Abstract
New non-symmetrical 1:1 supramolecular H-bonded (SMHB) interactions, Ix/II, were designed between the non-mesomorphic fatty acids (palmitic, oleic and linoleic acids) and 4-tetradecyloxyphenylazo pyridine. Mesophase behaviors of the formed complexes were examined via differential scanning calorimetry (DSC) and polarizing optical microscopy (POM). In order to confirm the H-bond interaction formations within the prepared SMHB complexes, FT-IR spectroscopy was established whereby Fermi bands confirm these interactions. Mesomorphic investigations for all complexes indicated that, independent of the terminal alkenyl chains of the natural acids, induced dimorphic smectic phases were observed. The stability of formed mesophases was found to depend on the degree of un-saturation of the terminal alkenyl group of acid component.
1. Introduction
Hydrogen-bonding (H-bonding) interactions are considered to be one of the good, fantastic strategies for the development of new supramolecular systems. Mesomorphic supramolecular H-bonded (SMHB) systems by intermolecular H-bonding interactions have attracted attention in application reports since the documents by Kato and Fréchet for designing this interaction between pyridyl and benzoic acid moieties to build SMHB liquid crystals [1,2,3,4]. These mesomorphic materials involved the non-covalent interactions and have essential applications for functional molecular geometries. The architectures of the LC molecules are mainly dependent on the molecular shape [5,6,7,8,9] and exhibit considerable role for mesophase phenomena. SMHB is one of the most known interactions in chemical and biological mechanisms in the association and aggregation of individual molecules. SMHB liquid crystals that are based on the pyridyl and the carboxylic components are widely reported [10,11,12,13,14,15].
Recently, cyano-substituted H-bonded complexes also have been investigated [16]. On the other hand, azobenzene derivatives are widely reported in many research fields [8,9,17,18,19]. Many azopyridine bases have been designed and evaluated extensively toward applications of liquid crystals [20,21,22]. Thermal stability of azobenzenes and their possibility of molecular-mobility, in response to light and heat, offer them for many photonic applications [23,24,25,26]. Additionally, their rigidity and linear geometry make them important for exhibiting LC phenomena [8,19]. Moreover, they can be incorporated in photoactive mesomorphic materials because they can easily undergo photo-induced trans/cis isomerization. Most of the investigated H-bonded LC systems are based on either rod-like [27,28,29,30,31,32] or angular [13] intermolecular H-bonding complexes.
Many SMHB complexes are prepared by mechanical grinding, solvent dissolving or heating of the H-bond blends without time-consuming or expensive chemical mechanisms [33,34,35]. Additionally, H-bonding between pyridines and benzoic acids was used to induce nematic (N), and smectic mesophases [36,37,38,39,40]. Recently, 1:2 molar ratio complexes were formed and investigated for their mesomorphic behavior [14,15]. These investigated mixtures process flexible acid core. It was found to exhibit induced N phase covering all terminal chain lengths of the base.
Continuing our investigations, the goal of present study is to examine the possibility of phase formation as a result of intermolecular H-bond interactions between both non-mesomorphic natural fatty acids Ix, and 4-tetradecyloxyphenylazo pyridine, [41] II. So, we could obtain new 1:1 SMHB complexes Ix/II (Figure 1) which are represented in Scheme 1. Various fatty acid derivatives have been used bearing the terminal alkenyl chains in order to monitor the effect of different unsaturation degree of alkenyl groups on the mesomorphic properties.
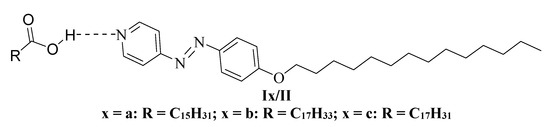
Figure 1.
The investigated supramolecular complexes Ix/II.
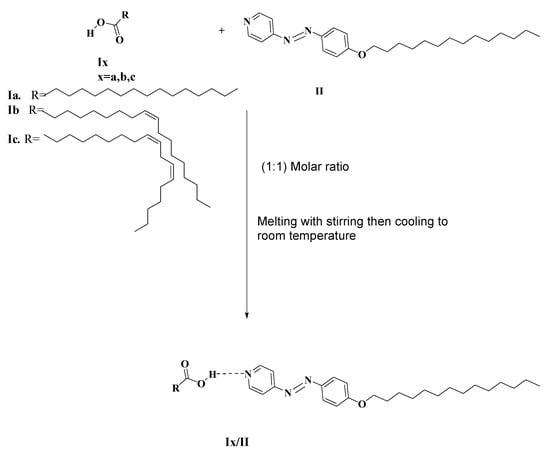
Scheme 1.
