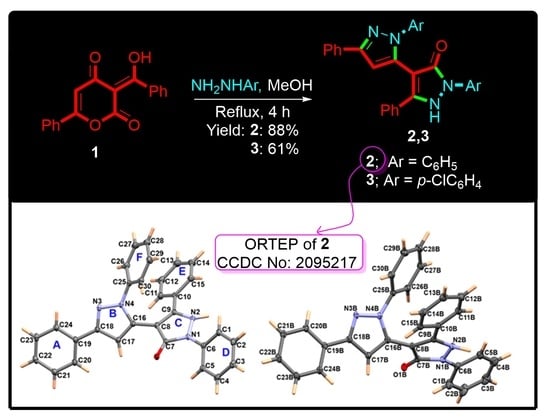
Synthesis and X-ray Crystal Structure of New Substituted 3-4′-Bipyrazole Derivatives. Hirshfeld Analysis, DFT and NBO Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of 2 and 3
2.2.1. 2,2′,5,5′-Tetraphenyl-1′,2′-Dihydro-2H,3′H-[3,4′-Bipyrazol]-3′-One (2)
2.2.2. 2,2′-Bis(4-Chlorophenyl)-5,5′-Diphenyl-1′,2′-Dihydro-2H,3′H-[3,4′-Bipyrazol]-3′-One (3)
2.3. Crystal Structure Determination
2.4. Computational Study Protocols
3. Results and Discussion
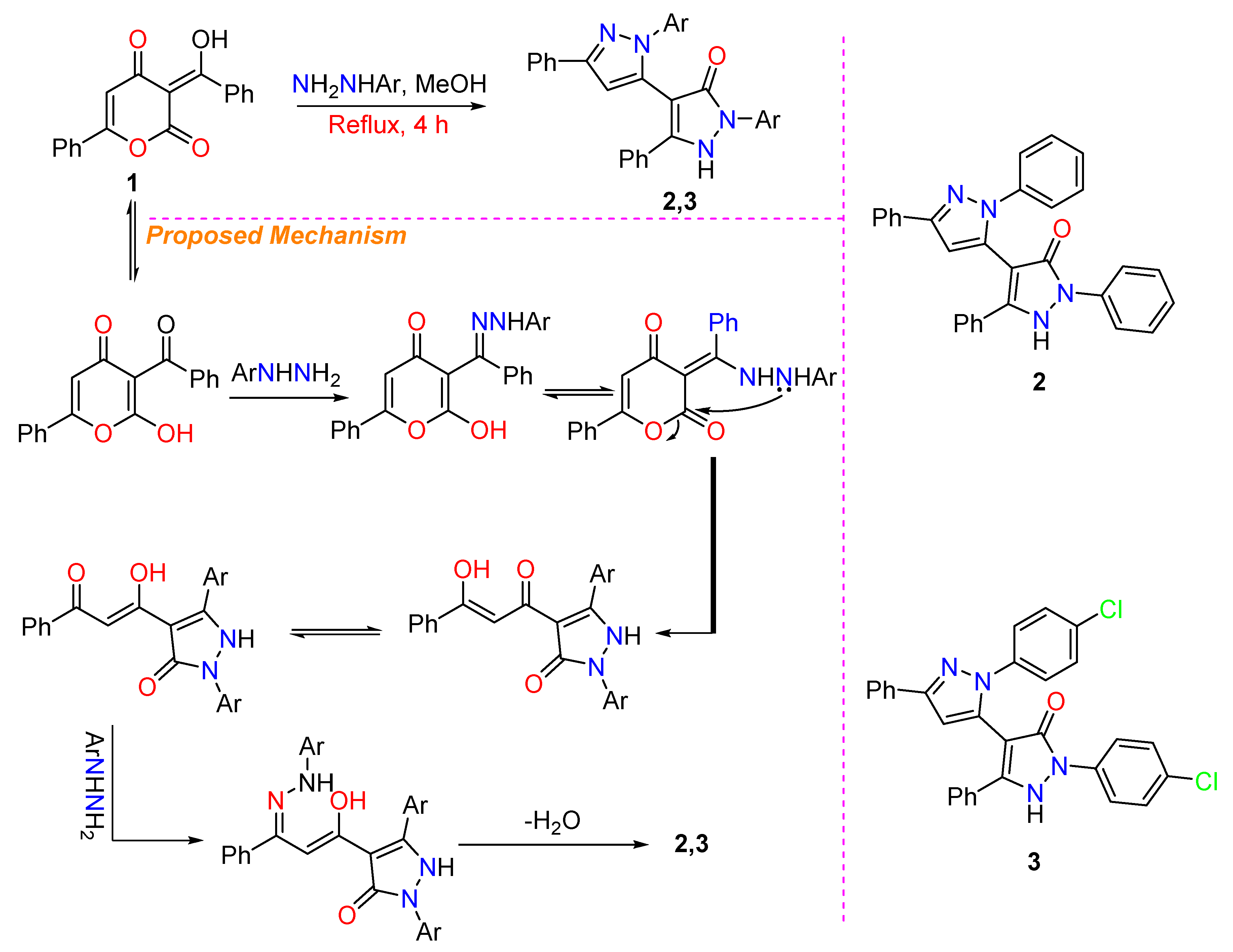
3.1. Synthesis of 2 and 3
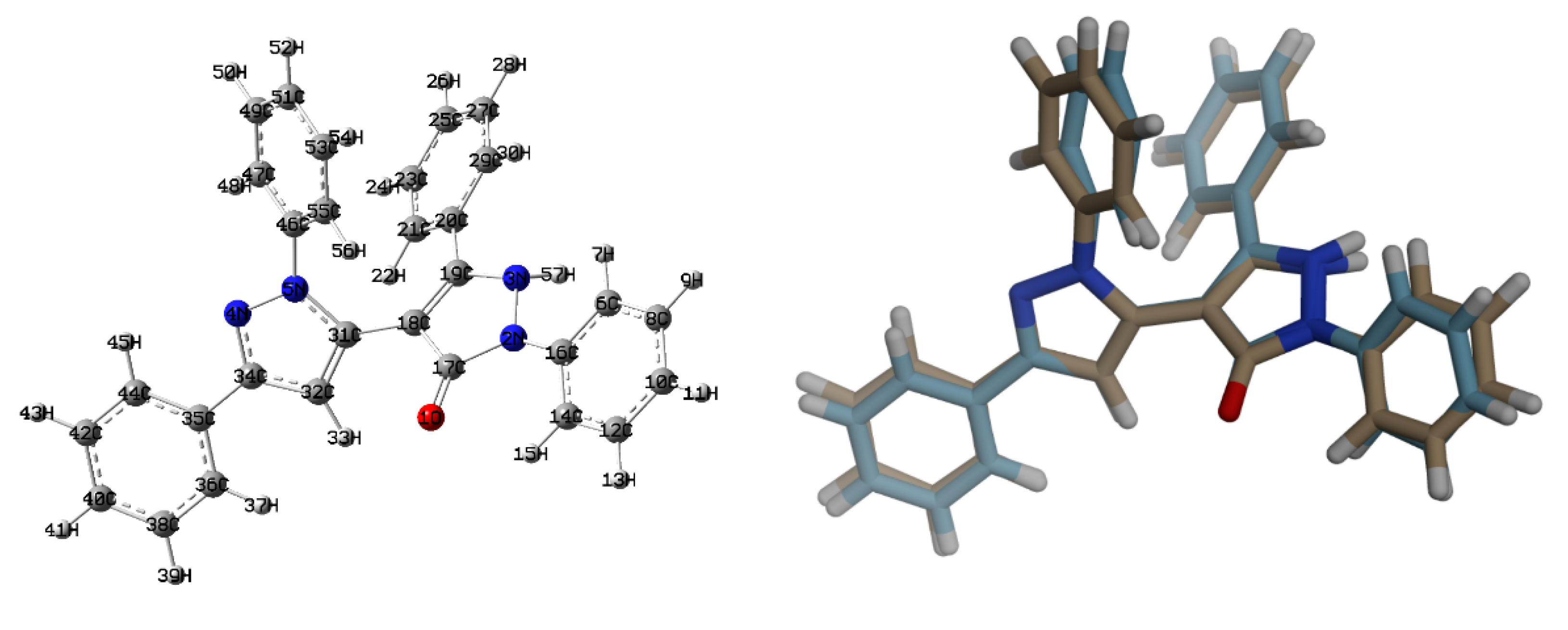
3.2. Crystal Structure Description of 2
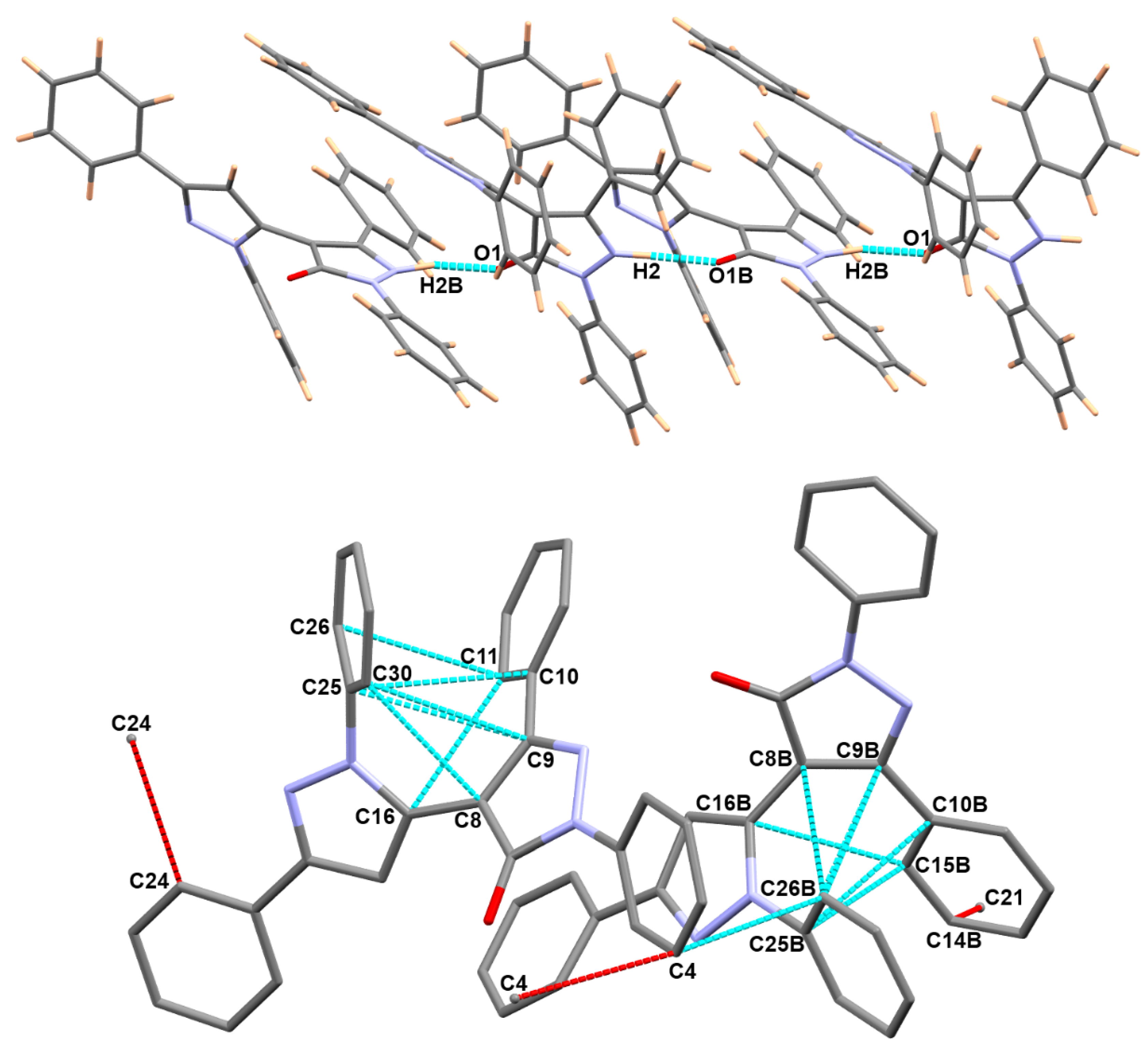
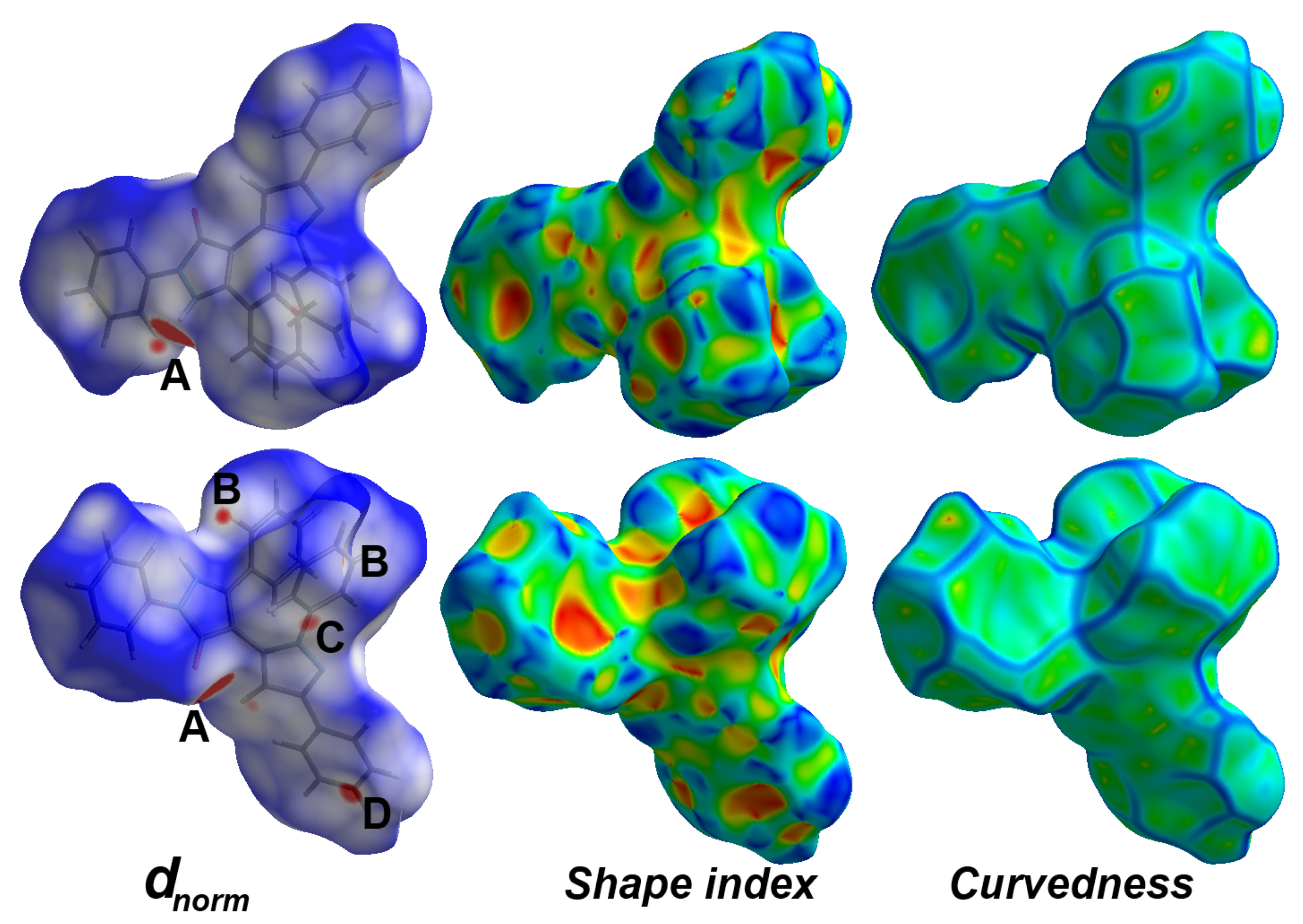
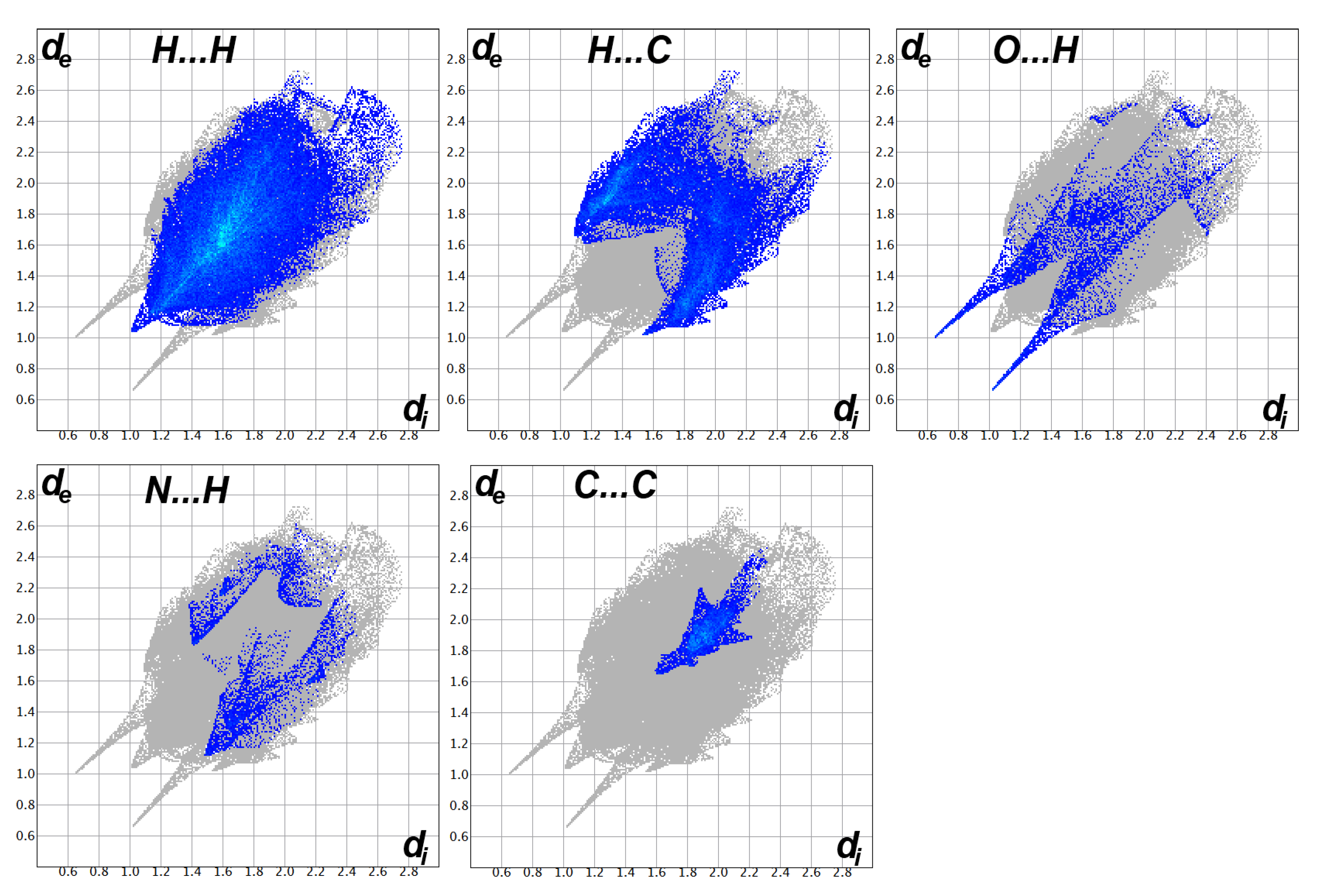
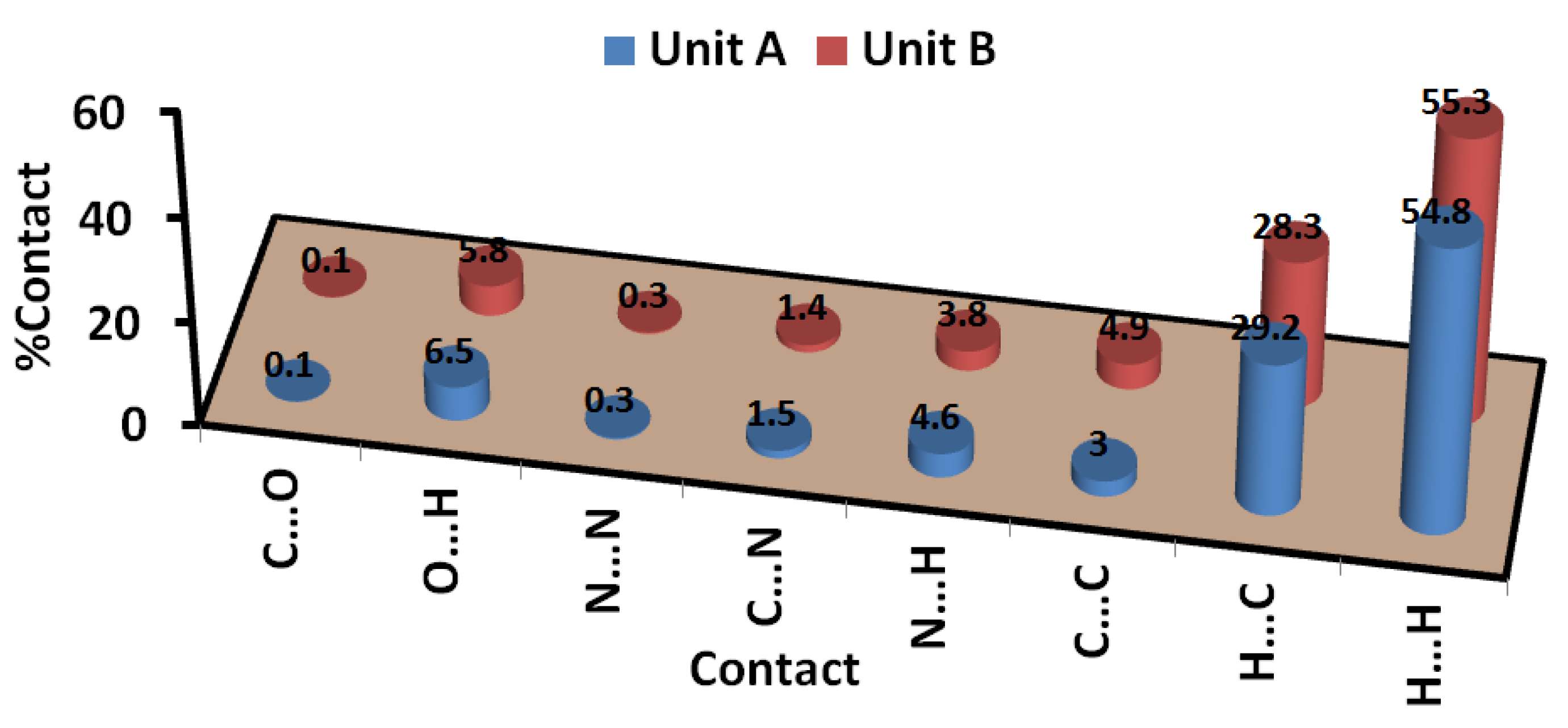
3.3. Analysis of Molecular Packing
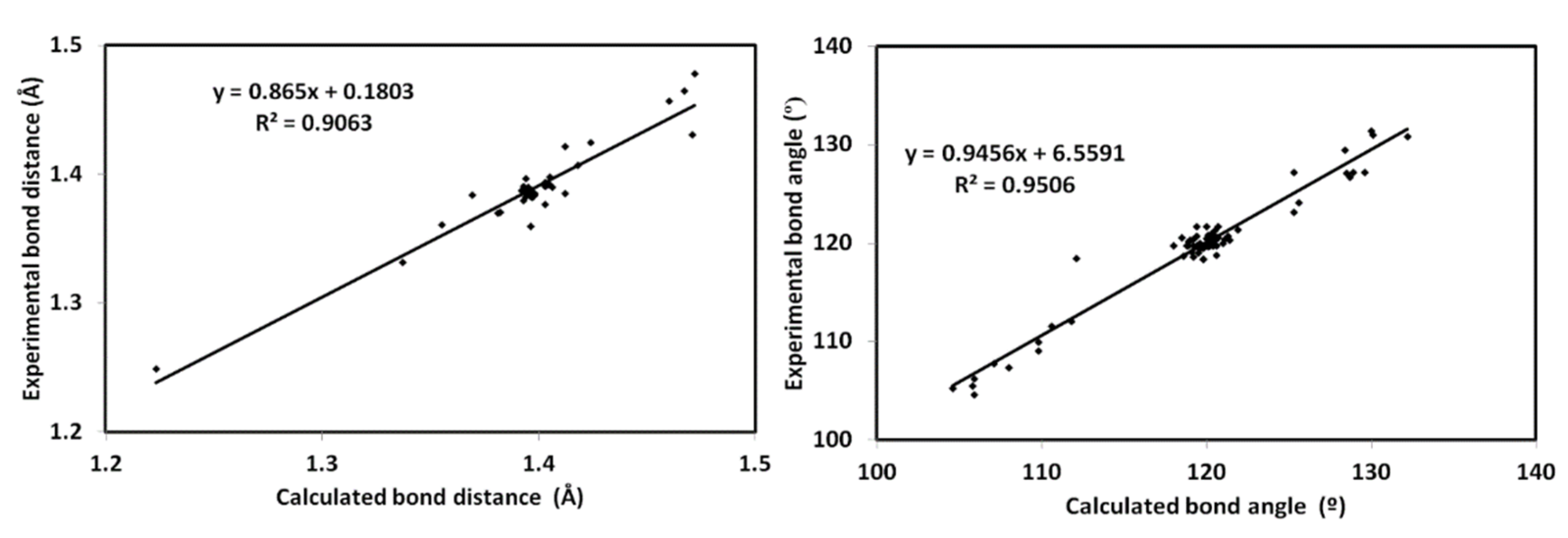
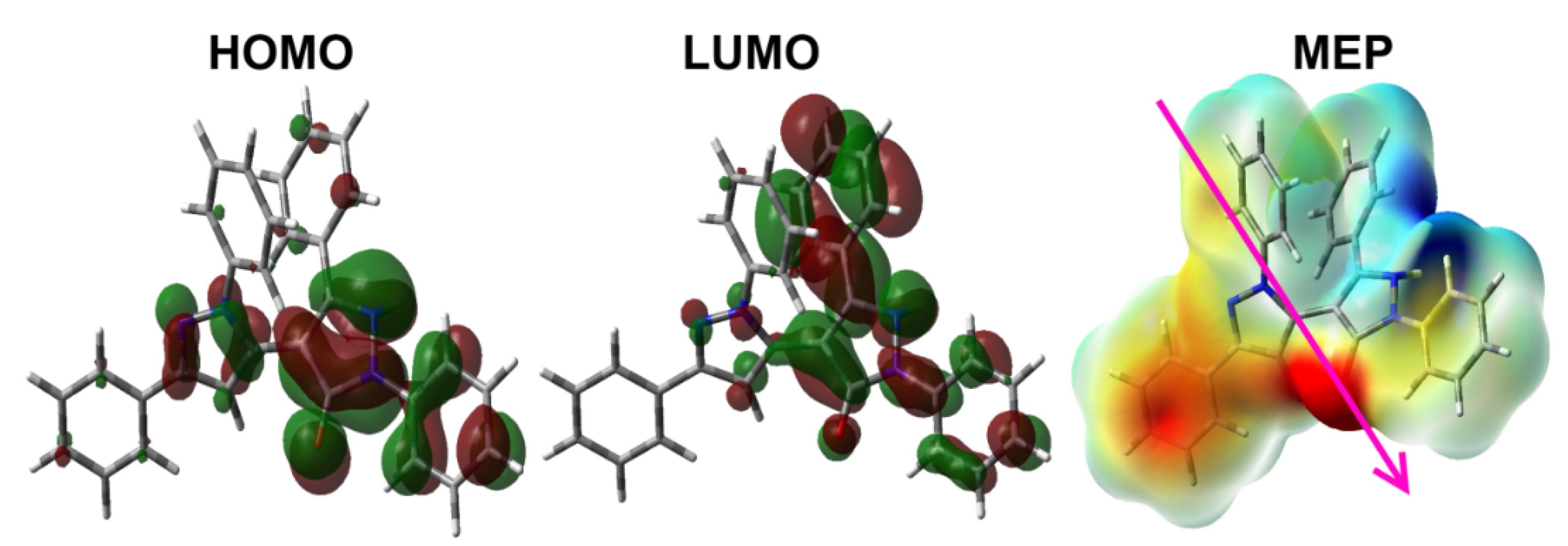
3.4. DFT Studies
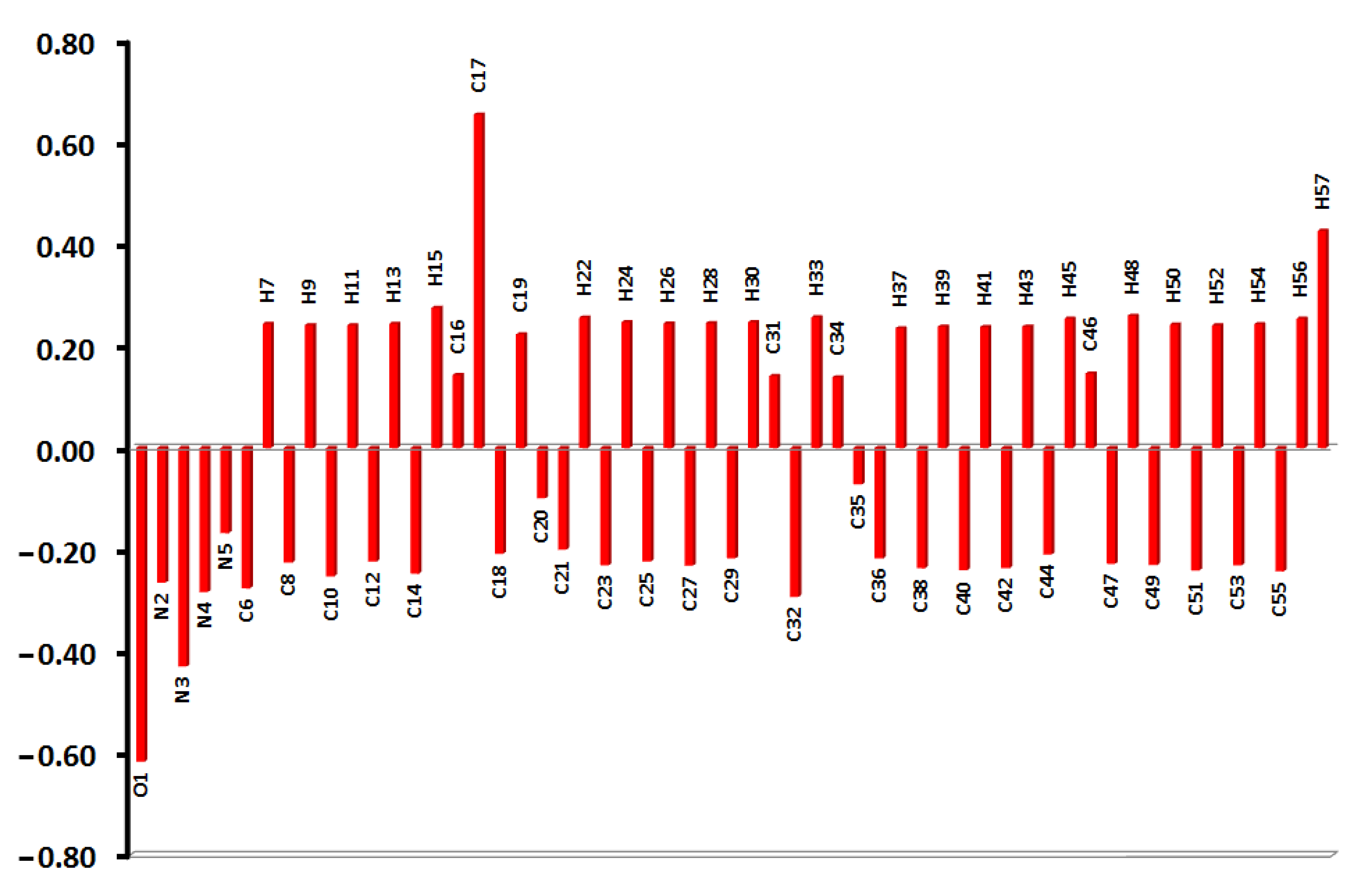
3.5. NBO Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.S. Recent advances in the synthesis of 2-pyrones. Mar. Drugs 2015, 13, 1581–1620. [Google Scholar] [CrossRef] [Green Version]
- Fisch, M.H.; Flick, B.H.; Arditti, J. Structure and antifungal activity of hircinol, loroglossol and orchinol. Phytochemistry 1973, 12, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.R.; Li, Q.; Bhushan, V. Intramolecular biaryl coupling: Asymmetric synthesis of the chiral B-ring diol unit of pradimicinone. Tetrahedron Lett. 1990, 31, 161–164. [Google Scholar] [CrossRef]
- Boger, D.L.; Mullican, M.D. Regiospecific total synthesis of juncusol. J. Org. Chem. 1984, 49, 4045–4050. [Google Scholar] [CrossRef]
