Abstract
The secondary structure of horse cytochrome c with mutations in the P76GTKMIFA83 site of the Ω-loop, exhibiting reduced efficiency of electron transfer, were studied. CD spectroscopy studies showed that the ordering of mutant structure increases by 3–6% compared to that of the WT molecules due to the higher content of β-structural elements. The IR spectroscopy data are consistent with the CD results and demonstrate that some α-helical elements change into β-structures, and the amount of the non-structured elements is decreased. The analysis of the 1H-NMR spectra demonstrated that cytochrome c mutants have a well-determined secondary structure with some specific features related to changes in the heme microenvironment. The observed changes in the structure of cytochrome c mutants are likely to be responsible for the decrease in the conformational mobility of the P76GTKMIFA83 sequence carrying mutations and for the decline in succinate:cytochrome c-reductase and cytochrome c-oxidase activities in the mitoplast system in the presence of these cytochromes c. We suggest that the decreased efficiency of the electron transfer of the studied cytochromes c may arise due to: (1) the change in the protein conformation in sites responsible for the interaction of cytochrome c with complexes III and IV and (2) the change in the heme conformation that deteriorates its optimal orientation towards donor and acceptor in complexes III and IV therefore slows down electron transfer. The results obtained are consistent with the previously proposed model of mitochondrial cytochrome c functioning associated with the deterministic mobility of protein globule parts.
1. Introduction
Cytochrome c is known to be involved in electron transfer from ubiquinol–cytochrome c-oxidoreductase (complex III) to cytochrome c-oxidase (complex IV) in the mitochondrial respiratory chain. Type c heme (hematoporphyrin) is the prosthetic group in cytochrome c that ensures its electron transfer function [1]. X-ray diffraction analysis and NMR spectroscopy revealed differences in the structures of the oxidized and reduced forms of cytochrome c [2,3]. IR spectroscopy studies proved that this transition is accompanied by rearrangements of some hydrogen bonds within the heme and diffusion of intramolecular water, as well as by changes in the ratio between the fraction of β-structural elements and α-helices [4,5,6]. Changes in the spatial structure of the protein are realized mainly in weakly structured and looped areas of the globule. The Ω-loops play a crucial role in structural rearrangements that precede electron transfer in the cytochrome c heme and other electron transport proteins or oxygen binding in hemoglobin and myoglobin [7,8,9,10]. Cytochrome c was found to contain three Ω-loop regions corresponding to residues 20–35, 36–61, and 70–85 of polypeptide chains [11,12,13]. The Ω-loop region corresponding to residues 70–85 and carrying the Met80 residue (one of the amino acid ligands of the Fe atom of the heme, which is more weakly coordinated to the Fe atom than His18 residue) is of particular interest. The Ω-loop corresponding to residues 70–85 of cytochrome c is more labile than other elements of protein spatial structure [11,12,14,15].
An analysis of the informational structure of proteins (ANIS method) [16,17] was employed to study the structure of cytochrome c (PDB ID 1hrc [18]). The P76GTKMIFA83 sequence from the Ω-loop region (70–85) was found to be the most conformationally labile element of cytochrome c [19,20]. Thus, a cytochrome c molecule was found to contain the only P76GTKMIFA83 site with the increased conformational mobility, and it is located within the Ω-loop region (residues 70–85). We have proposed a model for the function of cytochrome c. According to this model, the conformational changes of the loop moiety of the P76GTKMIFA83 sequence have a crucial effect on the conformational mobility of the heme by forming the optimal orientation with respect to the electron donor and acceptor within ubiquinol–cytochrome c oxidoreductase (complex III) and cytochrome c oxidase (complex IV), respectively, and ensuring electron transfer from complex III to complex IV.
To test the validity of this model, we used a number of cytochrome c mutants carrying multiple amino acid substitutions in the P76GTKMIFA83 site, reducing its ability to undergo conformational rearrangements. Previously, we demonstrated that the succinate–cytochrome c reductase and cytochrome c oxidase activities of rat liver mitoplasts in the presence of mutant variants of cytochrome c were decreased to various extents [20]. Resonance Raman spectroscopy (RRS) and surface-enhanced Raman spectroscopy (SERS) studies proved that the decline in the electron transfer properties of cytochrome c mutant forms correlates with changes in the heme conformation that is known to be important for the electron acceptance and donation [20]. Hence, these findings demonstrated that the P76GTKMIFA83 site plays crucial role in the electron transfer function of cytochrome c. Of significance, in our previous work, we did not study the influence of the P76GTKMIFA83 site on the overall conformation of the protein part of cytochrome c. With that, the conformation of the protein part of cytochrome c is important for its interaction with complexes III and IV, affecting the rate of the electron transfer between cytochromes c1 and c and cytochrome c and complex IV.
The purpose of this study was to investigate changes in the secondary structure of cytochrome c mutant variants possessing the reduced efficiency of electron transfer (T78S/K79P, I81Y/A83Y/G84N, P76I/G77L/I81L/F82L, and T78N/K79Y/M80I/I81M/G84N substitutions) by IR spectroscopy, circular dichroism (CD), and 1H-NMR and confirm the model of cytochrome c function.
2. Materials and Methods
Components for the culture media and buffer solutions for chromatography and electrophoresis (AppliChem, Darmstadt, Germany), ampicillin, cytochrome c from horse heart (Sigma, Schnelldorf, Germany), Xho I restriction endonuclease (Promega, Madison, WI, USA), BamH I restriction endonuclease (New England Biolabs Inc., Ipswich, MA, USA), Pfu-DNA polymerase, and T4-DNA ligase (Fermentas, Pabrade, Lithuania) were used in this study. Distilled water was additionally purified on a Milli-Q system (Millipore, Burlington, MA, USA).
2.1. Construction of the Mutant Genes of Cytochrome c
Construction of amino acid substitutions in the cytochrome c sequence and calculation of the structure of its mutant variants were described earlier [20] using the methods described in [21].
