Reinforcement of Recycled Aggregate by Microbial-Induced Mineralization and Deposition of Calcium Carbonate—Influencing Factors, Mechanism and Effect of Reinforcement
Abstract
:1. Introduction
2. Microbially Induced Calcium Carbonate Precipitation (MICP) Technology
- Bioactivity controlled calcium carbonate precipitate—the process of mineralization and precipitation is directly controlled by cell activities. Mineral precipitates are synthesized in cells or at designated locations on cells. The whole process of the nucleation and growth of mineralized products is greatly dependent on biological activities and requires intense mineralization conditions, so it can only be carried out under specific conditions [30,31];
- Biological metabolic control calcium carbonate precipitate—the process of mineralization precipitation is influenced by cell activities. Biological metabolic reactions related to biofilms cause the accumulation of organic substances or extracellular polymers on the cell surface, leading to the precipitation of calcium carbonate [31];
- Biologically induced calcium carbonate precipitate—mineralization precipitation is induced by cell activities. The metabolic activities of microorganisms cause changes in the surrounding chemical environment. Metal ions are attracted by the negatively charged CO32− generated by respiration on the cell wall surface, which promotes mineral precipitation on the cell wall surface [31,32].
3. Factors Influencing the Mineralized Sedimentation
3.1. Bacteria
3.2. Calcium Sources
3.3. Composition of the Culture Medium
3.4. Effect of pH
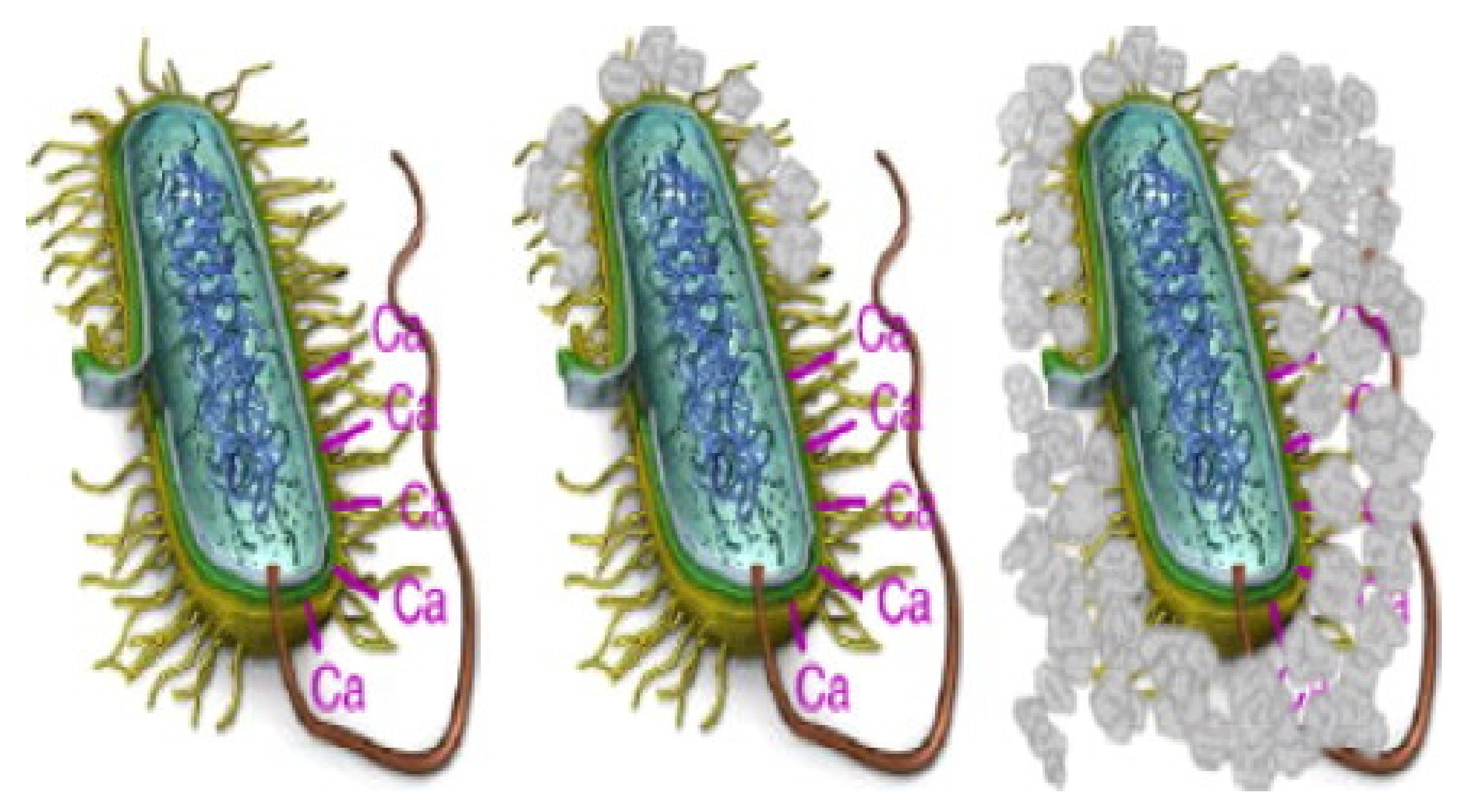
3.5. Availability of the Nucleation Center
3.6. Temperature