Steps of formation of 1:1 SMHB complexes (Ix/II).
2. Experimental
The azopyridine component, II, was synthesized according to a previous method [41] which is depicted in detail in Supplementary Information.
Preparation of 1:1 SMHB Complexes
Ix/II SMHB complexes were prepared from one mole of fatty acid (Ix) bearing different terminal alkenyl chains and one mole azopyridine base (II) having terminal tetradecyloxy chain. The two components of the mixture were melted together with stirring to prepare an intimate blend and then allowed to cool to 20 °C (Scheme 1). The preparation of the SMHB complexes (Ix/II) were affirmed via DSC and FT-IR spectroscopy.
3. Results and Discussion
3.1. FT-IR Spectroscopic Confirmation of Designed Complexes Ix/II
A representative example of FT-IR measurements were performed for individual molecules of the complex Ia/II, i.e., 4-tetradecyloxyphenylazo pyridine (II) and palmitic natural acid (Ia) as well as to their supramolecular complex (Ia/II). Collective FT-IR spectra are illustrated in Figure 2. FT-IR spectral data of the complex (Ia/II) confirm the formation of the H-bond interaction between the complementary acid and base components. As can be seen from Figure 2, the C=O group signal is assigned at 1699 cm−1 for the palmitic acid (Ia). The important confirmation of the formation of H-bonding interaction is the C=O and OH stretching vibration. It has been documented [41,42] that the induced three Fermi-bands (A-, B- and C-types) is an affirmation of the SMHB interactions. The Fermi-band of A-type for the complex Ia/II is overlapped near the C-H vibrational bands at 2928 to 2855 cm−1. In addition, the induced peak at 2650.0 cm−1 is assigned to B-type of the in-plane bending vibration of the OH group. Furthermore, the band at 1928.0 cm−1, related to C-type, is due to the interactions between the overtone of the torsional effects and the fundamental stretching vibrations of the OH group.
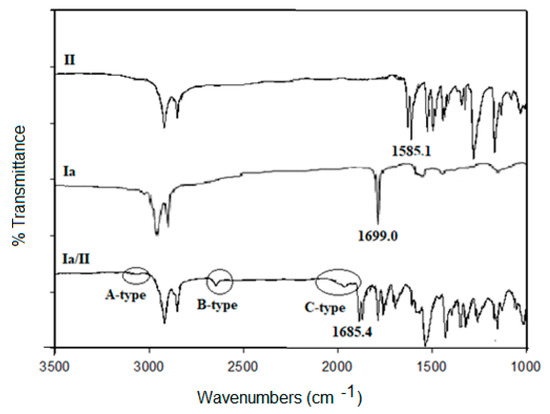
Figure 2.
Collective FT-IR spectra of Ia, II, and their complex Ia/II.
3.2. Mesomorphic Studies of 1:1 SMHB Complexes Ix/II
Optical and mesophase analyses for the designed un-symmetrical possible 1:1 complexes Ix/II, made from the three derivatives of the fatty acids Ix, and the base component II, were investigated via DSC and POM measurements. All determined data for the phase transitions of all formed complexes are collected in Table 1. DSC curves, taken from the second heating/cooling scans, for complex Ic/II are illustrated in Figure 3. Additionally, POM investigation images of Ic/II are displayed in Figure 4. DSC examinations are confirmed by the POM analyses. Transition temperatures of all characterized SMHB complexes are graphically illustrated in Figure 5 which is displayed to investigate the impact of the unsaturation degree of the flexible terminal alkenyl of the fatty acids on the mesomorphic transition behavior.

Table 1.
Phase transition temperatures (T, °C), enthalpy of transitions (∆H, kJ/mol), and normalized transition entropy (∆S) of supramolecular complexes Ix/II.
