Engineering Crystal Packing in RNA Structures I: Past and Future Strategies for Engineering RNA Packing in Crystals
Abstract
1. Introduction
2. The Propensity of RNA Helices to Form Intermolecular Stacks
3. Hairpin Loops and Their Utility in Crystal Packing Design
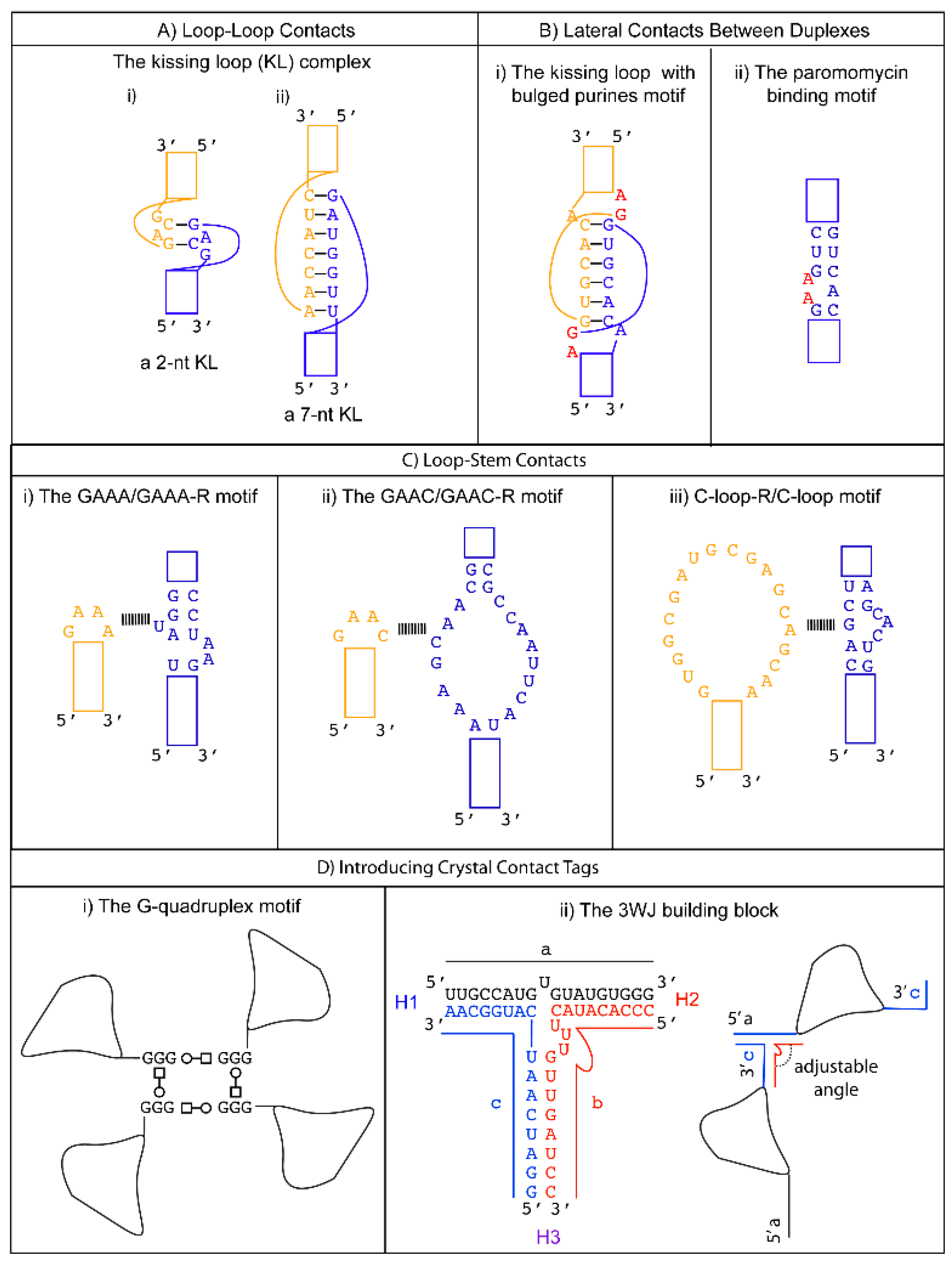
3.1. Promoting Loop-Loop Crystal Contacts: The Kissing Loop Complex
3.2. Promoting Loop to Stem Crystal Contacts: Loop to Receptor Motifs
3.2.1. Tetraloop-Tetraloop Receptor Motifs
The GAAA Loop and Its 11-nts Receptors (GAAA-R)
The GAAC Loop and Its 20-nts Receptor (GAAC-R)
3.2.2. The C-Loop and Its 20-nts Receptor (C-loop-R)
4. Designing Lateral Contacts between Duplexes
4.1. Kissing Loop with Two Bulged Purines
4.2. Paromomycin Binding Motif
5. Introducing Crystal Contact Tags
5.1. G-Quadruplex
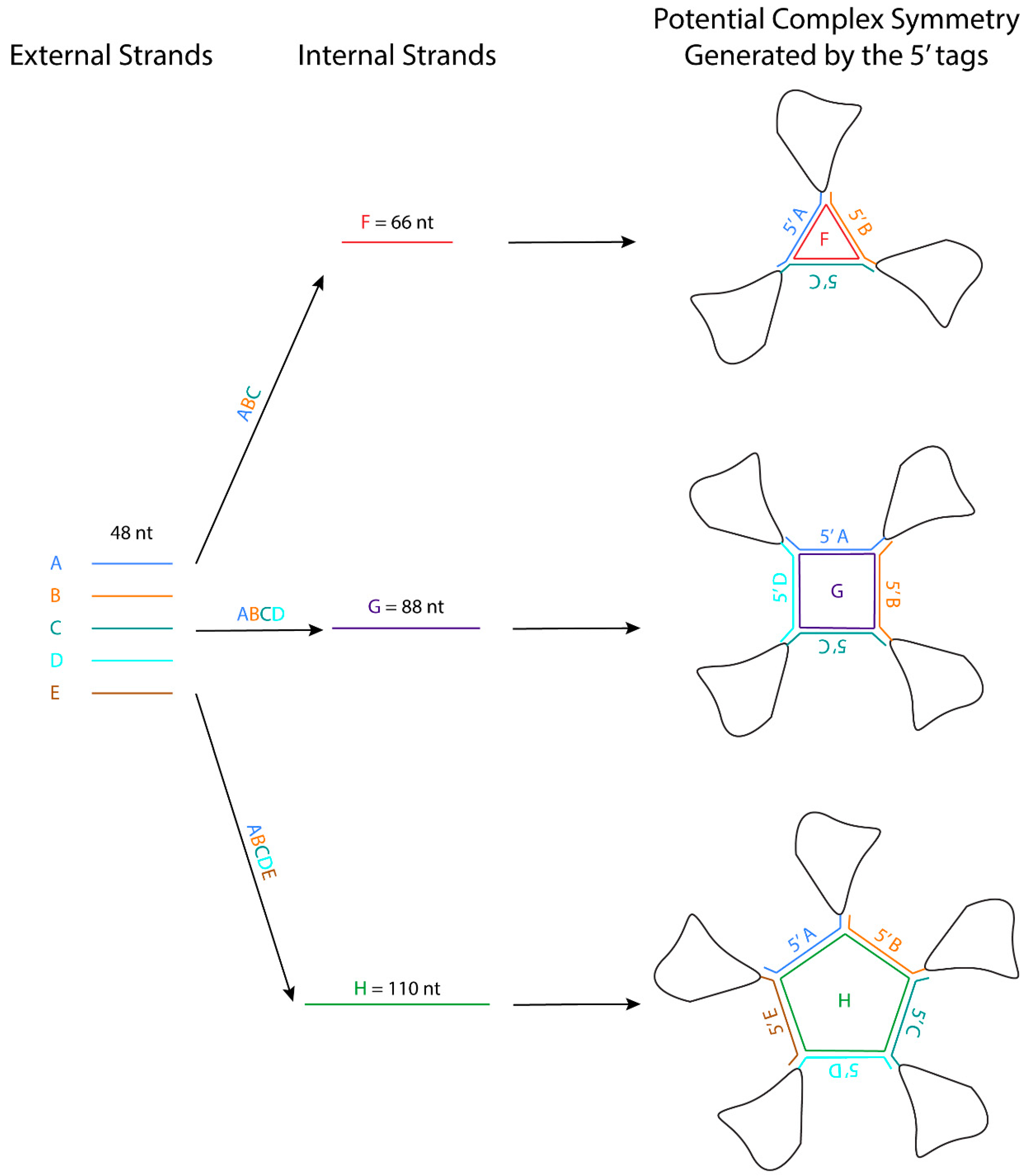
5.2. The Three-Way Junction (3WJ) Building Block
6. Introducing RNA Binding Proteins
6.1. RNA Binding Protein—U1A
6.2. Antibody Fragment
6.3. Peptide Nucleic Acid
7. Post-Crystallization Treatment
8. Future Directions of RNA Crystallography
9. Future Relevance of Engineering Crystal Packing in RNA Structures
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.H.; Quigley, G.; Suddath, F.L.; McPherson, A.; Sneden, D.; Kim, J.J.; Weinzierl, J.; Blattmann, P.; Rich, A. The three-dimensional structure of yeast phenylalanine transfer RNA: Shape of the molecule at 5.5-A resolution. Proc. Natl. Acad. Sci. USA 1972, 69, 3746–3750. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Quigley, G.J.; Suddath, F.L.; McPherson, A.; Sneden, D.; Kim, J.J.; Weinzierl, J.; Rich, A. Three-dimensional structure of yeast phenylalanine transfer RNA: Folding of the polynucleotide chain. Science 1973, 179, 285–288. [Google Scholar] [CrossRef]
- Kim, S.H.; Suddath, F.L.; Quigley, G.J.; McPherson, A.; Sussman, J.L.; Wang, A.H.; Seeman, N.C.; Rich, A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science 1974, 185, 435–440. [Google Scholar] [CrossRef]
- Pley, H.W.; Flaherty, K.M.; McKay, D.B. Three-dimensional structure of a hammerhead ribozyme. Nature 1994, 372, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.G.; Finch, J.T.; Klug, A. The crystal structure of an all-RNA hammerhead ribozyme: A proposed mechanism for RNA catalytic cleavage. Cell 1995, 81, 991–1002. [Google Scholar] [CrossRef]
- Ferre-D’Amare, A.R.; Zhou, K.; Doudna, J.A. Crystal structure of a hepatitis delta virus ribozyme. Nature 1998, 395, 567–574. [Google Scholar] [CrossRef]
- Cate, J.H.; Gooding, A.R.; Podell, E.; Zhou, K.; Golden, B.L.; Kundrot, C.E.; Cech, T.R.; Doudna, J.A. Crystal structure of a group I ribozyme domain: Principles of RNA packing. Science 1996, 273, 1678–1685. [Google Scholar] [CrossRef]
- Kastner, B.; Will, C.L.; Stark, H.; Luhrmann, R. Structural Insights into Nuclear pre-mRNA Splicing in Higher Eukaryotes. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef]
- Plaschka, C.; Newman, A.J.; Nagai, K. Structural Basis of Nuclear pre-mRNA Splicing: Lessons from Yeast. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef]