The mutations were introduced into the gene of horse cytochrome c in a composition with pBP(CYC1) expression plasmid vector by site-specific mutagenesis according to the QuikChangeTM Mutagenesis Kit method (Stratagene, CA, USA) [22]. The reaction mixture (50 µL) contained 10–15 ng of matrix DNA (a pBP(CYC1) plasmid containing the horse cytochrome c gene), oligonucleotide primers (125 ng) with mutations in the (76–83) region, four deoxynucleoside triphosphates (10 nmol of each), and Pfu polymerase (2.5 activity units). Twenty cycles of the amplification reaction were performed according to the following scheme: denaturation of the matrix DNA at 95 °C for 45 s, annealing at the calculated temperature for 60 s, and elongation at 74 °C for 10 min. When the reaction was completed, the Dpn I restrictase (10 activity units) was added, and the reaction mixture was incubated for 60 min at 37 °C. Further, aliquots of the prepared mixture were used for transformation of the XL-1 Blue supercompetent cells of E. coli according to the standard procedure. The production of mutant DNA during mutagenesis was analyzed by electrophoresis in 1% agarose gel. The nucleotide sequences of mutant genes in the plasmid DNA were determined on an ABI Prism 3100-Avant Genetic Analyzer (Applied Biosystems, Beverly, MA, USA). The selected mutant genes were cloned in the pBP(CYC1) expression vector modified for the expression of genes of horse cytochrome c [23].
2.2. Expression of the Mutant Genes of Cytochrome c, Proteins Isolation, and Purification
Expression of the mutant genes of cytochrome c was performed in the JM-109 strain of E. coli in an SB liquid-nutrient medium with ampicillin (the final concentration was 200 µg/mL) without addition of the inductor at 37 °C under vigorous stirring for 22–24 h [23].
After the growth of E. coli cells was complete, they were precipitated by centrifugation at 4000 g and 4 °C for 20 min. The cellular precipitate was resuspended in a buffer (25 mM NaPi, pH 6.0, 1 mM NaN3) and frozen at −20 °C for 20–30 min. The cells were homogenized by forcing through a French press (Spectronic Instruments, Inc., Rochester, NY, USA) at high pressure with subsequent centrifugation at 100,000 g for 20 min.
Isolation and purification of the target proteins were performed on an AKTA FPLC liquid chromatographic system (GE-HEALTHCARE, CIIIA) according to the previously elaborated scheme [20,24,25]. The cellular extract was applied to a Mono S HR 10/10 cation–exchange column (Bio-Rad, Hercules, CA, USA), which was equilibrated with a buffer containing 25 mM NaPi (pH 6.0) and 1 mM NaN3. Cytochrome c was eluted with a linear gradient of 1 M NaCl in the same buffer at a flow rate of 3 mL/min. The fraction obtained after purification on a Mono S column was analyzed on a spectrophotometer and by SDS-PAAG electrophoresis, dialyzed against the buffer for absorption chromatography (10 mM NaPi, pH 7.0, 1 mM NaN3), and applied to a column with CHT-I hydroxyapatite (Bio-Rad, Hercules, CA, USA). Cytochrome c was eluted with a linear gradient of 500 mM NaPi with pH 7.0, at a flow rate of 1 mL/min. The degree of purification and concentration of cytochrome c in the resulting fractions were determined on a spectrophotometer and by SDS-PAGE electrophoresis. The fractions with an A409/A280 purity of 4.5–5.0 (this value corresponded to a purity of ≥95% for the substance commercially prepared by Sigma, USA) were oxidized by treating with potassium ferricyanide added at the equimolar concentration, dialyzed three times against 10 mM ammonium carbonate buffer (pH 7.9), and lyophilized on an ALPHA I-5 device.
2.3. Preparation of Mitoplasts without Cytochrome c
Mitochondria from rat liver were prepared using the Johnson–Lardy method [26]. Mitoplasts (mitochondria lacking the outer membrane and cytochrome c) were prepared according to the Jacobs–Sanadi method [27]. Three-month-old male Wistar rats born in the vivarium of the Faculty of Biology, M.V. Lomonosov Moscow State University, were used. The animals were housed at 22 °C in a light-controlled environment (12:12 h light-dark cycle) and had free access to water and food. Living conditions, all procedures involving animals, and the protocol of the experiments were approved by the Bioethics Committee of M.V. Lomonosov Moscow State University according to the ethical and juridical norms of scientific researches in biology, medicine, and related areas corresponding to laws of the Russian Federation and international good laboratory practice (GLP) standards. All efforts were made to minimize animal suffering. Rats were deeply anesthetized using excessive Zoletil (30 mg/kg) and sacrificed by decapitation. Anesthesia and euthanasia were performed by a trained staff member who was approved and licensed by the Bioethics Committee of M.V. Lomonosov Moscow State University.
2.4. Measurement of the Succinate:Cytochrome c-Reductase and Cytochrome c-Oxidase Activities of the Mitoplasts
The succinate:cytochrome c-reductase activity of mitoplasts was measured on a spectrophotometer at 550 nm and 30 °C [28]. A sample (2 mL) contained the incubation medium (0.15 M sucrose, 20 mM KCl, 20 mM Tris-HCl, pH 7.4, 5 mM NaN3), 20 µL of the specimen of mitoplasts from rat liver (10 µg of the protein per ml), and oxidized cytochrome c. The reaction was initiated by adding potassium succinate to a final concentration of 10 mM. The activity was expressed in µmol of the reduced cytochrome c for 1 min per mg of the mitoplast protein. The standard measurement error was no higher than 10% in all cases.
The cytochrome c-oxidase activity of the mitoplasts was measured with an ammeter using a closed platinum electrode at 20 °C [28]. A sample (1.3 mL) contained the incubation medium (0.15 M sucrose, 20 mM KCl, 20 mM Tris-HCl, pH 7.4, 10 mM ascorbic acid), 15 µL of the mitoplast specimen (65 µg of the protein per ml), and oxidized cytochrome c. The reaction was initiated by adding tetramethyl-p-phenylene diamine (TMPD) to a final concentration of 0.2 mM. The activity was expressed in µg atoms of the adsorbed oxygen for 1 min per mg of the mitoplast protein. The standard measurement error was no higher than 15% in all cases.
2.5. Calculation of the Kinetic Parameters of the Reactions
The enzymatic reactions (succinate:cytochrome c-reductase and cytochrome c-oxidase) were considered to correspond to Michaelis–Menten kinetics during the measurements. The graph of the dependence of the reaction rate on the substrate concentration was plotted using the Origin 7.0 software program (Microcal, United States) in Lineweaver–Burk double opposite coordinates (1/A, 1/S):
where Km is the Michaelis constant, [S]0 is the initial concentration of the substrate in the reaction mixture, and A and Amax are the measured and the maximum reaction rates, respectively. The kinetic parameters of the reactions (Km and Amax) were calculated from this equation. Results are expressed as mean ± SD.
