of ZnO/Metal Hybrid Nanostructures from Generation of Reactive Oxygen Species and Charge Carriers. ACS Appl. Mater. Interfaces 2014, 6, 15527–15535. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Dvorský, R.; Reli, M. Nanocomposites of SnO2 and g-C3N4: Preparation, characterization and photocatalysis under visible LED irradiation. Ceram. Int. 2018, 44, 3837–3846. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.-y.; Zada, A. Graphitic carbon nitride, a polymer photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Wang, G.; Long, X.; Qi, K.; Dang, S.; Zhong, M.; Xiao, S.; Zhou, T. Two-dimensional CdS/g-C6N6 heterostructure used for visible light photocatalysis. Appl. Surf. Sci. 2019, 471, 162–167. [Google Scholar] [CrossRef]
- Zhao, K.; Khan, I.; Qi, K.; Liu, Y.; Khataee, A. Ionic liquid assisted preparation of phosphorus-doped g-C3N4 photocatalyst for decomposition of emerging water pollutants. Mater. Chem. Phys. 2020, 253, 123322. [Google Scholar] [CrossRef]
- Chang, S.; Sang, Y.; Liu, H. Efficient Photocatalytic Degradation of RhB by Constructing Sn3O4 Nanoflakes on Sulfur-Doped NaTaO3 Nanocubes. Crystals 2021, 11, 59. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Syrek, K.; Mączka, K.; Sulka, G.D. Photocatalytic Decolorization of Methyl Red on Nanoporous Anodic ZrO2 of Different Crystal Structures. Crystals 2021, 11, 215. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alanazi, H.S.; Alsyahi, A.A.; Ahmad, N. Hydrothermal Synthesis, Characterization and Exploration of Photocatalytic Activities of Polyoxometalate: Ni-CoWO4 Nanoparticles. Crystals 2021, 11, 456. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhu, L.; He, H.; Hu, L.; Huang, J.; Hu, F.; He, B.; Ye, Z. Shape control of colloidal Mn doped ZnO nanocrystals and their visible light photocatalytic properties. Nanoscale 2013, 5, 10461–10471. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Shi, B.; Stanislaw, N.; Mu, C.; Qi, K. Facile synthesis of hierarchical ZnO microstructures with enhanced photocatalytic activity. Mater. Sci.-Pol. 2017, 35, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Li, D.; Zou, Z.; Xia, D. Facile Preparation of BiOCl/ZnO Heterostructure with Oxygen-Rich Vacancies and Its Enhanced Photocatalytic Performance. Chem. Sel. 2019, 4, 12245–12251. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.-y.; Selvaraj, R.; Wang, W.; Yan, Z. Comparison of Pt and Ag as co-catalyst on g-C3N4 for improving photocatalytic activity: Experimental and DFT studies. Desalin. Water Treat. 2019, 153, 244–252. [Google Scholar] [CrossRef]
- An, C.; Peng, S.; Sun, Y. Facile Synthesis of Sunlight-Driven AgCl:Ag Plasmonic Nanophotocatalyst. Adv. Mater. 2010, 22, 2570–2574. [Google Scholar] [CrossRef]
- Zhang, S.; Khan, I.; Qin, X.; Qi, K.; Liu, Y.; Bai, S. Construction of 1D Ag-AgBr/AlOOH Plasmonic Photocatalyst for Degradation of Tetracycline Hydrochloride. Front. Chem. 2020, 8, 117. [Google Scholar] [CrossRef]
- Boltersdorf, J.; Leff, A.C.; Forcherio, G.T.; Baker, D.R. Plasmonic Au–Pd Bimetallic Nanocatalysts for Hot-Carrier-Enhanced Photocatalytic and Electrochemical Ethanol Oxidation. Crystals 2021, 11, 226. [Google Scholar] [CrossRef]
- Zada, A.; Muhammad, P.; Ahmad, W.; Hussain, Z.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Surface Plasmonic-Assisted Photocatalysis and Optoelectronic Devices with Noble Metal Nanocrystals: Design, Synthesis, and Applications. Adv. Funct. Mater. 2019, 30, 1906744. [Google Scholar] [CrossRef]
- Christopher, P.; Ingram, D.B.; Linic, S. Enhancing Photochemical Activity of Semiconductor Nanoparticles with Optically Active Ag Nanostructures: Photochemistry Mediated by Ag Surface Plasmons. J. Phys. Chem. C 2010, 114, 9173–9177. [Google Scholar] [CrossRef]
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Lv, Y.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater. 2010, 182, 123–129. [Google Scholar] [CrossRef]
- Rupa, A.V.; Manikandan, D.; Divakar, D.; Sivakumar, T. Effect of deposition of Ag on TiO2 nanoparticles on the photodegradation of Reactive Yellow-17. J. Hazard. Mater. 2007, 147, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.-Y.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- He, G.; Xing, C.; Xiao, X.; Hu, R.; Zuo, X.; Nan, J. Facile synthesis of flower-like Bi12O17Cl2/β-Bi2O3 composites with enhanced visible light photocatalytic performance for the degradation of 4-tert-butylphenol. Appl. Catal. B Environ. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Kong, L.; Yu, B.; Xu, X.; Pan, J.; Su, Y.; Hu, J. Room Temperature Ferromagnetism and Photoluminescence in Cu-Doped ZnO Nanocrystals. J. Nanosci. Nanotechnol. 2014, 14, 6012–6015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, T.; Xu, Q.; Li, D.; Meng, S.; Chen, M. Perovskite oxide ultrathin nanosheets/g-C3N4 2D-2D heterojunction photocatalysts with significantly enhanced photocatalytic activity towards the photodegradation of tetracycline. Appl. Catal. B Environ. 2017, 201, 617–628. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zada, A.; Cui, N.; Liu, N.; Liu, M.; Yang, Y.; Jiang, D.; Jiang, J.; Liu, S. Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants. Crystals 2021, 11, 981. https://doi.org/10.3390/cryst11080981
Zhang Z, Zada A, Cui N, Liu N, Liu M, Yang Y, Jiang D, Jiang J, Liu S. Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants. Crystals. 2021; 11(8):981. https://doi.org/10.3390/cryst11080981
Chicago/Turabian StyleZhang, Zhihao, Amir Zada, Nan Cui, Naiwen Liu, Minghui Liu, Yuzhuo Yang, Delong Jiang, Jianhui Jiang, and Shuyuan Liu. 2021. "Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants" Crystals 11, no. 8: 981. https://doi.org/10.3390/cryst11080981
APA StyleZhang, Z., Zada, A., Cui, N., Liu, N., Liu, M., Yang, Y., Jiang, D., Jiang, J., & Liu, S. (2021). Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants. Crystals, 11(8), 981. https://doi.org/10.3390/cryst11080981