4. Reinforcement Mechanism
4.1. Mineralization Mechanism of Ureolytic Bacteria
4.2. Mineralization Mechanism of Nonureolytic Bacteria
4.2.1. Fermentation of Fatty Acids
4.2.2. Carbonic Anhydrase Catalysis
4.2.3. Denitrification
4.2.4. Sulphur Cycle
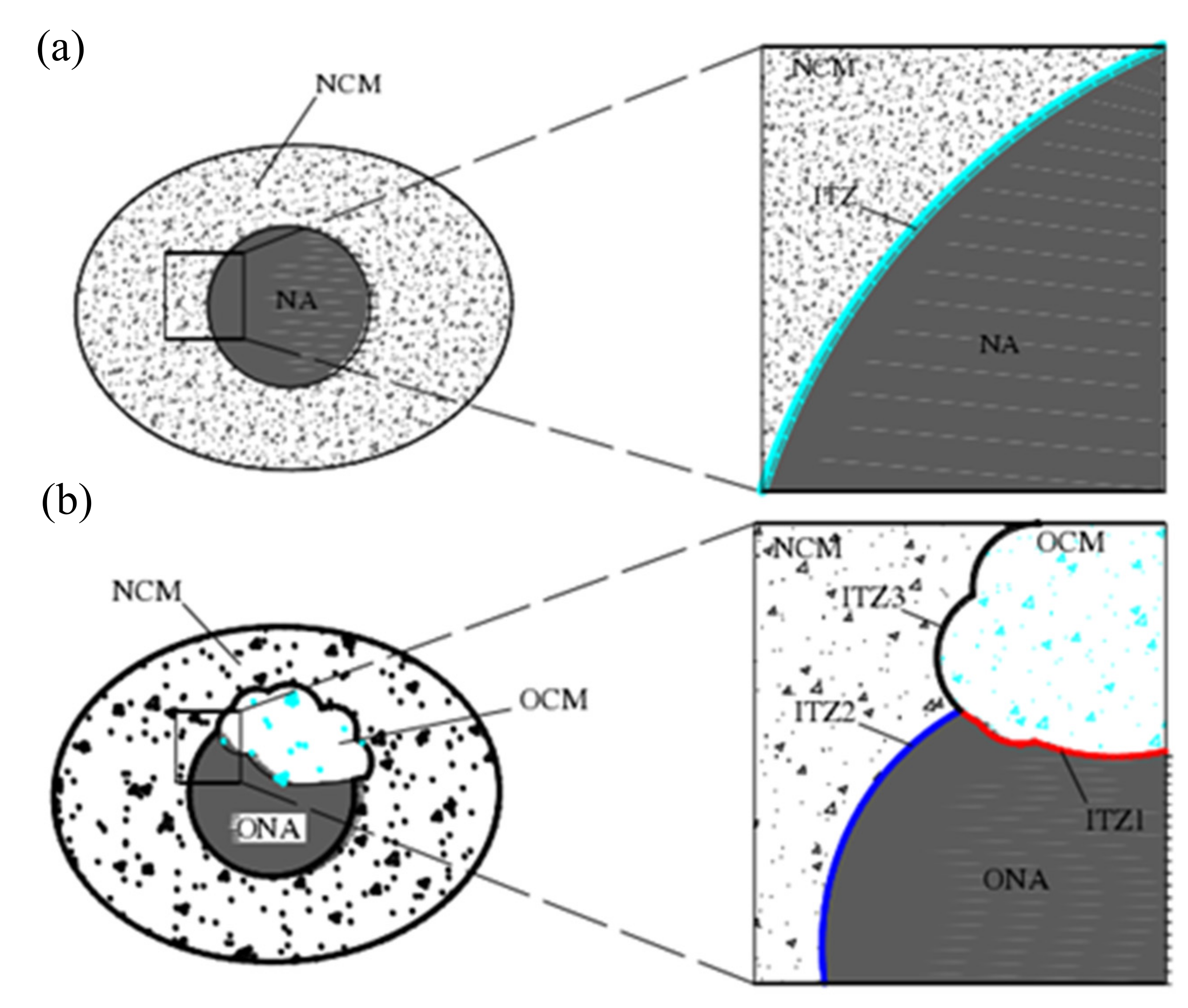
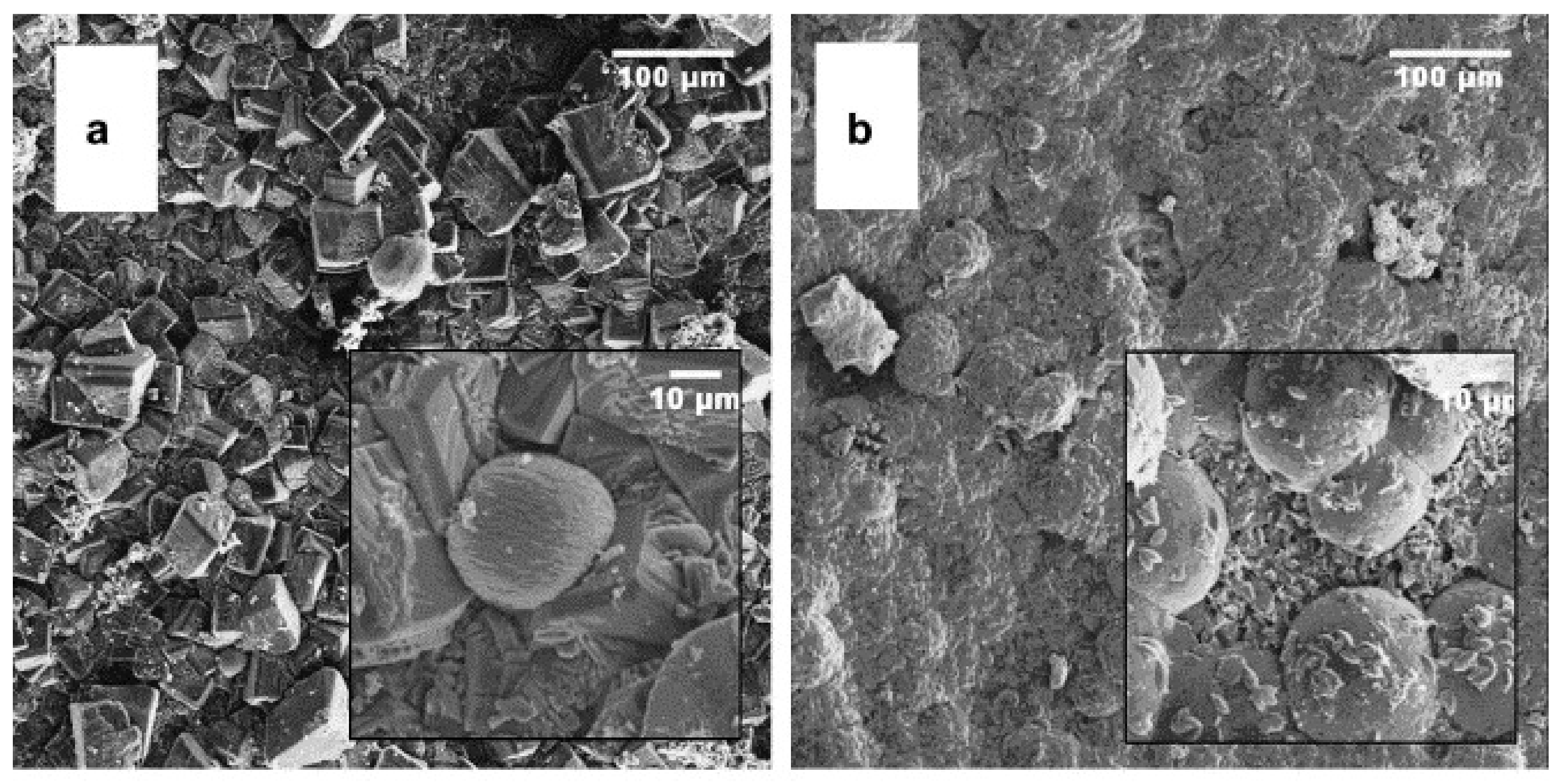
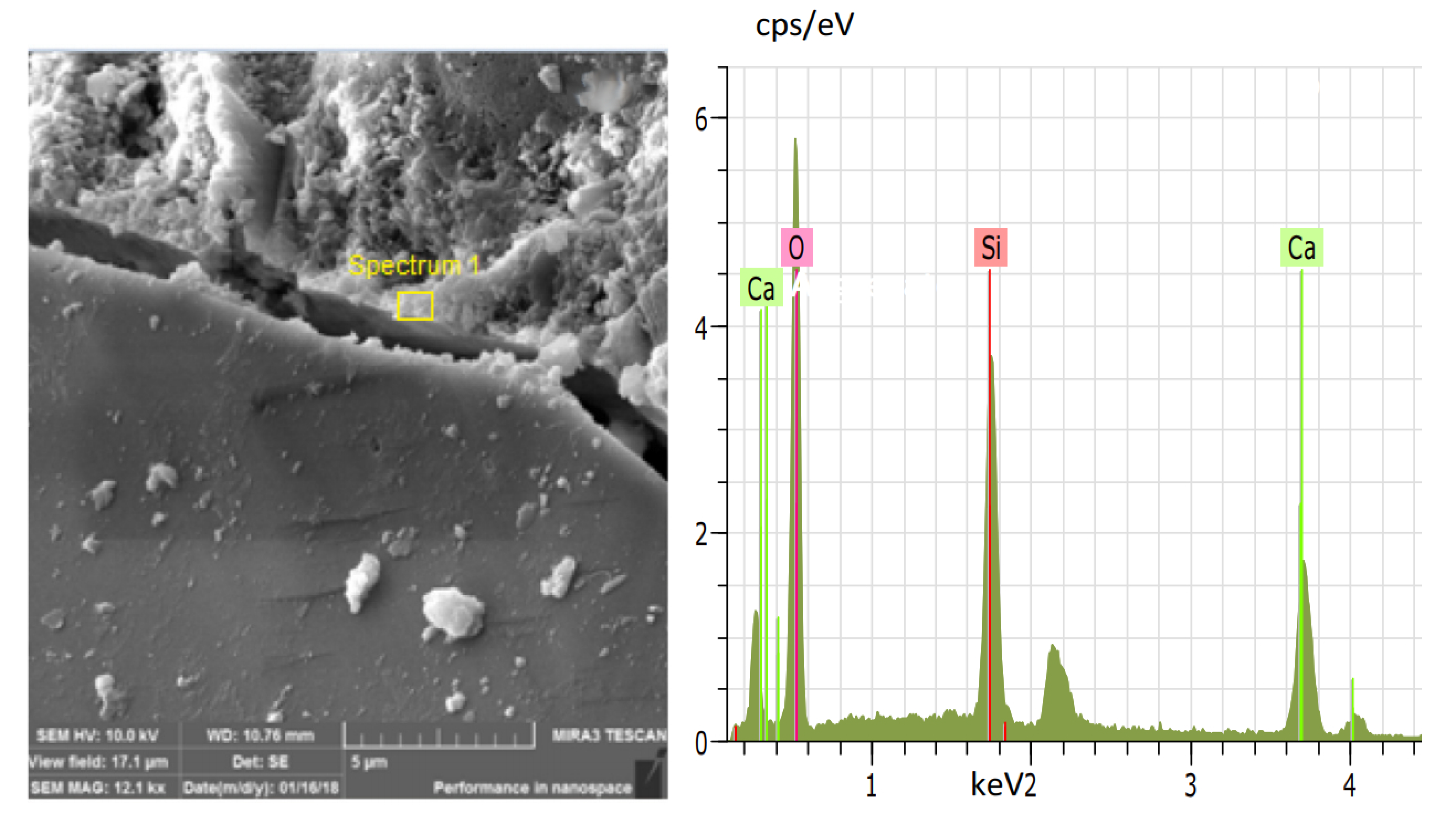
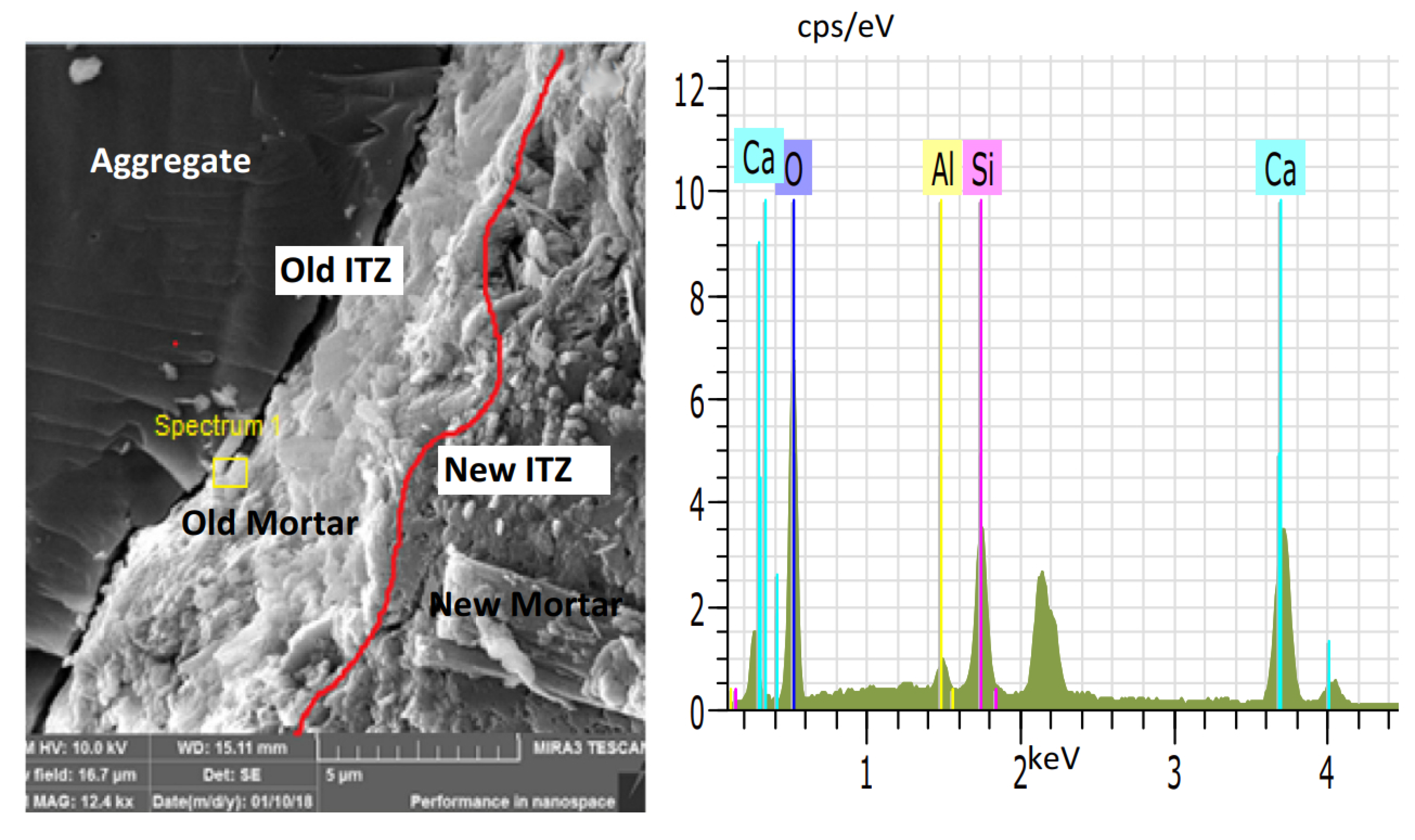
5. Reinforcement Effect of Recycled Aggregate
6. Conclusions and Prospect
- As a relatively new and popular strengthening method in recent years, the MICP technique for modifying recycled aggregate is favored by researchers due to its environmental protection and low energy consumption performance. This technique can obtain a good result for improving the properties of recycled aggregate and concrete, especially microproperties. However, its