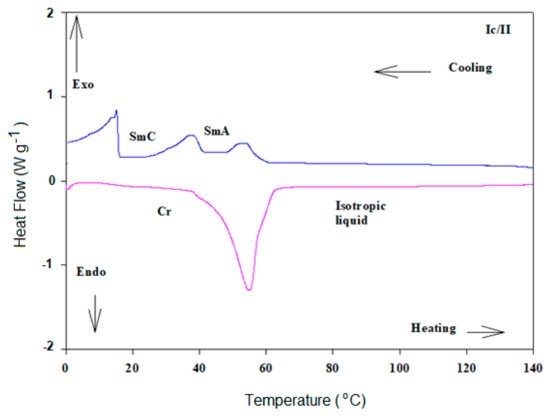
Figure 3.
DSC thermograms of second heating/cooling rounds of Ic/II complex at heating rate 10 °C min−1.
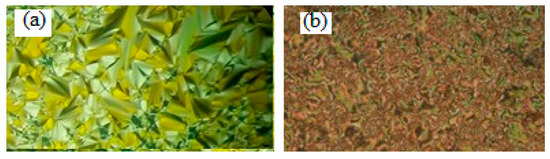
Figure 4.
Smectic phases’ textures upon cooling under POM for complex Ic/II (a) SmA phase at 44.0 °C and (b) SmC phase at 25.0 °C.
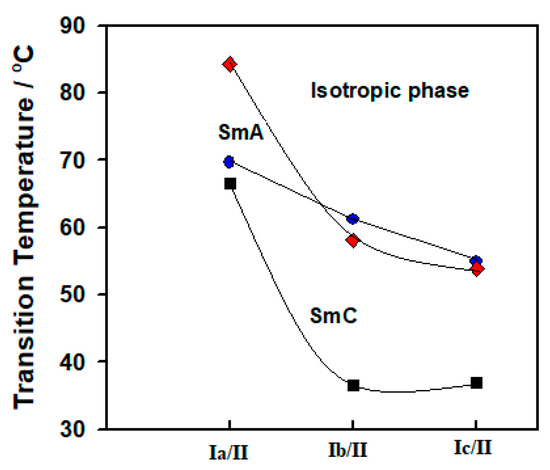
Figure 5.
Transition temperatures of designed complexes Ix/II.
Before the discussion of the mesophase behavior of designed complexes, it should be noted that each of the fatty acids (Ix) and the 4-tetradecyloxyphenylazo pyridine (II) are non-mesomorphic (i.e., are converted directly from the solid crystal to the isotropic liquid phase upon heating). As can be seen from Figure 5 and Table 1, all the designed SMHB complexes are mesomorphic having induced dimorphic phases. Data also revealed that the 1:1 complexes possess regular melting points with respect to the terminal chains. Their melting points were found to decrease with increasing the number of double bonds within the terminal chain of acid component (Ix). The linoleic acid complex (Ic/II) shows the lowest value of melting point upon heating, 54.9 °C. Moreover, Table 1 and Figure 5 show that the palmitic acid complex (Ia/II) exhibit induced only smectic A phase upon heating (enantiotropic property), while on cooling, it showed dimorphic induced SmA phase followed by SmC phase with relatively good temperature ranges. The smectic phase stabilities are 84.3 and 66.5 °C, respectively for SmA and SmC upon cooling of Ia/II. Thus, interactions of the H-bonding proved to be effective in the enhancement of thermal stability of the SmA and leads to formation of induced dimorphic phases. In case of the other two complexes Ib/II and Ic/II, their 1:1 molar ratios are dimorphic possessing monotropic SmA and SmC phases with lower thermal stabilities. The SmA and SmC phases’ stabilities for Ib/II are 58.1 and 36.6 °C, respectively through cooling cycle. While for Ic/II, the SmA and SmC mesophases stabilities are 53.9 and 37.0 °C, respectively.