- Yan, C.; Wan, R.; Shi, Y. Molecular Mechanisms of pre-mRNA Splicing through Structural Biology of the Spliceosome. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Ke, A.; Doudna, J.A. Crystallization of RNA and RNA-protein complexes. Methods 2004, 34, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Shoffner, G.M.; Wang, R.; Podell, E.; Cech, T.R.; Guo, F. In Crystallo Selection to Establish New RNA Crystal Contacts. Structure 2018, 26, 1275–1283.e3. [Google Scholar] [CrossRef]
- Russell, R.; Zhuang, X.; Babcock, H.P.; Millett, I.S.; Doniach, S.; Chu, S.; Herschlag, D. Exploring the folding landscape of a structured RNA. Proc. Natl. Acad. Sci. USA 2002, 99, 155–160. [Google Scholar] [CrossRef]
- Chen, S.J.; Dill, K.A. RNA folding energy landscapes. Proc. Natl. Acad. Sci. USA 2000, 97, 646–651. [Google Scholar] [CrossRef]
- Cordero, P.; Das, R. Rich RNA Structure Landscapes Revealed by Mutate-and-Map Analysis. PLoS Comput. Biol. 2015, 11, e1004473. [Google Scholar] [CrossRef]
- Lynch, D.C.; Schimmel, P.R. Cooperative binding of magnesium to transfer ribonucleic acid studied by a fluorescent probe. Biochemistry 1974, 13, 1841–1852. [Google Scholar] [CrossRef]
- Bernetti, M.; Hall, K.B.; Bussi, G. Reweighting of molecular simulations with explicit-solvent SAXS restraints elucidates ion-dependent RNA ensembles. Nucleic Acids Res. 2021. [Google Scholar] [CrossRef]
- Klosterman, P.S.; Shah, S.A.; Steitz, T.A. Crystal structures of two plasmid copy control related RNA duplexes: An 18 base pair duplex at 1.20 A resolution and a 19 base pair duplex at 1.55 A resolution. Biochemistry 1999, 38, 14784–14792. [Google Scholar] [CrossRef]
- Mueller, U.; Muller, Y.A.; Herbst-Irmer, R.; Sprinzl, M.; Heinemann, U. Disorder and twin refinement of RNA heptamer double helices. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Brunger, A.T. The 1.8 A crystal structure of a statically disordered 17 base-pair RNA duplex: Principles of RNA crystal packing and its effect on nucleic acid structure. J. Mol. Biol. 1999, 285, 1577–1588. [Google Scholar] [CrossRef]
- Batey, R.T.; Kieft, J.S. Improved native affinity purification of RNA. RNA 2007, 13, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Golden, B.L.; Podell, E.R.; Gooding, A.R.; Cech, T.R. Crystals by design: A strategy for crystallization of a ribozyme derived from the Tetrahymena group I intron. J. Mol. Biol. 1997, 270, 711–723. [Google Scholar] [CrossRef]
- Lippa, G.M.; Liberman, J.A.; Jenkins, J.L.; Krucinska, J.; Salim, M.; Wedekind, J.E. Crystallographic analysis of small ribozymes and riboswitches. Methods Mol. Biol. 2012, 848, 159–184. [Google Scholar] [CrossRef]
- MacElrevey, C.; Spitale, R.C.; Krucinska, J.; Wedekind, J.E. A posteriori design of crystal contacts to improve the X-ray diffraction properties of a small RNA enzyme. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.E.; Garst, A.D.; Batey, R.T. Strategies in RNA crystallography. Methods Enzymol. 2009, 469, 119–139. [Google Scholar] [CrossRef]
- Schultz, S.C.; Shields, G.C.; Steitz, T.A. Crystallization of Escherichia coli catabolite gene activator protein with its DNA binding site. The use of modular DNA. J. Mol. Biol. 1990, 213, 159–166. [Google Scholar] [CrossRef]