2.6. CD Spectroscopy
The CD spectra of cytochrome c and its mutant variants were recorded at 25 °C in 10 mM NH4HCO3 buffer, pH 7.9, using a J-810 spectropolarimeter (Jasco, Tokyo, Japan) in the range of 190 to 250 nm. The concentration of the studied proteins was 0.08 mM. For each sample, an average of the three spectra was taken for further calculation. The secondary structure analysis was performed on the basis of the CD spectra using CONTILL software.
2.7. 1H-NMR Spectroscopy
The 1H-NMR spectra of cytochrome c and its mutant variants were recorded at 25 °C in deionized H2O with 10% D2O on an AVANCE-600 spectrometer (Bruker, Karlsruhe, Germany) with an operating proton frequency of 600 MHz. The concentration of the studied proteins was 0.16 mM, and the pH value of the cytochromes solution in deionized H2O was 6.8.
2.8. IR Spectroscopy of Mutant Cytochrome c
To study the mutant cytochromes by IR spectroscopy, a weighed portion of a 2–3 mg protein preparation was mixed with KBr in a ratio of 1:100 and pressed under a pressure of 200 bar in vacuum. The spectra of the prepared samples were recorded in the range of 400 to 4000 cm–1 on an IRPrestige 21 IR spectrophotometer (Shimadzu, Kyoto, Japan). For each sample, 40 scans were performed; the measurement results were processed using IRsolution software.
3. Results and Discussion
3.1. Kinetic Parameters Succinate Cytochrome c-Reductase and Cytochrome c-Oxidase Reactions
We constructed a number of mutant forms of cytochrome c with substitutions in the sequence P76GTKMIFA83 of the Ω-loops 70–85 [20]. Some obtained proteins are characterized with significantly reduced electron-transport activity and conformational changes of hemoporphyrin [20]. We identified three mutant variants: T78S/K79P, I81Y/A83Y/G84N, and T78N/K79Y/M80I/I81M/G84N, in the presence of which a decrease in mitoplast succinate:cytochrome c-reductase activity was observed to 3–6% of the activity in the presence of wild cytochrome c type. The cytochrome c-oxidase activity of mitoplasts in the presence of these mutants was reduced to a much lesser extent and amounted to 47.5 to 23.6% of their activity in the presence of wild-type cytochrome c.
Next, we obtained a series of additional mutant variants, with substitutions aimed at a greater decrease in the electron transport activity, in particular, cytochrome c-oxidase activity, as the final stage of electron transfer in the electron transport chain (ETC). The construction of amino acid substitutions was carried out on the basis of the method described in [21]. The succinate:cytochrome c-reductase activity of rat liver mitoplasts was measured spectrophotometrically at 550 nm, based on reduction of the completely oxidized exogenous cytochrome c [20,28], and cytochrome c-oxidase activity was measured amperometrically [20,28]. In the succinate:cytochrome c-reductase reaction, potassium succinate served as an electron donor, transferring electrons to the respiratory chain to complex II-succinate dehydrogenase (SDH), and then to ubiquinone, complex III, and exogenous cytochrome c. It was shown that, in the presence of a mutant variant of cytochrome c with substitutions P76I/G77L/I81L/F82L, succinate:cytochrome c-reductase activity of mitoplasts was 2.4%, and cytochrome c-oxidase activity was 21.6% of the corresponding activities in the presence of wild-type cytochrome c. Figure 1 shows the Amax and Km values for four mutant variants with maximally reduced activities. The data presented (Figure 1) indicate that the Km values of the cytochrome c-oxidase reaction did not show significant changes for tested mutant forms of cytochrome c except variant P76I/G77L/I81L/F82L. With this, we suggest that the significant decrease of Amax values in the reaction of the oxidation of cytochrome c mutants is due to the change in the rate of the electron transfer along electron carriers. At the same time, the decrease of the Km values of the succinate:cytochrome c-reductase reaction was significant for the mutant cytochrome T78N/K79Y/M80I/I81M/F82N (about two times). Such a decrease may indicate a change in its ability to form active complexes with the corresponding redox partner from ubiquinol:cytochrome c-oxidoreductase. It should be noted that such a decrease is combined with the observed decrease in the rate of the reduction reaction of this cytochrome c mutant.
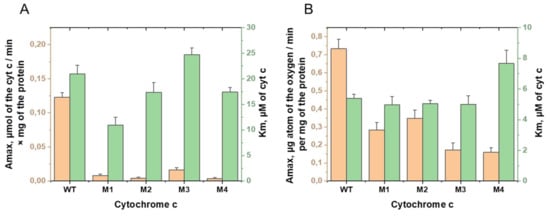
Figure 1.
Comparison of the kinetic parameters Amax (orange columns) and Km (green columns) of the (A) succinate:cytochrome c-reductase and (B) cytochrome c-oxidase reactions in rat liver mitoplasts in the presence of the externally added WT and mutant cytochromes c T78N/K79Y/M80I/I81M/F82N (M1), T78S/K79P (M2), I81Y/A83Y/G84N (M3), and P76I/G77L/I81L/F82L (M4).
Thus, the result of the two stages of our work was the production of mutant forms of cytochrome c with reduced electron transfer function. These changes were pronounced to a greater extent in the mutant variants with substitutions T78S/K79P, I81Y/A83Y/G84N, T78N/K79Y/M80I/I81M/G84N, and P76I/G77L/I81L/F82L.
We investigated the effect of amino acid substitutions in the disordered loop region of cytochrome c P76GTKMIFA83 on its secondary structure. The main results of the IR spectroscopy, CD, and 1H-NMR, studies focused on the oxidized forms of the cytochrome c mutants T78N/K79Y/M80I/I81M/F82N (M1), T78S/K79P (M2), I81Y/A83Y/G84N (M3), and P76I/G77L/I81L/F82L (M4) are presented below.
3.2. The CD Spectra of Cytochrome c and Its Mutant Forms
The analysis of the CD spectra (Figure 2) revealed that the content of the α-helical elements in the secondary structure of mutant cytochromes decreased by 9–14% compared to the WT cytochrome c (Table 1). At the same time, the content of the fraction with the non-ordered structure in mutant variants was 3–6% lower than that in WT, while the number of β-layers was higher than that in WT cytochrome c by 33–50% (Table 1). These findings allow one to say that the increased content of β-structural motifs achieved by introduction of amino acid substitutions into cytochrome c reduced the conformational mobility of the studied protein fragment P76GTKMIFA83. Overall, the CD spectroscopy data demonstrate that the degree of ordering of the structure of mutant variants has slightly increases compared to that in the wild-type cytochrome c.