- Rabideau, P.W.; Harvey, R.G. Metal-ammonia reduction. VII. Stereospecific re- duction in the phenanthrene series. J. Org. Chem. 1970, 35, 25–30. [Google Scholar] [CrossRef]
- Goel, A.; Ram, V.J. Natural and synthetic 2 H-pyran-2-ones and their versatility in organic synthesis. Tetrahedron 2009, 65, 7865–7913. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kobayashi, S.; Nishikiori, T.; Nakagawa, T.; Tatsuta, K. NK10958P, a novel plant growth regulator produced by Streptomyces sp. J. Antibiot. 1997, 50, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Birkbeck, A.A.; Enders, D. The total synthesis of (+)-pectinatone: An iterative alkylation approach based on the SAMP-hydrazone method. Tetrahedron Lett. 1998, 39, 7823–7826. [Google Scholar] [CrossRef]
- Costa, S.S.; Jossang, A.; Bodo, B. 4′′′′-Acetylsagittatin A, a kaempferol triglycoside from Kalanchoe streptantha. J. Nat. Prod. 1996, 59, 327–329. [Google Scholar] [CrossRef]
- Parker, S.R.; Cutler, H.G.; Jacyno, J.M.; Hill, R.A. Biological activity of 6-pentyl-2-H-pyran-2-one and its analogs. J. Agric. Food Chem. 1997, 45, 2774–2776. [Google Scholar] [CrossRef]
- Nair, M.G.; Chandra, A.; Thorogood, D.L. Griseulin, a new nitro-containing bioactive metabolite produced by Streptomyces spp. J. Antibiot. 1993, 46, 1762–1763. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Nishiyama, S.; Yamamura, S. Structural revision of griseulin, a bioactive pyrone possessing a nitrophenyl unit. Chem. Lett. 1994, 1747–1748. [Google Scholar] [CrossRef]
- Xue, F.; Li, X.; Wan, B. A class of benzene backbone-based olefin-sulfoxide ligands for Rh-catalyzed enantioselective addition of arylboronic acids to enones. J. Org. Chem. 2011, 76, 7256–7262. [Google Scholar] [CrossRef]