- Coonrod, L.A.; Lohman, J.R.; Berglund, J.A. Utilizing the GAAA tetraloop/receptor to facilitate crystal packing and determination of the structure of a CUG RNA helix. Biochemistry 2012, 51, 8330–8337. [Google Scholar] [CrossRef]
- Ferré-D’Amaré, A.R.; Zhou, K.; Doudna, J.A. A general module for RNA crystallization. J. Mol. Biol. 1998, 279, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Tamjar, J.; Katorcha, E.; Popov, A.; Malinina, L. Structural dynamics of double-helical RNAs composed of CUG/CUG- and CUG/CGG-repeats. J. Biomol. Struct. Dyn. 2012, 30, 505–523. [Google Scholar] [CrossRef]
- Zhang, J.; Ferre-D’Amare, A.R. Dramatic improvement of crystals of large RNAs by cation replacement and dehydration. Structure 2014, 22, 1363–1371. [Google Scholar] [CrossRef]
- Zhang, J.; Ferre-D’Amare, A.R. Improving RNA Crystal Diffraction Quality by Postcrystallization Treatment. Methods Mol. Biol. 2021, 2323, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Varani, G. Engineering RNA-binding proteins for biology. FEBS J. 2013, 280, 3734–3754. [Google Scholar] [CrossRef]
- Khisamutdinov, E.F.; Jasinski, D.L.; Guo, P. RNA as a boiling-resistant anionic polymer material to build robust structures with defined shape and stoichiometry. ACS Nano 2014, 8, 4771–4781. [Google Scholar] [CrossRef] [PubMed]
- Ferre-D’Amare, A.R.; Doudna, J.A. Methods to crystallize RNA. Curr. Protoc. Nucleic Acid Chem. 2001. [Google Scholar] [CrossRef]
- Holbrook, S.R. RNA structure: The long and the short of it. Curr. Opin. Struct. Biol. 2005, 15, 302–308. [Google Scholar] [CrossRef]
- Dibrov, S.M.; Parker, M.A.; Bergdahl, B.M.; Hermann, T. Crystal structure of a benzimidazole hepatitis C virus inhibitor free and in complex with the viral RNA target. J. Chem. Crystallogr. 2013, 43, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Mooers, B.H.; Singh, A. The crystal structure of an oligo(U):pre-mRNA duplex from a trypanosome RNA editing substrate. RNA 2011, 17, 1870–1883. [Google Scholar] [CrossRef]
- Mooers, B.H. Fusion RNAs in crystallographic studies of double-stranded RNA from trypanosome RNA editing. Methods Mol. Biol. 2015, 1240, 191–216. [Google Scholar] [CrossRef]
- Mooers, B.H. Direct-methods structure determination of a trypanosome RNA-editing substrate fragment with translational pseudosymmetry. Acta Crystallogr. D Struct. Biol. 2016, 72, 477–487. [Google Scholar] [CrossRef]
- Drenth, J. Principles of Protein X-Ray Crystallography, 3rd ed.; Springer-Verlag: New York, NY, USA, 2007. [Google Scholar]
- Holbrook, S.R.; Cheong, C.; Tinoco, I., Jr.; Kim, S.H. Crystal structure of an RNA double helix incorporating a track of non-Watson-Crick base pairs. Nature 1991, 353, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, S.; McLean, J.; Hermann, T. Structure of an RNA dimer of a regulatory element from human thymidylate synthase mRNA. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 97–104. [Google Scholar] [CrossRef]