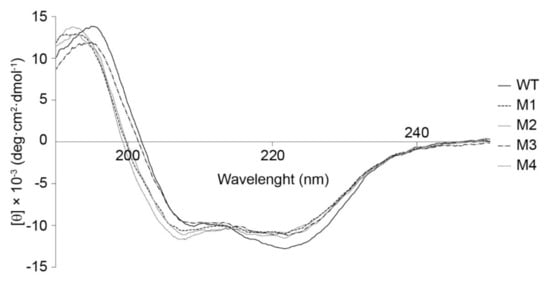
Figure 2.
The CD spectra of cytochrome c (WT) and its mutant variants (M1-T78N/K79Y/M80I/I81M/F82N, M2-T78S/K79P, M3-I81Y/A83Y/G84N, M4-P76I/G77L/I81L/F82L).

Table 1.
The secondary structure analysis of cytochrome c (WT) and its mutant variants. The proportion of α-helix, β-layer, and β-turn in the molecule’s structure are given. RMSD and NMRSD represent root-mean-square deviation and normalized-mean-square deviation between the calculated and the experimental CD spectra, respectively.
Hence, CD spectroscopy studies of the secondary structure of mutant cytochrome c variants carrying T78S/K79P, I81Y/A83Y/G84N, T78N/K79Y/M80I/I81M/F82N, and P76I/G77L/I81L/F82L substitutions demonstrated that the introduction of these mutations altered the contents of fractions with different types of structures (in particular, the fraction of α-helix increases while β-layer fraction decreases), which seems to change the conformational mobility.
3.3. The 1H-NMR Spectra of Oxidized Cytochrome c and Its Mutant Forms
3.3.1. 1H-NMR Spectra of Cytochrome c Protein
This study demonstrated that the 1H-NMR spectra of the mutant forms of cytochrome c have some unique features compared to those of wild-type (WT) cytochrome c (Figure 3). The range of chemical shifts of recombinant proteins includes both positive and negative values, thus demonstrating that the proteins under study are well-structured. The 1H-NMR spectra contained the broadening of amide proton signals, as well as signals at (−1.5–0) ppm. NMR line broadening at (0–3.5) ppm was detected for the mutant carrying T78N/K79Y/M80I/I81M/F82N substitutions. The dispersion of chemical shifts of amide (5.5–12 ppm), Hα (3.5–5.5 ppm), and Cα (−4–3.5 ppm) protons is potentially indicative of the high content of secondary structure elements.
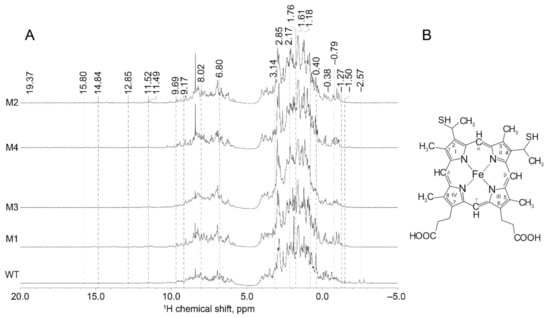
Figure 3.
(A) The 1H-NMR spectra of cytochrome c (WT) and its mutant variants (M1-T78N/K79Y/M80I/I81M/F82N, M2-T78S/K79P, M3-I81Y/A83Y/G84N, M4-P76I/G77L/I81L/F82L); (B) Structural formula of heme c with numeration of C atoms.
The chemical shift at 12.85 ppm in the spectrum of cytochrome c is ascribed to the Met80 CβH proton [29,30]. It was found that the intensity of this signal was either significantly reduced or not detected in the spectra of all the mutant variants. This effect is potentially related to changes in the position of Met 80. In connection with this, the following changes in -NH and Cα protons of Met 80 residue in the mutant variants were revealed [31]: the signal at 9.17 ppm was shifted towards higher frequencies (downfield) for all the mutant variants of cytochrome c and for the variant carrying P76I/G77L/I81L/F82L substitutions in particular.
In the spectrum of WT cytochrome c, the signal lying close to that of the Cα proton (at 2.85 ppm) is shifted towards lower frequencies (upfield) for the mutant carrying P76I/G77L/I81L/F82L substitutions, while it is split for the remaining cytochromes. This phenomenon is possibly related to the “anchoring” of Met80 in the polypeptide chain fragment (residues 76–83). CβH2 of 6-propionate may additionally contribute to this signal. Indeed, the peak at 1.18 ppm characterizing the same proton in the mutants carrying T78S/K79P and T78N/K79Y/M80I/I81M/G84N substitutions was shifted upfield and was split in all other cytochrome variants (Figure 3).
3.3.2. 1H-NMR Spectra of Cytochrome c Hematoporphyrin
Here we present a description of 1H-NMR spectra (Figure 3) that directly characterizes the heme molecule in oxidized cytochrome c [29]. The signal from the meso-β proton in the spectrum of cytochrome c (at −0.79 ppm) in the mutant variants is shifted downfield, which is more pronounced for the mutant variants carrying the T78S/K79P and T78N/K79Y/M80I/I81M/G84N substitutions. The position of the peak at 8.02 ppm, ascribed to the signal from the Meso-γ proton, is shifted downfield in the T78S/K79P mutant and upfield in the mutants carrying I81Y/A83Y/G84N and T78N/K79Y/M80I/I81M/G84N substitutions. The peak at 6.80 ppm (ascribed to the C1 proton in the pyrrole semiring) in the mutant cytochrome variants (except for the form carrying a T78S/K79P substitution) is shifted downfield, while the peak at 9.69 ppm (ascribed to C5 proton in the pyrrole semiring) is split in all the variants. The peak at −1.27 ppm (ascribed to the CH proton in bridge 2) in the spectrum of the T78N/K79Y/M80I/I81M/G84N and P76I/G77L/I81L/F82L mutants consists of two and six lines, respectively. Peak splitting into several lines is probably caused by the unstable position of these protons in hematoporphyrin.