Generally, the mesophase phenomenon of any LC system depends mainly on its mesomeric characters; intermolecular-interactions, and their molecular geometry. In the present investigated SMHB complexes Ix/II, the mesomorphic stabilities depend on many factors: 1. Lateral-adhesion of components which increases with the increment of the degree of unsaturation of the acid terminal chains. Additionally, the alkenyl chain length play considerable role in the induced mesophase. 2. Molecular shape which is affected by the steric hindrance of the terminal fatty acid chains. 3. End-to-end interactions which depend on the polarity and the degree of unsaturation of the terminal acid wings that result in changes in the polarizability. On the other hand, the linking moieties greatly impact the conjugation within the mesogenic unit of the molecule. It can be concluded that the high molecular anisotropy will influence the induced smectic phases which resulted from different conformation of the terminal alkenyl natural fatty acid chains that increases the length of the mesogenic part thus enhances the mesophase stability. Furthermore, the higher attractions between the longer alkenyl chains of natural acid increase the terminal interactions and alkenyl group aggregations which affect also their mesophase behavior.
3.3. Measurements of Entropy Changes
As evaluated from DSC data, the normalized entropy changes (∆S/R) were calculated for the investigated non-symmetric 1:1 complexes (Ix/II) and are included in Table 1. Graphical representation of the entropy changes from I-SmA and SmA-SmC transitions upon cooling scan for all designed complexes Ix/II are illustrated in Figure 6. As can be seen from Table 1 and Figure 6, increment of the entropy change is increased as the number of double bonds within the acid alkenyl chain is increased. Moreover, the ∆S/R of I-SmA transitions has lower magnitude compared to SmA-SmC entropy changes. These data are in agreement with the previous findings [43]. The increment of the entropy changes with the degree of saturation of terminal fatty acids may be due to the different bi-axiality of the mesogenic portion of the formed complex which results in the change of the conformational entropy, in addition to the different structural interactions between individual components of the complex, which are impacted by the molecular geometry of the molecule.
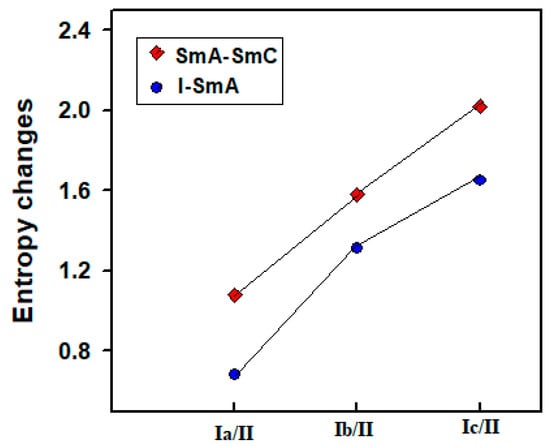
Figure 6.
Entropy changes upon cooling of mesomorphic transitions of designed complexes Ix/II.
3.4. Effect of Replacement of the Natural Fatty Acid Component on the Mesophase Behavior of Their Complex with 4-Alkoxy Phenylazo Benzoic Acid
To investigate the effect of exchange of the natural fatty acid component on the phase transition properties, a comparison was constructed between the mesomorphic behavior of present investigated SMHB complexes Ix/II, comprising a fatty acid component, and the previously investigated complexes IIIn/II [41] possessing 4-n-alkoxy phenylazo benzoic acid (Figure 7). The established comparison revealed that the replacement of the more rigid alkoxyphenylazo benzoic acid derivative with the natural fatty acid destabilizes the mesomorphic thermal stability and disrupts the N phase. The high stability of IIIn/II complexes is coming from the high polarizability of acid molecules, as well as the rigidity of individual components that leads to the high H-bonded intermolecular interactions within the latter case [44].
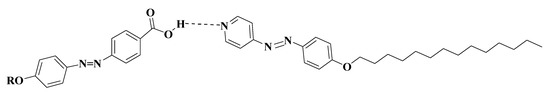
Figure 7.
The supramolecular complexes IIIn/II.
3.5. Thermogravimetric Characterizations
The thermogravimetric analysis (TG) of the designed complex Ia/II was investigated as an example. TG thermogram and its corresponding derivative (DTG) of Ia/II is illustrated in Figure 8. As can be seen from Figure 8, depending on the molecular geometry of the complex, the decomposition takes place via two degradation steps. The maximum degradation rate (Tmax) at 288 °C indicates that the complex is possessing good thermal stability. Additionally, it was found that the second decomposition step takes place between 300 °C and 350 °C with maximum rate at ≈320 °C. It can be concluded that the present evaluated complexes possess high thermal stabilities highly above the stability of the mesophase.