- Beuning, P.J.; Tessmer, M.R.; Baumann, C.G.; Kallick, D.A.; Musier-Forsyth, K. Sequence-dependent conformational differences of small RNAs revealed by native gel electrophoresis. Anal. Biochem. 1999, 273, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.A.; Koculi, E. Analysis of RNA folding by native polyacrylamide gel electrophoresis. Methods Enzymol. 2009, 469, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Oubridge, C.; Ito, N.; Evans, P.R.; Teo, C.H.; Nagai, K. Crystal structure at 1.92 A resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature 1994, 372, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Price, S.R.; Evans, P.R.; Nagai, K. Crystal structure of the spliceosomal U2B”-U2A’ protein complex bound to a fragment of U2 small nuclear RNA. Nature 1998, 394, 645–650. [Google Scholar] [CrossRef]
- Hoang, C.; Ferre-D’Amare, A.R. Cocrystal structure of a tRNA Psi55 pseudouridine synthase: Nucleotide flipping by an RNA-modifying enzyme. Cell 2001, 107, 929–939. [Google Scholar] [CrossRef]
- Ennifar, E.; Nikulin, A.; Tishchenko, S.; Serganov, A.; Nevskaya, N.; Garber, M.; Ehresmann, B.; Ehresmann, C.; Nikonov, S.; Dumas, P. The crystal structure of UUCG tetraloop. J. Mol. Biol. 2000, 304, 35–42. [Google Scholar] [CrossRef]
- Tomizawa, J. Control of ColE1 plasmid replication: The process of binding of RNA I to the primer transcript. Cell 1984, 38, 861–870. [Google Scholar] [CrossRef]
- Paillart, J.C.; Skripkin, E.; Ehresmann, B.; Ehresmann, C.; Marquet, R. A loop-loop “kissing” complex is the essential part of the dimer linkage of genomic HIV-1 RNA. Proc. Natl. Acad. Sci. USA 1996, 93, 5572–5577. [Google Scholar] [CrossRef]
- Shetty, S.; Kim, S.; Shimakami, T.; Lemon, S.M.; Mihailescu, M.R. Hepatitis C virus genomic RNA dimerization is mediated via a kissing complex intermediate. RNA 2010, 16, 913–925. [Google Scholar] [CrossRef]
- Moras, D.; Comarmond, M.B.; Fischer, J.; Weiss, R.; Thierry, J.C.; Ebel, J.P.; Giege, R. Crystal structure of yeast tRNAAsp. Nature 1980, 288, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Tinoco, I., Jr. A retroviral RNA kissing complex containing only two G.C base pairs. Proc. Natl. Acad. Sci. USA 2000, 97, 9396–9401. [Google Scholar] [CrossRef]
- Lee, A.J.; Crothers, D.M. The solution structure of an RNA loop-loop complex: The ColE1 inverted loop sequence. Structure 1998, 6, 993–1005. [Google Scholar] [CrossRef]
- Marino, J.P.; Gregorian, R.S., Jr.; Csankovszki, G.; Crothers, D.M. Bent helix formation between RNA hairpins with complementary loops. Science 1995, 268, 1448–1454. [Google Scholar] [CrossRef]
- Kondo, Y.; Oubridge, C.; van Roon, A.M.; Nagai, K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5’ splice site recognition. eLife 2015, 4, e04986. [Google Scholar] [CrossRef]
- Pomeranz Krummel, D.A.; Oubridge, C.; Leung, A.K.; Li, J.; Nagai, K. Crystal structure of human spliceosomal U1 snRNP at 5.5 A resolution. Nature 2009, 458, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.W.; Kondo, Y.; Krummel, D.A.P.; Li, J.; Price, S.R.; van Roon, A.-M.M. Engineering Crystal Packing in RNA-Protein Complexes II: A Historical Perspective from the Structural Studies of the Spliceosome. Crystals 2021, in press. [Google Scholar]