- Gianni, J.; Pirovano, V.; Abbiati, G. Silver triflate/p-TSA co-catalysed synthesis of 3-substituted isocoumarins from 2-alkynylbenzoates. Org. Biomol. Chem. 2018, 16, 3213–3219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, in press. [Google Scholar] [CrossRef]
- Shaaban, M.R.; Mayhoub, A.S.; Farag, A.M. Recent advances in the therapeutic applications of pyrazolines. Expert. Opin. Ther. Pat. 2012, 22, 253–291. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M.Z. Unveiling a versatile heterocycle: Pyrazoline—A review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef] [Green Version]
- Tok, F.; Abas, B.I.; Çevik, O.; Koçyiğit-Kaymakçıoğlu, B. Design, synthesis and biological evaluation of some new 2-pyrazoline derivatives as potential anticancer agents. Bioorg. Chem. 2020, 102, 104063. [Google Scholar] [CrossRef]
- Yusuf, M.; Jain, P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab. J. Chem. 2014, 7, 553–596. [Google Scholar] [CrossRef] [Green Version]
- Kariuki, B.M.; Abdel-Wahab, B.F.; El-Hiti, G.A. Synthesis and structural characterization of isostructural 4-(4-Aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals 2021, 11, 795. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Al-Majid, A.M.; Barakat, A.; Alshahrani, S.; Siddiqui, Y. 1,1′-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as building block in heterocyclic synthesis. Novel synthesis of some pyrazoles, and pyrimidines derivatives. Molecules 2011, 16, 6502–6511. [Google Scholar] [CrossRef] [Green Version]
- Mabkhot, Y.N.; Barakat, A.; Al-Majid, A.M.; Al-Othman, Z.A.; Alamary, A.S. A Facile and convenient synthesis of some novel hydrazones, schiff’s base and pyrazoles incorporating thieno [2,3-b]thiophenes. Int. J. Mol. Sci. 2011, 12, 7824–7834. [Google Scholar] [CrossRef] [Green Version]
- Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Al-Showiman, S.S.; Barakat, A.; Ghabbour, H.A.; Frey, W. Synthesis, in-vitro antibacterial, antifungal, and molecular modeling of potent anti-microbial agents from a combined pyrazole and thiophene pharmacophore. Molecules 2015, 20, 8712–8729. [Google Scholar] [CrossRef] [Green Version]
- Elshaier, Y.A.M.M.; Barakat, A.; Al-Qahtany, B.M.; Al-Majid, A.M.; Al-Agamy, M.H. Synthesis of pyrazole-thiobarbituric acid derivatives: Antimicrobial activity and docking studies. Molecules 2016, 21, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, A.; Al-Qahtani, B.M.; Al-Majid, A.M.; Ali, M.; Mabkhot, Y.N.; Al-Agamy, M.H.M.; Wadood, A. Synthesis, characterization, anti-microbial activity and molecular docking studies of combined pyrazol-barbituric acid pharmacophore. Trop. J. Pharm. Res. 2016, 15, 1319–1326. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Majid, A.; Al-Qahtani, B.M.; Ali, M.; Teleb, M.; Al-Agamy, M.H.M.; Naz, S.; Ul-Haq, Z. Synthesis, antimicrobial activity, pharmacophore modeling and molecular docking studies of new pyrazole-dimedone hybrid architectures. Chem. Cent. J. 2018, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Lellek, V.; Chen, C.-Y.; Yang, W.; Liu, J.; Ji, X.; Faessler, R. An efficient synthesis of substituted pyrazoles from one-pot reaction of ketones, aldehydes, and hydrazine monohydrochloride. Synlett 2018, 29, 1071–1075. [Google Scholar] [CrossRef] [Green Version]