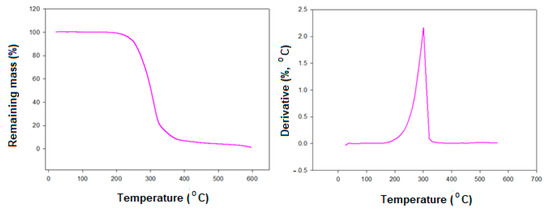
Figure 8.
TG (a) and DTG (b) thermograms of the complex Ia/II.
3.6. X-ray Diffraction (XRD) Measurements
The phase behavior of the present complexes (Ix/II) was affirmed by XRD analyses. XRD used as another tool to confirm the mesomorphic assignments [45,46]. Figure 9 shows the XRD for the complex Ia/II, as an example, for the present investigated series. The measurement was performed on cooling of the complex mixture from the isotropic liquid state. Figure 9 indicated that XRD analysis pattern showed two peaks at angles 2Ɵ = 50.0 and 25.0°, which are assigned to the presence of two smectic transition phases observed upon cooling. Thus, the XRD evaluations affirmed the presence of two monomorphic peaks of the SmA and SmC mesophases.
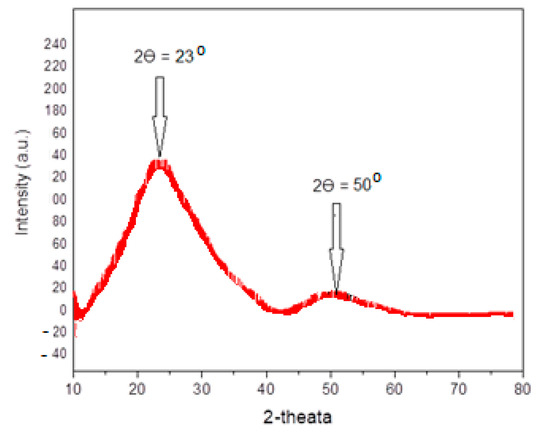
Figure 9.
XRD patterns of the complex Ia/II upon cooling from the isotropic liquid state.
4. Conclusions
Newly designed liquid crystal SMHB complexes based on natural fatty acids, formed from two non-mesomorphic components, were prepared and investigated mesomorphically. The SMHB interactions were confirmed by FT-IR spectroscopic analysis via the formation of the induced Fermi-bands. The optical and mesomorphic properties of designed 1:1 complexes were examined by POM and DSC tools. The results revealed that dimorphic smectic phases are induced in all prepared complexes. The thermal stabilities of the mesophase of the induced smectic A and C phases were found to depend on the unsaturation degree of the terminal alkenyl natural acid chains. Furthermore, the smectic C phase for all complexes were present near the room temperature upon cooling.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11080940/s1.
Author Contributions
Formal analysis, A.M.M., H.A.A., F.S.A. and A.A.A.; Funding acquisition, F.S.A. and H.A.A.; Methodology, A.A.A. and H.A.A.; Project administration, F.S.A.; Resources and Software, M.M.N. and H.A.A.; Writing—original draft, H.A.A., A.A.A., F.S.A., A.M.M. and M.M.N.; Writing—review and editing, H.A.A., A.M.M. and M.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sunil, B.; Srinatha, M.; Shanker, G.; Hegde, G.; Alaasar, M.; Tschierske, C. Effective tuning of optical storage devices using photosensitive bent-core liquid crystals. J. Mol. Liq. 2020, 304, 112719. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef]
- Brand, H.R.; Cladis, P.E.; Pleiner, H. Symmetry and defects in the CM phase of polymeric liquid crystals. Macromolecules 1992, 25, 7223–7226. [Google Scholar] [CrossRef]
- Cook, A.G.; Baumeister, U.; Tschierske, C. Supramolecular dendrimers: Unusual mesophases of ionic liquid crystals derived from protonation of DAB dendrimers with facial amphiphilic carboxylic acids. J. Mater. Chem. 2005, 15, 1708–1721. [Google Scholar] [CrossRef]
- Khushaim, M.S.; Alalawy, H.H.; Naoum, M.M.; Ahmed, H.A. Experimental and computational simulations of nematogenic liquid crystals based on cinnamic acid in pure and mixed state. Liq. Cryst. 2021, 1–12. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Naoum, M.M.; Al-Zahrani, S.A.; Ahmed, H.A. New Nitro-Laterally Substituted Azomethine Derivatives;Synthesis, Mesomorphic and Computational Characterizations. Molecules 2021, 26, 1927. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.; Ahmed, H.; Alalawy, H.; Al Alwani, M. Synthesis, Mesomorphic and Computational Characterizations of Nematogenic Schiff Base Derivatives in Pure and Mixed State. Molecules 2021, 26, 2038. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-atawy, M.A. Synthesis, Mesomorphic and Geometrical approaches of New non-symmetrical System based on central Naphthalene moiety. Liq. Cryst. 2021. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Alhaddad, O.A.; Ahmed, H.A. Experimental and geometrical structure characterizations of new synthesized laterally fluorinated nematogenic system. Liq. Cryst. 2021. [Google Scholar] [CrossRef]