- Ennifar, E.; Dumas, P. Polymorphism of bulged-out residues in HIV-1 RNA DIS kissing complex and structure comparison with solution studies. J. Mol. Biol. 2006, 356, 771–782. [Google Scholar] [CrossRef]
- Kondo, J.; Pachamuthu, K.; Francois, B.; Szychowski, J.; Hanessian, S.; Westhof, E. Crystal structure of the bacterial ribosomal decoding site complexed with a synthetic doubly functionalized paromomycin derivative: A new specific binding mode to an a-minor motif enhances in vitro antibacterial activity. ChemMedChem 2007, 2, 1631–1638. [Google Scholar] [CrossRef]
- Vicens, Q.; Westhof, E. Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure 2001, 9, 647–658. [Google Scholar] [CrossRef]
- Leontis, N.B.; Westhof, E. Geometric nomenclature and classification of RNA base pairs. RNA 2001, 7, 499–512. [Google Scholar] [CrossRef]
- D’Ascenzo, L.; Leonarski, F.; Vicens, Q.; Auffinger, P. Revisiting GNRA and UNCG folds: U-turns versus Z-turns in RNA hairpin loops. RNA 2017, 23, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Nozinovic, S.; Furtig, B.; Jonker, H.R.; Richter, C.; Schwalbe, H. High-resolution NMR structure of an RNA model system: The 14-mer cUUCGg tetraloop hairpin RNA. Nucleic Acids Res. 2010, 38, 683–694. [Google Scholar] [CrossRef]
- Allain, F.H.; Varani, G. Structure of the P1 helix from group I self-splicing introns. J. Mol. Biol. 1995, 250, 333–353. [Google Scholar] [CrossRef]
- D’Ascenzo, L.; Vicens, Q.; Auffinger, P. Identification of receptors for UNCG and GNRA Z-turns and their occurrence in rRNA. Nucleic Acids Res. 2018, 46, 7989–7997. [Google Scholar] [CrossRef]
- Pley, H.W.; Flaherty, K.M.; McKay, D.B. Model for an RNA tertiary interaction from the structure of an intermolecular complex between a GAAA tetraloop and an RNA helix. Nature 1994, 372, 111–113. [Google Scholar] [CrossRef]
- Murphy, F.L.; Cech, T.R. GAAA tetraloop and conserved bulge stabilize tertiary structure of a group I intron domain. J. Mol. Biol. 1994, 236, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.; Michel, F.; Westhof, E. Involvement of a GNRA tetraloop in long-range RNA tertiary interactions. J. Mol. Biol. 1994, 236, 1271–1276. [Google Scholar] [CrossRef]
- Costa, M.; Michel, F. Frequent use of the same tertiary motif by self-folding RNAs. EMBO J. 1995, 14, 1276–1285. [Google Scholar] [CrossRef]
- Costa, M.; Michel, F. Rules for RNA recognition of GNRA tetraloops deduced by in vitro selection: Comparison with in vivo evolution. EMBO J. 1997, 16, 3289–3302. [Google Scholar] [CrossRef]
- Geary, C.; Baudrey, S.; Jaeger, L. Comprehensive features of natural and in vitro selected GNRA tetraloop-binding receptors. Nucleic Acids Res. 2008, 36, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Kambach, C.; Kondo, Y.; Kampmann, M.; Jinek, M.; Nagai, K. Use of RNA tertiary interaction modules for the crystallisation of the spliceosomal snRNP core domain. J. Mol. Biol. 2010, 402, 154–164. [Google Scholar] [CrossRef]