It was found that the spectra of mutant variants carrying the T78S/K79P and P76I/G77L/I81L/F82L substitutions either do not contain the peak at −2.57 ppm (ascribed to the vibrations of the methyl group in bridge 4) or this peak is weaker than that in WT cytochrome c. This result is possibly related to the shift in the methyl group of hematoporphyrin. Let us mention that the peak at 2.17 ppm is shifted downfield for all the mutant variants, which probably indicates that the position of the CH proton in bridge 4 was changed. The peak at 3.14 ppm is also shifted downfield in all the mutations except for the T78N/K79Y/M80I/I81M/G84N variant, which may indicate that the distance from the methyl group in bridge 4 was changed.
The line at −1.50 ppm (ascribed to CαH in 6-propionate) in the mutant variants was found to be split into at least two peaks with lower intensities. This proton may also give rise to a different signal at 1.76 ppm that is shifted downfield for the mutant variants carrying T78S/K79P and I81Y/A83Y/G84N substitutions. In our study, the 11.49 ppm peak that shifted downfield was detected around this value for all the recombinant proteins. The line at 1.61 ppm, characterizing CβH2 in 7-propionate, was shifted downfield in mutant cytochrome variants; the greatest shift was observed for the T78S/K79P variant. This proton in the spectrum of cytochrome c is also characterized by the mode of −0.38 ppm (peak at 0.40 ppm) and is split in the spectra of mutant variants.
Hence, an analysis of the 1H-NMR spectra of recombinant proteins indicates that the mutant forms of cytochrome c are well-structured and have specific features pertaining to the heme microenvironment, which is altered, indicating alterations in the overall heme geometry. Importantly, the spectra of the mutant variants also contained additional bands that were not observed in the 1H-NMR spectrum of wild-type cytochrome c. Furthermore, it was found that the spectra of recombinant proteins do not contain any chemical shifts of methyl groups, which can possibly be attributed to hindrance of methyl group rotation in heme. We suppose that this phenomenon, as well as changes in the positions of a number of atoms, is caused by alterations in the heme conformation. This result is in complete agreement with our previous study of the oxidized mutants of cytochrome c by means of surface-enhanced Raman spectroscopy (SERS) [20], demonstrating a worsening of vibrations of methyl groups and of asymmetric vibrations of pyrrol semirings and the appearance of heme molecules in the deformed ruffled conformation that deteriorates electron transfer from cytochrome c1 to cytochrome c [30].
3.4. The IR Spectra of Cytochrome c and Its Mutant Forms
The IR spectra of wild-type and mutant forms of cytochrome c are shown on Figure 4, and frequency shifts of the main absorption peaks are listed in Table 2. Vibrations of C = O bonds in the polypeptide chain were detected within the amide I band that had the maximum position at 1655 cm−1 in the spectrum of the wild-type cytochrome c. Such a peak maximum is typical for a α-helical protein structure [32]. On the contrary, the amide I band in the IR spectra of mutant variants split into two poorly resolved peaks, demonstrating that the α-helical structure was predominantly retained in the mutant variants and that additional β-sheet and β-turn structural elements emerged in the proteins. Thus, the amide I band in the spectra of mutant variants includes two overlapping signals from different types of secondary structural elements [11]: at 1631–1636 cm−1 and at 1681–1695 cm−1 (Figure 4, Table 2). For the T78S/K79P and I81Y/A83Y/G84N mutant variants, the bands at 1636 cm−1 and 1634, cm−1, respectively, indicate that either there is a β-sheet fraction interacting with the heme propionates [33,34] or the β-sheet fraction acquires an antiparallel orientation [6,11,35,36]. The bands at 1682 cm−1 and 1684 cm−1 in the T78S/K79P and I81Y/A83Y/G84N mutant variants, respectively, indicate that the proteins have acquired a type II β-turn [37,38].
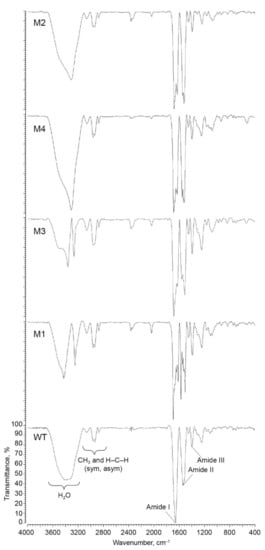
Figure 4.
The IR spectra of cytochrome c (WT) and its mutant variants (M1-T78N/K79Y/M80I/I81M/F82N, M2-T78S/K79P, M3-I81Y/A83Y/G84N, M4-P76I/G77L/I81L/F82L).

Table 2.
Analysis data of the IR spectra of cytochrome c (WT) and its mutant variants.
We believe that the band at 1695 cm−1 for the T78N/K79Y/M80I /I81M/G84N mutant variant can be ascribed to the type III β-turn 310 [33], which links the α-helix to the β-polypeptide chain [20].
For the P76I/G77L/I81L/F82L variant, the band at 1632 cm−1 indicates that there is an additional fraction of β-sheets and β-turns in the protein (e.g., the band at 1686 cm−1 attests to the presence of high-frequency β-turns) [11].
The amide II band (1600–1500 cm−1) is assigned to the in-plane anti-phase vibrations of the N–H and C–N bonds in the peptide bonds of the protein [35]. In our experiments, the spectrum of the wild-type cytochrome c contains a band at 1533 cm−1, which indicates the existence of an antiparallel β-sheets in its structure [6,35]. In the spectra of mutant variants (except for the I81Y/A83Y/G84N variant), this peak was split into two (peaks at 1504–1529 cm−1 and 1550–1574 cm−1). The band at 1530 cm−1 in the T78S/K79P mutant variant is known to arise from changes in the β-structure of cytochrome c [39]. Let us mention that, for both the T78S/K79P and the P76I/G77L/I81L/F82L mutant, the band at 1551 cm−1 is assigned to antisymmetric vibrations υAS(COO–) of carboxylic groups [5,34] or to the presence of an α-helical structure [39].
The band at 1518 cm−1 in the I81Y/A83Y/G84N mutant variant characterizes the C=C vibration of the bond in the aromatic ring of tyrosine [5,6,39,40,41], while the band at 1574 cm−1 of the T78N/K79Y/M80I/I81M/G84N variant is very close to the signal assigned to the vibrations of ionized carboxylic groups (1570 cm−1) [42], or glutamates lying closer to the cytochrome c surface [34], or heme propionates. As cytochrome c binds to zinc and cadmium, the band at 1504 cm−1 becomes an additional characteristic of the vibrations of carboxylic groups [34]. The band at 1522 cm−1 in the P76I/G77L/I81L/F82L mutant variant arises from vibrations of the Leu residue inserted into the α-helix, as well as symmetric vibrations of the Lys residue, which are potentially caused by the formation of hydrogen bonds between ε-amino groups of lysine residues and other side-chain groups of cytochrome c [37,39].