- Kato, T.; Fukumasa, M.; Frechet, J. Supramolecular Liquid-Crystalline Complexes Exhibiting Room-Temperature Mesophases and Electrooptic Effects. Hydrogen-Bonded Mesogens Derived from Alkylpyridines and Benzoic Acids. Chem. Mater. 1995, 7, 368–372. [Google Scholar] [CrossRef]
- Fukumasa, M.; Kato, T.; Uryu, T.; Frechet, J. The Simplest Structure of the Hydrogen-Bonded Mesogen Built from 4-Alkoxybenzoic Acid and 4-Alkylpyridine. Chem. Lett. 1993, 22, 65–68. [Google Scholar] [CrossRef]
- Kato, T.; Fujishima, A.; Fréchet, J.M.J. Self-assembly of a twin liquid crystalline complex through intermolecular hydrogen bondings. Chem. Lett. 1990, 19, 919–922. [Google Scholar] [CrossRef]
- Kato, T.; Adachi, H.; Fujishima, A.; Frechet, J. Self-Assembly of Liquid Crystalline Complexes Having Angular Structures through Intermolecular Hydrogen Bonding. Chem. Lett. 1992, 21, 265–268. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Khushaim, M.S. Nematic Phase Induced from Symmetrical Supramolecular H-Bonded Systems Based on Flexible Acid Core. Crystals 2020, 10, 801. [Google Scholar] [CrossRef]
- Ahmed, H.; Khushaim, M.S. Nematogenic Laterally Substituted Supramolecular H-Bonded Complexes Based on Flexible Core. Crystals 2020, 10, 878. [Google Scholar] [CrossRef]
- Hagar, M.E.F.; Alnoman, R.B.; Jaremko, M.; Emwas AH, M.; Sioud, S.; Al-Ola, K.A.; Ahmed, H.A. New liquid crystal assemblies based on cyano-hydrogen bonding interactions. Front. Chem. 2021, 9, 679885. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Ahmed, H.A.; Alhaddad, O.A. New azobenzene-based natural fatty acid liquid crystals with low melting point: Synthesis, DFT calculations and binary mixtures. Liq. Cryst. 2019, 46, 2223–2234. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.; Ahmed, H.; El-Atawy, M. Synthesis, Optical and DFT Characterizations of Laterally Fluorinated Phenyl Cinnamate Liquid Crystal Non-Symmetric System. Symmetry 2021, 13, 1145. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Ahmed, H.A.; El-Atawy, M.A.; Abu Al-Ola, K.A.; Omar, A.Z. Synthetic, Mesomorphic, and DFT Investigations of New Nematogenic Polar Naphthyl Benzoate Ester Derivatives. Materials 2021, 14, 2587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Quan, M.; Zhang, L.; Yang, H.; Lu, Y. Photothermal effect of azopyridine compounds and their applications. RSC Adv. 2014, 5, 4675–4680. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Cuadrado, A.; Ortega, M.; Nonell, S.; Velasco, D. A photoswitchable bis-azo derivative with a high temporal resolution. Chem. Commun. 2014, 50, 11462–11464. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M. Azomesogens with a heterocyclic moiety. Bull. Mater. Sci. 2000, 23, 237–238. [Google Scholar] [CrossRef]
- Ikeda, T.; Wu, Y. Photoinduced alignment behavior of polymer liquid crystals containing azobenzene moieties in the side chain. Pure Appl. Chem. 1999, 71, 2131–2136. [Google Scholar] [CrossRef][Green Version]