- Alex, K.; Tillack, A.; Schwarz, N.; Beller, M. Zinc-catalyzed synthesis of pyrazolines and pyrazoles via hydrohydrazination. Org. Lett. 2008, 10, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.A.M.; Haukka, M.; Barakat, A.; Boraei, A. A novel synthetic approach to pyran-2,4-dione scaffold production:Microwave-assisted dimerization, cyclization, and expeditiousregioselective conversion intob-enamino-pyran-2,4-diones. Tetrahedron Lett. 2020, 61, 152660. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Haukka, M.; Sarhan, A.A.M.; Soliman, S.M.; Barakat, A. Intramolecular hydrogen bond, hirshfeld analysis, AIM; DFT studies of pyran-2,4-dione derivatives. Crystals 2021, 11, 896. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, E. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Joe, I.H.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Pinzaru, S.C. Theoretical and vibrational spectral investigation of sodium salt of acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar]
- Sebastian, S.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef]
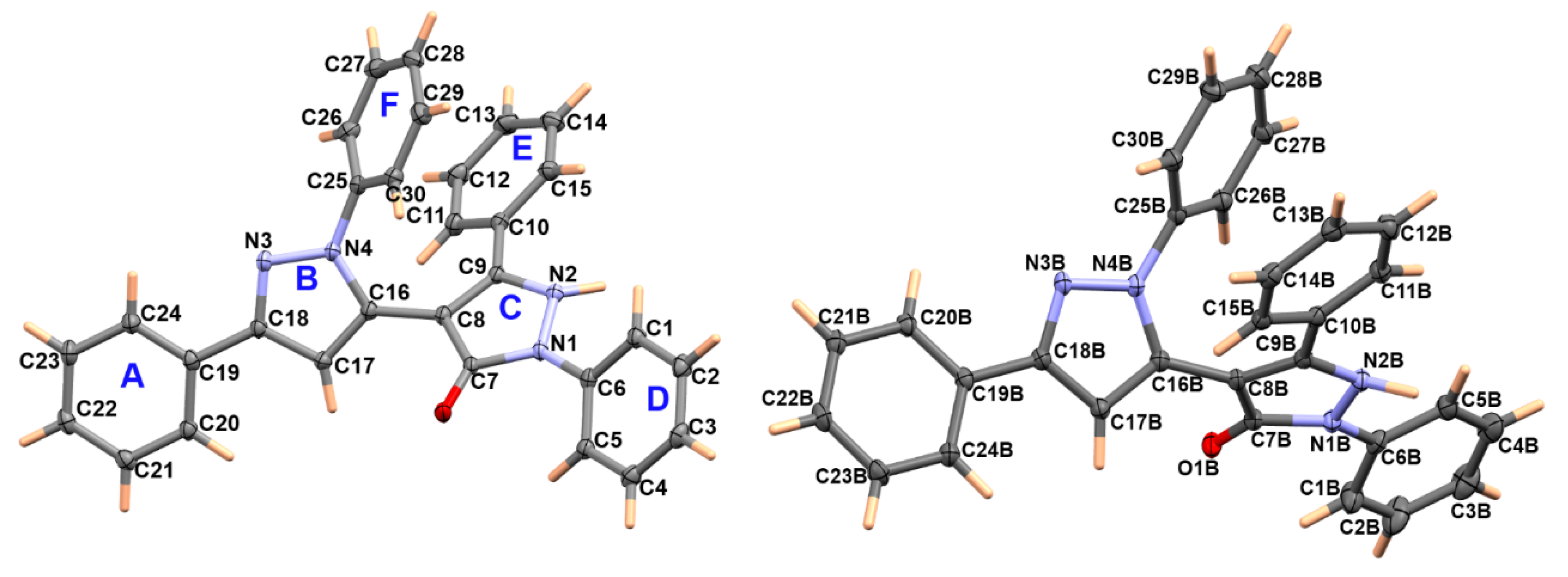
| 2 | |
|---|---|
| CCDC no. | 2095217 |
| empirical formula | C30H22N4O |
| Fw | 454.51 |
| temp (K) | 120(2) |
| λ(Å) | 1.54184 |
| cryst syst | Triclinic |
| space group | P?1 |
| a (Å) | 11.0023(4) |
| b (Å) | 11.9332(5) |
| c (Å) | 18.0128(7) |
| α(deg) | 85.276(3) |
| β (deg) | 84.517(3) |
| γ(deg) | 85.240(3) |
| V (Å3) | 2339.40(16) |
| Z | 4 |
| ρcalc (Mg/m3) | 1.290 |
| μ(Mo Kα) (mm−1) | 0.634 |
| No. reflns. | 17900 |
| Unique reflns. | 9577 |
| Completeness to θ = 67.684° (%) | 99.8 |
| GOOF (F2) | 1.034 |
| Rint | 0.0419 |
| R1 a (I ≥ 2σ) | 0.0485 |
| wR2 b (I ≥ 2σ) | 0.1153 |
| Atoms | Distance | Atoms | Distance |
| O1–C7 | 1.249(2) | O1B–C7B | 1.250(2) |