- Leung, A.K.; Nagai, K.; Li, J. Structure of the spliceosomal U4 snRNP core domain and its implication for snRNP biogenesis. Nature 2011, 473, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Reiter, N.J.; Osterman, A.; Torres-Larios, A.; Swinger, K.K.; Pan, T.; Mondragon, A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature 2010, 468, 784–789. [Google Scholar] [CrossRef]
- Toor, N.; Keating, K.S.; Taylor, S.D.; Pyle, A.M. Crystal structure of a self-spliced group II intron. Science 2008, 320, 77–82. [Google Scholar] [CrossRef]
- Keating, K.S.; Toor, N.; Pyle, A.M. The GANC tetraloop: A novel motif in the group IIC intron structure. J. Mol. Biol. 2008, 383, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, J.; Furuta, H.; Ikawa, Y. An in vitro-selected RNA receptor for the GAAC loop: Modular receptor for non-GNRA-type tetraloop. Nucleic Acids Res. 2013, 41, 3748–3759. [Google Scholar] [CrossRef]
- Lescoute, A.; Leontis, N.B.; Massire, C.; Westhof, E. Recurrent structural RNA motifs, Isostericity Matrices and sequence alignments. Nucleic Acids Res. 2005, 33, 2395–2409. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, S.P.; Ikawa, Y.; Nakamura, Y. Selection of a novel class of RNA-RNA interaction motifs based on the ligase ribozyme with defined modular architecture. Nucleic Acids Res. 2008, 36, 3600–3607. [Google Scholar] [CrossRef]
- Tamura, M.; Holbrook, S.R. Sequence and structural conservation in RNA ribose zippers. J. Mol. Biol. 2002, 320, 455–474. [Google Scholar] [CrossRef]
- Kondo, J.; Dock-Bregeon, A.C.; Willkomm, D.K.; Hartmann, R.K.; Westhof, E. Structure of an A-form RNA duplex obtained by degradation of 6S RNA in a crystallization droplet. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 634–639. [Google Scholar] [CrossRef]
- Reblova, K.; Fadrna, E.; Sarzynska, J.; Kulinski, T.; Kulhanek, P.; Ennifar, E.; Koca, J.; Sponer, J. Conformations of flanking bases in HIV-1 RNA DIS kissing complexes studied by molecular dynamics. Biophys. J. 2007, 93, 3932–3949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarzynska, J.; Reblova, K.; Sponer, J.; Kulinski, T. Conformational transitions of flanking purines in HIV-1 RNA dimerization initiation site kissing complexes studied by CHARMM explicit solvent molecular dynamics. Biopolymers 2008, 89, 732–746. [Google Scholar] [CrossRef]
- Ogle, J.M.; Brodersen, D.E.; Clemons, W.M., Jr.; Tarry, M.J.; Carter, A.P.; Ramakrishnan, V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 2001, 292, 897–902. [Google Scholar] [CrossRef]
- Banco, M.T.; Ferre-D’Amare, A.R. The emerging structural complexity of G-quadruplex RNAs. RNA 2021, 27, 390–402. [Google Scholar] [CrossRef]
- Feklistov, A.; Darst, S.A. Crystallographic analysis of an RNA polymerase σ-subunit fragment complexed with -10 promoter element ssDNA: Quadruplex formation as a possible tool for engineering crystal contacts in protein-ssDNA complexes. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 950–955. [Google Scholar] [CrossRef]
- Yoshizawa, S. Nanotechnology Tools for the Study of RNA, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 139. [Google Scholar]