- Eich, M.; Wendorff, J.H.; Reck, B.; Ringsdorf, H. Reversible digital and holographic optical storage in polymeric liquid crystals. Die Makromol. Chem. Rapid Commun. 1987, 8, 59–63. [Google Scholar] [CrossRef]
- Eich, M.; Wendorff, J.H. Erasable holograms in polymeric liquid crystals. Die Makromol. Chem. Rapid Commun. 1987, 8, 467–471. [Google Scholar] [CrossRef]
- Anderle, K.; Birenheide, R.; Werner, M.J.A.; Wendorff, J.H. Molecular addressing? Studies on light-induced reorientation in liquid-crystalline side chain polymers. Liq. Cryst. 1991, 9, 691–699. [Google Scholar] [CrossRef]
- Ikeda, T.; Mamiya, J.-I.; Yu, Y. Photomechanics of Liquid-Crystalline Elastomers and Other Polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. ChemInform Abstract: Azobenzene Photoswitches for Biomolecules. ChemInform 2011, 42, 4422–4437. [Google Scholar] [CrossRef]
- Tanaka, D.; Ishiguro, H.; Shimizu, Y.; Uchida, K. Thermal and photoinduced liquid crystalline phase transitions with a rod–disc alternative change in the molecular shape. J. Mater. Chem. 2012, 22, 25065–25071. [Google Scholar] [CrossRef]
- Alaasar, M.; Poppe, S.; Tschierske, C. Photoresponsive halogen bonded polycatenar liquid crystals. J. Mol. Liq. 2019, 277, 233–240. [Google Scholar] [CrossRef]
- Wolf, J.R.; Dyer, D.J. Hydrogen bonded liquid crystalline heterodimers incorporating alkoxystilbazoles and alkoxy-4-pyridones. Liq. Cryst. Today 2015, 24, 47–55. [Google Scholar] [CrossRef]
- He, W.; Pan, G.; Yang, Z.; Zhao, D.; Niu, G.; Huang, W.; Yuan, X.; Guo, J.; Cao, H.; Yang, H. Wide Blue Phase Range in a Hydrogen-Bonded Self-Assembled Complex of Chiral Fluoro-Substituted Benzoic Acid and Pyridine Derivative. Adv. Mater. 2009, 21, 2050–2053. [Google Scholar] [CrossRef]
- Miranda, M.D.; Chávez, F.V.; Maria, T.M.; Eusebio, M.E.S.; Sebastião, P.; Silva, M.R. Self-assembled liquid crystals by hydrogen bonding between bipyridyl and alkylbenzoic acids: Solvent-free synthesis by mechanochemistry. Liq. Cryst. 2014, 41, 1743–1751. [Google Scholar] [CrossRef]
- Kohlmeier, A.; Dietmar, J. Hydrogen-bonded block mesogens derived from semiperfluorinated benzoic acids and the non-mesogenic 1,2-bis(4-pyridyl)ethylene. Liq. Cryst. 2007, 34, 65–71. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Kato, T.; Kamikawa, Y. Hydrogen-Bonded Systems: Discrete Defined Aggregates by Intermolecular H-Bonding, Amides, Carboxylic Acids, and Heterocycles. Handb. Liq. Cryst. 2014, 5, 1–28. [Google Scholar]
- Jansze, S.M.; Martinez-Felipe, A.; Storey, J.; Marcelis, A.T.M.; Imrie, C.T. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. Int. Ed. 2014, 54, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Janietz, D.; Bauer, M. Chromophoric poly (vinyl alcohol derivative) s, 1. Synthesis and spectroscopical characterization of some poly (vinyl alcohol) s with alkoxyazobenzenecarbonyl substituents. Macromol. Chem. Phys. 1991, 192, 2635–2640. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; TM Marcelis, A.; MD Storey, J.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1:1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Alnoman, R.; Al-Nazawi, F.K.; Ahmed, H.A.; Hagar, M. Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Y.; Jia, Y.-G.; Yao, D.-S.; Dong, X.-W. Preparation and properties of siloxane liquid crystalline elastomers with a mesogenic crosslinking agent. Liq. Cryst. 2004, 31, 339–345. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