| N1–N2 | 1.377(2) | N1B–N2B | 1.387(2) |
| N1–C7 | 1.385(2) | N1B–C7B | 1.395(2) |
| N1–C6 | 1.422(2) | N1B–C6B | 1.410(2) |
| N2–C9 | 1.360(2) | N2B–C9B | 1.354(2) |
| N3–C18 | 1.332(3) | N3B–C18B | 1.345(2) |
| N3–N4 | 1.361(2) | N3B–N4B | 1.366(2) |
| N4–C16 | 1.370(2) | N4B–C16B | 1.365(2) |
| N4–C25 | 1.425(2) | N4B–C25B | 1.431(2) |
| Atoms | Angle | Atoms | Angle |
| N2–N1–C7 | 110.01(15) | C5–C6–N1 | 118.42(17) |
| N2–N1–C6 | 121.68(15) | O1–C7–N1 | 123.25(16) |
| C7–N1–C6 | 127.14(15) | O1–C7–C8 | 131.41(17) |
| C9–N2–N1 | 107.80(15) | N1–C7–C8 | 105.28(15) |
| C18–N3–N4 | 104.58(15) | C9–C8–C7 | 107.45(16) |
| N3–N4–C16 | 112.08(15) | C9–C8–C16 | 130.99(17) |
| N3–N4–C25 | 120.64(16) | C7–C8–C16 | 121.41(16) |
| C16–N4–C25 | 127.25(16) | N2–C9–C8 | 109.10(16) |
| C6–C1–C2 | 118.41(19) | N2–C9–C10 | 119.81(16) |
| C3–C2–C1 | 120.4(2) | C8–C9–C10 | 130.96(17) |
| C2–C3–C4 | 120.37(19) | C15–C10–C11 | 119.13(19) |
| C3–C4–C5 | 120.4(2) | C15–C10–C9 | 120.99(17) |
| C4–C5–C6 | 118.60(19) | C11–C10–C9 | 119.84(17) |
| C1–C6–C5 | 121.75(18) | C12–C11–C10 | 120.09(19) |
| C1–C6–N1 | 119.82(18) | ||
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| N2-H2…O1B | 0.95(3) | 1.73(3) | 2.681(2) | 173(3) |
| N2B-H2B…O1 #1 | 1.01(3) | 1.65(3) | 2.648(2) | 166(3) |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| C8…C30 | 3.195 | C8B…C26B | 3.127 |
| C9…C25 | 3.287 | C9B…C25B | 3.342 |
| C9…C30 | 3.316 | C9B…C26B | 3.296 |
| C10…C25 | 3.307 | C10B…C25B | 3.331 |
| C11…C16 | 3.303 | C15B…C16B | 3.142 |
| C11…C26 | 3.335 | C15B…C25B | 3.336 |
| C4…C26B | 3.264 | C4…C4 | 3.273 ii |
| C24…C24 | 3.39 i | C21…C14B | 3.369 iii |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| O1B…H2 | 1.677 | H30…C4B | 2.776 |