- Guo, P.; Haque, F. RNA Nanotechnology and Therapeutics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Grabow, W.W.; Jaeger, L. RNA self-assembly and RNA nanotechnology. Acc. Chem. Res. 2014, 47, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, D.; Haque, F.; Binzel, D.W.; Guo, P. Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano 2017, 11, 1142–1164. [Google Scholar] [CrossRef]
- Xia, K.; Shen, J.; Li, Q.; Fan, C.; Gu, H. Near-Atomic Fabrication with Nucleic Acids. ACS Nano 2020, 14, 1319–1337. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Shu, D.; Shu, Y.; Haque, F.; Abdelmawla, S.; Guo, P. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 2011, 6, 658–667. [Google Scholar] [CrossRef]
- Ferre-D’Amare, A.R. Use of the spliceosomal protein U1A to facilitate crystallization and structure determination of complex RNAs. Methods 2010, 52, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Koldobskaya, Y.; Duguid, E.M.; Shechner, D.M.; Suslov, N.B.; Ye, J.; Sidhu, S.S.; Bartel, D.P.; Koide, S.; Kossiakoff, A.A.; Piccirilli, J.A. A portable RNA sequence whose recognition by a synthetic antibody facilitates structural determination. Nat. Struct. Mol. Biol. 2011, 18, 100–106. [Google Scholar] [CrossRef]
- Koide, S. Engineering of recombinant crystallization chaperones. Curr. Opin. Struct. Biol. 2009, 19, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.D.; Tereshko, V.; Frederiksen, J.K.; Koide, A.; Fellouse, F.A.; Sidhu, S.S.; Koide, S.; Kossiakoff, A.A.; Piccirilli, J.A. Synthetic antibodies for specific recognition and crystallization of structured RNA. Proc. Natl. Acad. Sci. USA 2008, 105, 82–87. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef]
- Kiliszek, A.; Banaszak, K.; Dauter, Z.; Rypniewski, W. The first crystal structures of RNA-PNA duplexes and a PNA-PNA duplex containing mismatches--toward anti-sense therapy against TREDs. Nucleic Acids Res. 2016, 44, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Verona, M.D.; Verdolino, V.; Palazzesi, F.; Corradini, R. Focus on PNA Flexibility and RNA Binding using Molecular Dynamics and Metadynamics. Sci. Rep. 2017, 7, 42799. [Google Scholar] [CrossRef]
- Arnott, S.; Hukins, D.W.; Dover, S.D. Optimised parameters for RNA double-helices. Biochem. Biophys. Res. Commun. 1972, 48, 1392–1399. [Google Scholar] [CrossRef]
- Pflugrath, J.W. Practical macromolecular cryocrystallography. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 622–642. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Sica, F.; Mattia, C.A.; Merlino, A. Increasing the X-ray diffraction power of protein crystals by dehydration: The case of bovine serum albumin and a survey of literature data. Int. J. Mol. Sci. 2012, 13, 3782–3800. [Google Scholar] [CrossRef]
- Klein, D.J.; Ferre-D’Amare, A.R. Crystallization of the glmS ribozyme-riboswitch. Methods Mol. Biol. 2009, 540, 129–139. [Google Scholar] [CrossRef]
- Zhang, J.; Ferre-D’Amare, A.R. Co-crystal structure of a T-box riboswitch stem I domain in complex with its cognate tRNA. Nature 2013, 500, 363–366. [Google Scholar] [CrossRef]
- Voth, A.R.; Hays, F.A.; Ho, P.S. Directing macromolecular conformation through halogen bonds. Proc. Natl. Acad. Sci. USA 2007, 104, 6188–6193. [Google Scholar] [CrossRef] [PubMed]