A peak at 1240 cm−1 was observed in the spectrum of cytochrome c within the amide III band (1320–1200 cm−1). In the mutant variants carrying T78S/K79P and I81Y/A83Y/G84N substitutions, the position of this peak remained unchanged. In the T78N/K79Y/M80I/I81M/G84N and P76I/G77L/I81L/F82L mutant variants, this band was shifted towards higher frequencies by 2 cm−1.
All cytochromes (WT and mutant forms) possess four peaks in the high frequency range (around 2832–2836, 2870, 2960, and 3060 cm−1) corresponding to the symmetric and asymmetric stretching vibrations of -CH2- bonds and methyl groups of protein. We did not observe significant changes in the peak positions or relative intensities that demonstrate the absence of the significant deterioration in these types of peptide bond stretching.
For the cytochrome c variants carrying T78S/K79P, I81Y/A83Y/G84N, and P76I/G77L/I81L/F82L substitutions, we found that the band at 3339 cm−1 was significantly shifted towards lower frequencies by 33 cm−1, 35 cm−1, and 37 cm−1, respectively. For the T78N/K79Y/M80I/I81M/G84N mutant variant, the original band was split into two (at 3422 cm−1 and 3244 cm−1). The changes in this spectral region are possibly related to the H–O–H vibrations of residual water molecules in cytochrome c specimens.
Hence, mutations reducing the conformational mobility of the target P76GTKMIFA83 site led to structural changes in both the Ω-loop (residues 70–85) and other polypeptide chain regions. The IR spectroscopy data are consistent with the findings obtained by CD spectroscopy and demonstrate that some α-helical elements were deformed and partially destructed, while the content of the β-structures was increased, and the amount of unordered structure fraction was slightly decreased. It is known that changes in the redox state of cytochrome c are accompanied by reversible variations of fractions of broadened β-structures, β-turns, and α-helices in addition to changes in the oxidation state of Fe atoms in the heme [4]. Thus, the reduced form of cytochrome c is characterized by the presence of β-turns and high-frequency β-folds (1693 cm−1), which arise from rearrangements of some β-structural elements and their transformation into antiparallel β-sheets. Furthermore, the signals from the α-helices are shifted towards shorter wavelengths upon reduction. This phenomenon is attributed either to changes in the microenvironment of α-helices due to broadening of β-structures or formation of some 310-helical structures as a result of 5–1 closure of hydrogen bonds. The oxidized form of cytochrome c contains fewer β-structural elements compared to the reduced form. The IR spectroscopy data infer that the presence of high-frequency β-folds and transformed α-helices is a feature that is shared by mutant variants and the reduced form of wild-type cytochrome c. These changes in the secondary structure of mutant variants were probably responsible for the greater decline in interaction between recombinant proteins and complex III compared to complex IV [19,20]. Thus, the succinate:cytochrome c-reductase activity for the P76I/G77L/I81L/F82L mutant was less than 3%, but about 22% of cytochrome c-oxidase activity of the wild-type cytochrome c was retained.
The decline in both activities (succinate:cytochrome c-reductase activity and cytochrome c-oxidase activity) of mitoplasts, which is observed in the presence of these cytochrome c variants, is presumably related to the reduced conformational mobility of the mutation-carrying P76GTKMIFA83 site within the Ω-loop (residues 70–85). The limited mobility of this Ω-loop apparently makes it difficult for the heme of mutant cytochrome variants to acquire a conformation ensuring the optimal heme orientation with respect to the cytochrome c1 heme for the electron tunneling. This conclusion agrees with the SERS and resonance Raman spectroscopy data obtained earlier for these cytochrome c mutant forms and demonstrates that the hemes of all mutant forms in both redox states has a higher probability of the ruffled conformation than the heme of WT cytochrome c [20].
4. Conclusions
The results of this study demonstrate that the conformational mobility of the P76GTKMIFA83 site of the Ω-loop (residues 70–85) is a prerequisite for electron transfer by cytochrome c in the mitochondrial respiratory chain. The structure of cytochrome c is a dynamic system consisting mainly of α-helices. Parts of this dynamic system are rearranged during the functioning of the protein, and conformation changes occur in the loop regions (the P76GTKMIFA83 sequence being the most significant among them). The conformational changes arising in the protein and heme parts of the WT cytochrome c upon binding to partner proteins are needed to ensure a conformation that would be optimal for highly efficient electron transfer. Thus, changes in the cytochrome c protein part under interaction of cytochrome c with complex III are necessary for the formation of the protein supercomplexes and for the drawing together of hemes c and c1, whereas heme c mobility is necessary for its optimal orientation towards heme c1 when the supercomplex cytochrome c-complex III is formed. Mutations introduced into the region of the Ω-loop corresponding to residue 70–85 lead to both the changes in the protein secondary structure and to the conformational alterations of the heme, which changes both interaction of cytochrome c with complex III and heme c orientation towards heme c1. Thus, the decreased conformational mobility of the P76GTKMIFA83 site seems to hinder the rearrangement of the polypeptide chain, leading to the formation of β-structural elements that obstruct the interaction of oxidized cytochrome c with complex III and to the conformational changes of the heme, which reduce its ability to accept electrons. This result demonstrates the high importance of the non-ordered Ω-loop for the electron transfer activity of cytochrome c.