| O1…H2B | 1.658 | H2…C7B | 2.613 |
| O1…H5B | 2.465 | H12B…C28 | 2.761 |
| H23…C12 | 2.726 | H2B…C7 | 2.608 |
| H14B…C21 | 2.769 | H3…H11B | 2.056 |
| H17…C21B | 2.559 | C4…C26B | 3.264 |
| NBO i | NBO j | E(2) | Donor NBO i | Acceptor NBO j | E(2) |
|---|---|---|---|---|---|
| BD(1) C17–C18 | BD*(1) C19–C20 | 6.61 | BD(2) N4–C34 | BD*(2) C31–C32 | 12.59 |
| BD(1) C18–C31 | BD*(1) C18–C19 | 5.00 | BD(2) N4–C34 | BD*(2) C35–C36 | 9.42 |
| BD(1) C31–C32 | BD*(1) N5–C46 | 5.06 | BD(2) C6–C16 | BD*(2) C8–C10 | 20.71 |
| BD(1) C31–C32 | BD*(1) C34–C35 | 5.39 | BD(2) C6–C16 | BD*(2) C12–C14 | 17.34 |
| BD(1) C32–C34 | BD*(1) C18–C31 | 6.78 | BD(2) C8–C10 | BD*(2) C6–C16 | 19.45 |
| BD(2) C8–C10 | BD*(2) C12–C14 | 20.87 | |||
| LP(1) N4 | BD*(1) N5–C31 | 7.25 | BD(2) C12–C14 | BD*(2) C6–C16 | 21.86 |
| LP(1) N4 | BD*(1) C32–C34 | 5.38 | BD(2) C12–C14 | BD*(2) C8–C10 | 19.25 |
| BD(2) C18–C19 | BD*(2) C20–C29 | 7.95 | |||
| LP(1) N2 | BD*(2) C6–C16 | 29.57 | BD(2) C18–C19 | BD*(2) C31–C32 | 5.57 |
| LP(1) N3 | BD*(2) C18–C19 | 20.16 | BD(2) C20–C29 | BD*(2) C18–C19 | 13.95 |
| LP(1) N5 | BD*(2) N4–C34 | 28.45 | BD(2) C20–C29 | BD*(2) C21–C23 | 19.16 |
| LP(1) N5 | BD*(2) C31–C32 | 36.90 | BD(2) C20–C29 | BD*(2) C25–C27 | 19.32 |
| LP(1) N5 | BD*(2) C46–C55 | 17.17 | BD(2) C21–C23 | BD*(2) C20–C29 | 19.89 |
| BD(2) C21–C23 | BD*(2) C25–C27 | 20.53 | |||
| BD(2) C25–C27 | BD*(2) C20–C29 | 21.42 | |||
| BD(2) C25–C27 | BD*(2) C21–C23 | 19.25 | |||
| BD(2) C31–C32 | BD*(2) N4–C34 | 27.10 | |||
| BD(2) C31–C32 | BD*(2) C18–C19 | 6.37 | |||
| BD(2) C35–C36 | BD*(2) N4–C34 | 20.01 | |||
| BD(2) C35–C36 | BD*(2) C38–C40 | 20.92 | |||
| BD(2) C35–C36 | BD*(2) C42–C44 | 19.13 | |||