- Ennifar, E.; Bernacchi, S.; Wolff, P.; Dumas, P. Influence of C-5 halogenation of uridines on hairpin versus duplex RNA folding. RNA 2007, 13, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Nannenga, B.L.; Iadanza, M.G.; Gonen, T. Three-dimensional electron crystallography of protein microcrystals. eLife 2013, 2, e01345. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Zidek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021. [Google Scholar] [CrossRef]
- Desmet, J.; De Maeyer, M.; Hazes, B.; Lasters, I. The dead-end elimination theorem and its use in protein side-chain positioning. Nature 1992, 356, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kappel, K.; Zhang, K.; Su, Z.; Watkins, A.M.; Kladwang, W.; Li, S.; Pintilie, G.; Topkar, V.V.; Rangan, R.; Zheludev, I.N.; et al. Accelerated cryo-EM-guided determination of three-dimensional RNA-only structures. Nat. Methods 2020, 17, 699–707. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, K.; Kappel, K.; Li, S.; Palo, M.Z.; Pintilie, G.D.; Rangan, R.; Luo, B.; Wei, Y.; Das, R.; et al. Cryo-EM structures of full-length Tetrahymena ribozyme at 3.1 A resolution. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Banatao, D.R.; Cascio, D.; Crowley, C.S.; Fleissner, M.R.; Tienson, H.L.; Yeates, T.O. An approach to crystallizing proteins by synthetic symmetrization. Proc. Natl. Acad. Sci. USA 2006, 103, 16230–16235. [Google Scholar] [CrossRef] [PubMed]
- Khisamutdinov, E.F.; Li, H.; Jasinski, D.L.; Chen, J.; Fu, J.; Guo, P. Enhancing immunomodulation on innate immunity by shape transition among RNA triangle, square and pentagon nanovehicles. Nucleic Acids Res. 2014, 42, 9996–10004. [Google Scholar] [CrossRef]
- Claverie, J.M. Fewer genes, more noncoding RNA. Science 2005, 309, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Rouskin, S.; Zubradt, M.; Washietl, S.; Kellis, M.; Weissman, J.S. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature 2014, 505, 701–705. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujari, N.; Saundh, S.L.; Acquah, F.A.; Mooers, B.H.M.; Ferré-D’Amaré, A.R.; Leung, A.K.-W. Engineering Crystal Packing in RNA Structures I: Past and Future Strategies for Engineering RNA Packing in Crystals. Crystals 2021, 11, 952. https://doi.org/10.3390/cryst11080952
Pujari N, Saundh SL, Acquah FA, Mooers BHM, Ferré-D’Amaré AR, Leung AK-W. Engineering Crystal Packing in RNA Structures I: Past and Future Strategies for Engineering RNA Packing in Crystals. Crystals. 2021; 11(8):952. https://doi.org/10.3390/cryst11080952
Chicago/Turabian StylePujari, Narsimha, Stephanie L. Saundh, Francis A. Acquah, Blaine H. M. Mooers, Adrian R. Ferré-D’Amaré, and Adelaine Kwun-Wai Leung. 2021. "Engineering Crystal Packing in RNA Structures I: Past and Future Strategies for Engineering RNA Packing in Crystals" Crystals 11, no. 8: 952. https://doi.org/10.3390/cryst11080952
APA StylePujari, N., Saundh, S. L., Acquah, F. A., Mooers, B. H. M., Ferré-D’Amaré, A. R., & Leung, A. K.-W. (2021). Engineering Crystal Packing in RNA Structures I: Past and Future Strategies for Engineering RNA Packing in Crystals. Crystals, 11(8), 952. https://doi.org/10.3390/cryst11080952