Author Contributions
Conceptualization, R.V.C., N.A.B., D.A.D., A.B.R., M.P.K., and G.V.M.; data curation, R.V.C., T.V.B., and G.V.M.; formal analysis, R.V.C., T.V.B., N.A.B., A.R.B., A.I.Y., and A.N.N.; funding acquisition, R.V.C., N.A.B., D.A.D., G.V.M., and M.P.K.; investigation, R.V.C., T.V.B., V.V.R., K.S.K., and A.I.Y.; methodology, R.V.C., N.A.B., K.S.K., V.V.R., G.V.M., and D.A.D.; project administration, D.A.D., M.P.K., and G.V.M.; resources, D.A.D., G.V.M., and M.P.K.; software, N.A.B., A.R.B., and A.N.N.; supervision, D.A.D., G.V.M., A.B.R., and M.P.K.; validation, R.V.C., T.V.B., N.A.B., A.R.B., and A.I.Y.; visualization, R.V.C. and T.V.B.; writing—original draft, R.V.C., T.V.B., and N.A.B.; Writing—review and editing, R.V.C., N.A.B., D.A.D., and G.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Program for Molecular and Cellular Biology of the Russian Academy of Sciences. N.A.B. acknowledges financial support from RFBR (grant RFBR 20-04-01011_a), which was used for the development of the software for the spectral analysis and for the data analysis. G.V.M. and A.I.Y. acknowledge financial support from the Russian Sciences Foundation (grant RSF 19-79-30062), which was used for the IR experiments. The research was carried out as part of the Scientific Project of the State Order of the Government of the Russian Federation to Lomonosov Moscow State University, No. 121032500076-1. This research has been supported by the Interdisciplinary Scientific and Educational School of Moscow University “Molecular Technologies of the Living Systems and Synthetic Biology”.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Bioethics Committee of M.V. Lomonosov Moscow State University according to the ethical and juridical norms of scientific researches in biology, medicine and related areas corresponding to laws of Russian Federation and international GLP standards (Good Laboratory Practice).
Data Availability Statement
All raw data are available from corresponding authors under request.
Acknowledgments
The authors are grateful to the Moscow State University (Russia) for the opportunity to use the NMR facilities of the BioSpectroTomography Center of the Faculty of Fundamental Medicine. The authors are also grateful to S.S. Zhokhov for the expert technical assistance in the NMR measurements. The authors are grateful to V.G. Grivennikova and A.D. Vinogradov from the Biochemistry Department, biological faculty of the M.V. Lomonosov Moscow State University for their help in measuring the biological activity of cytochromes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys Acta 2012, 1823, 1604–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banci, L.; Bertini, I.; Gray, H.B.; Luchinat, C.; Reddig, T.; Rosato, A.; Turano, P. Solution structure of oxidized horse heart cytochrome c. Biochemistry 1997, 36, 9867–9877. [Google Scholar] [CrossRef] [Green Version]
- Takano, T.F.; Dickerson, R.E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J. Mol. Biol. 1981, 153, 79–94. [Google Scholar] [CrossRef]
- Calvert, J.F.; Hill, J.L.; Dong, A. Redox-dependent conformational changes are common structural features of cytochrome c from various species. Arch Biochem. Biophys. 1997, 346, 287–293. [Google Scholar] [CrossRef]
- Moss, D.; Nabedryk, E.; Breton, J.; Mantele, W. Redox-linked conformational changes in proteins detected by a combination of infrared spectroscopy and protein electrochemistry. Evaluation of the technique with cytochrome c. Eur. J. Biochem. 1990, 187, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, D.D.; Mantele, W. Electrochemically induced conformational changes in cytochrome c monitored by Fourier transform infrared difference spectroscopy: Influence of temperature, pH, and electrode surfaces. Biochemistry 1993, 32, 1118–1126. [Google Scholar] [CrossRef]
- Inoue, T.; Suzuki, S.; Nishio, N.; Yamaguchi, K.; Kataoka, K.; Tobari, J.; Yong, X.; Hamanaka, S.; Matsumura, H.; Kai, Y. The significance of the flexible loop in the azurin (Az-iso2) from the obligate methylotroph Methylomonas sp. strain J. J. Mol. Biol. 2003, 333, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; McCammon, J.A. Dynamics, hydration, and motional averaging of a loop-gated artificial protein cavity: The W191G mutant of cytochrome c-peroxidase in water as revealed by molecular dynamics simulations. Biochemistry 2007, 46, 10629–10642. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, J.F.; Rose, G.D. Loops in globular proteins: A novel category of secondary structure. Science 1986, 234, 849–855. [Google Scholar] [CrossRef]
- Alvarez-Paggi, D.; Hannibal, L.; Castro, M.A.; Oviedo-Rouco, S.; Demicheli, V.; Tórtora, V.; Tomasina, F.; Radi, R.; Murgida, D.H. Multifunctional Cytochrome c: Learning New Tricks from an Old Dog. Chem. Rev. 2017, 117, 13382–13460. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Q.L.; Li, H.; Weng, Y.X.; Wang, W.C.; Qiu, X.G. Infrared spectroscopic discrimination between the loop and alpha-helices and determination of the loop diffusion kinetics by temperature-jump time-resolved infrared spectroscopy for cytochrome c. Biophys J. 2007, 93, 2756–2766. [Google Scholar] [CrossRef] [Green Version]
- Hoang, L.; Bedard, S.; Krishna, M.M.; Lin, Y.; Englander, S.W. Cytochrome c folding pathway: Kinetic native-state hydrogen exchange. Proc. Natl. Acad. Sci. USA 2002, 99, 12173–12178. [Google Scholar] [CrossRef] [Green Version]
- Hoang, L.; Maity, H.; Krishna, M.M.; Lin, Y.; Englander, S.W. Folding units govern the cytochrome c alkaline transition. J. Mol. Biol. 2003, 331, 37–43. [Google Scholar] [CrossRef]
- Maity, H.; Maity, M.; Krishna, M.M.; Mayne, L.; Englander, S.W. Protein folding: The stepwise assembly of foldon units. Proc. Natl. Acad. Sci. USA 2005, 102, 4741–4746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Rumbley, J.N.; Englander, S.W.; Wand, A.J. Fast structural dynamics in reduced and oxidized cytochrome c. Protein Sci. 2009, 18, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Nekrasov, A.; Anashkina, A.; Zinchenko, A. A new paradigm of protein structural organization. Theoretical Approaches to BioInformation Systems. Book of Abstracts. In Proceedings of the TABIS 2013, Belgrade, Serbia, 17–22 September 2013; p. 1. Available online: https://www.researchgate.net/profile/Alexei-Nekrasov/publication/270684947_A_New_Paradigm_of_Protein_Structural_Organization/links/54b285da0cf220c63cd25c21/A-New-Paradigm-of-Protein-Structural-Organization.pdf#page=15. (accessed on 12 August 2021).