| BD(2) C38–C40 | BD*(2) C35–C36 | 19.85 | |||
| BD(2) C38–C40 | BD*(2) C42–C44 | 19.52 | |||
| BD(2) C42–C44 | BD*(2) C35–C36 | 19.90 | |||
| BD(2) C42–C44 | BD*(2) C38–C40 | 20.31 | |||
| BD(2) C46–C55 | BD*(2) C47–C49 | 19.29 | |||
| BD(2) C46–C55 | BD*(2) C51–C53 | 20.04 | |||
| BD(2) C47–C49 | BD*(2) C46–C55 | 20.93 | |||
| BD(2) C47–C49 | BD*(2) C51–C53 | 20.13 | |||
| BD(2) C51–C53 | BD*(2) C46–C55 | 20.39 | |||
| BD(2) C51–C53 | BD*(2) C47–C49 | 20.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boraei, A.T.A.; Haukka, M.; Sarhan, A.A.M.; Soliman, S.M.; Al-Majid, A.M.; Barakat, A. Synthesis and X-ray Crystal Structure of New Substituted 3-4′-Bipyrazole Derivatives. Hirshfeld Analysis, DFT and NBO Studies. Crystals 2021, 11, 953. https://doi.org/10.3390/cryst11080953
Boraei ATA, Haukka M, Sarhan AAM, Soliman SM, Al-Majid AM, Barakat A. Synthesis and X-ray Crystal Structure of New Substituted 3-4′-Bipyrazole Derivatives. Hirshfeld Analysis, DFT and NBO Studies. Crystals. 2021; 11(8):953. https://doi.org/10.3390/cryst11080953
Chicago/Turabian StyleBoraei, Ahmed T. A., Matti Haukka, Ahmed A. M. Sarhan, Saied M. Soliman, Abdullah Mohammed Al-Majid, and Assem Barakat. 2021. "Synthesis and X-ray Crystal Structure of New Substituted 3-4′-Bipyrazole Derivatives. Hirshfeld Analysis, DFT and NBO Studies" Crystals 11, no. 8: 953. https://doi.org/10.3390/cryst11080953
APA StyleBoraei, A. T. A., Haukka, M., Sarhan, A. A. M., Soliman, S. M., Al-Majid, A. M., & Barakat, A. (2021). Synthesis and X-ray Crystal Structure of New Substituted 3-4′-Bipyrazole Derivatives. Hirshfeld Analysis, DFT and NBO Studies. Crystals, 11(8), 953. https://doi.org/10.3390/cryst11080953