- Anashkina, A.A.; Nekrasov, A.N. The method for identification of hierarchical organization of protein sequences. Russ. J. Numer. Anal. Math. Model. 2014, 29, 266–273. [Google Scholar] [CrossRef]
- Bushnell, G.W.; Louie, G.V.; Brayer, G.D. High-resolution three-dimensional structure of horse heart cytochrome c. J. Mol. Biol. 1990, 214, 585–595. [Google Scholar] [CrossRef]
- Ostroverkhova, T.V.; Chertkova, R.V.; Nekrasov, A.N.; Dolgikh, D.A.; Kirpichnikov, M.P. Design of mutant variants of horse cytochrome c by analysis of informational structure method and testing its biological activity. Mosc. Univ. Biol. Sci. Bull. 2011, 66, 65–67. [Google Scholar] [CrossRef]
- Chertkova, R.V.; Brazhe, N.A.; Bryantseva, T.V.; Nekrasov, A.N.; Dolgikh, D.A.; Yusipovich, A.I.; Sosnovtseva, O.; Maksimov, G.V.; Rubin, A.B.; Kirpichnikov, M.P. New insight into the mechanism of mitochondrial cytochrome c function. PLoS ONE 2017, 12, e0178280. [Google Scholar] [CrossRef]
- Nekrasov, A.N.; Alekseeva, L.G.; Pogosyan, R.A.; Dolgikh, D.A.; Kirpichnikov, M.P.; de Brevern, A.G.; Anashkina, A.A. A minimum set of stable blocks for rational design of polypeptide chains. Biochimie 2019, 160, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Pepelina, T.Y.; Chertkova, R.V.; Ostroverkhova, T.V.; Dolgikh, D.A.; Kirpichnikov, M.P.; Grivennikova, V.G.; Vinogradov, A.D. Site-directed mutagenesis of cytochrome c: Reactions with respiratory chain components and superoxide radical. Biochemistry (Mosc.) 2009, 74, 625–632. [Google Scholar] [CrossRef]
- Dolgikh, D.A.; Latypov, R.F.; Abdullaev, Z.K.; Kolov, V.; Roder, H.; Kirpichnikov, M.P. Expression of mutant horse cytochrome c genes in Escherichia coli. Russ. J. Bioorg. Chem. 1998, 24, 756–759. [Google Scholar]
- Pepelina, T.Yu.; Chertkova, R.V.; Dolgikh, D.A.; Kirpichnikov, M.P. The role of individual lysine residues of horse cytochrome c in the formation of reactive complexes with components of the respiratory chain. Russ. J. Bioorg. Chem. 2010, 36, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chertkova, R.V.; Sharonov, G.V.; Feofanov, A.V.; Bocharova, O.V.; Latypov, R.F.; Chernyak, B.V.; Arseniev, A.S.; Dolgikh, D.A.; Kirpichnikov, M.P. Proapoptotic activity of cytochrome c in living cells: Effect of K72 substitutions and species differences. Mol. Cell. Biochem. 2008, 314, 85–93. [Google Scholar] [CrossRef]
- Johnson, D.; Lardy, H. Isolation of Mitochondria. Meth Enzym. 1967, 10, 94–96. [Google Scholar]
- Jacobs, E.E.; Sanadi, D.R. The reversible removal of cytochrome c from mitochondria. J. Biol. Chem. 1960, 235, 531–534. [Google Scholar] [CrossRef]
- Vinogradov, A.; Leikin, Yu.; Lipskaya, T. Mitochondrial biochemistry. Bioenergetics. In Manual of Practical Study to Animal Biochemistry; Lomonosov Moscow State University publishers: Moscow, Russia, 1977; pp. 19–22. [Google Scholar]
- Feng, Y.Q.; Roder, H.; Englander, S.W. Assignment of paramagnetically shifted resonances in the 1H NMR spectrum of horse ferricytochrome c. Biophys. J. 1990, 57, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.; Turner, D.L. Proton NMR studies of horse ferricytochrome c. Completion of the assignment of the well resolved hyperfine shifted resonances. FEBS Lett. 1987, 226, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Lee, A.D.; Williams, R.J.; Williams, G. The effects of multiple amino acid substitutions on the polypeptide backbone of tuna and horse cytochromes c. Eur. J. Biochem. 1989, 182, 57–65. [Google Scholar] [CrossRef]
- Dong, A.; Huang, P.; Caughey, W.S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef]
- Ataka, K.; Heberle, J. Functional vibrational spectroscopy of a cytochrome c monolayer: SEIDAS probes the interaction with different surface-modified electrodes. J. Am. Chem. Soc. 2004, 126, 9445–9457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Benabbas, A.; Zeng, W.; Kleingardner, J.G.; Bren, K.L.; Champion, P.M. Investigations of heme distor-tion, low-frequency vibrational excitations, and electron transfer in cytochrome c. Proc. Natl. Acad. Sci. USA 2014, 111, 6570–6575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar]
- Holzbaur, I.E.; English, A.M.; Ismail, A.A. FTIR study of the thermal denaturation of horseradish and cytochrome c peroxidases in D2O. Biochemistry 1996, 35, 5488–5494. [Google Scholar] [CrossRef]
- Dong, A.C.; Huang, P.; Caughey, W.S. Redox-dependent changes in beta-extended chain and turn structures of cytochrome c in water solution determined by second derivative amide I infrared spectra. Biochemistry 1992, 31, 182–189. [Google Scholar] [CrossRef]
- Dong, A.; Caughey, W.S. Infrared methods for study of hemoglobin reactions and structures. Methods Enzymol. 1994, 232, 139–175. [Google Scholar]
- Venyaminov, S.Y.; Kalnin, N.N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. II. Amide absorption bands of polypeptides and fibrous proteins in alpha-, beta-, and random coil conformations. Biopolymers 1990, 30, 1259–1271. [Google Scholar] [CrossRef]
- Ruegg, M.; Metzger, V.; Susi, H. Computer analyses of characteristic infrared bands of globular proteins. Biopolymers 1975, 14, 1465–1471. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Cabiaux, V.; Ruysschaert, J.M. Secondary structure and dosage of soluble and membrane proteins by attenuated total reflection Fourier-transform infrared spectroscopy on hydrated films. Eur. J. Biochem. 1990, 193, 409–420. [Google Scholar] [CrossRef]
- Tonge, P.; Moore, G.R.; Wharton, C.W. Fourier-transform infra-red studies of the alkaline isomerization of mitochondrial cytochrome c and the ionization of carboxylic acids. Biochem. J. 1989, 258, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).