mineralization period is too long, and almost all studies have shown that it takes more than 7 days to achieve significant effects [48,81]. It is difficult to reduce the cost of a culture medium due to the high requirements of a biological culture [45]. All of these factors have limited the development of this technique.
- Microbial mineralization and deposition occur under the combined action of many factors, such as bacteria, calcium source, medium composition, pH value, nucleation center location, and temperature. At present, studies on modified aggregates mostly focus on the influence of calcium source, medium composition and pH value. In fact, as a process of biological growth and metabolism, comprehensive exploration of the optimal range of influencing factors should be the first step before the modification of aggregates. For example, when selecting different strains, the influence of different pH values on the experimental results should be determined to achieve the best precipitation effect. In particular, there is still no good solution for fixing the nucleation site on the weak spot of the aggregate surface, and the influence of these factors should be considered first to obtain a better modification effect.
- The mechanism of mineralization precipitation of different types of bacteria is slightly different. Generally, it is the process of generating calcium carbonate. When using the MICP technique to modify recycled aggregate, researchers should choose the most suitable strain according to their own needs and laboratory conditions to achieve the best modification effect.
- According to current studies, it is difficult to overcome the disadvantages of using the MICP technique, such as consumption of time and lack of pertinence, to strengthen recycled aggregates. In future studies, researchers could try to combine some physical or chemical strengthening methods with the MICP technique for composite strengthening of recycled aggregate according to the mechanism and the advantages and disadvantages of different strengthening methods, which may be the development tendency of reinforcing recycled aggregate in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Wu, R. Using graphene oxide to improve the properties of ultra-high-performance concrete with fine recycled aggregate. Constr. Build. Mater. 2020, 259, 120657. [Google Scholar] [CrossRef]
- Jin, R.; Li, B.; Zhou, T.; Wanatowski, D.; Piroozfar, P. An empirical study of perceptions towards construction and demolition waste recycling and reuse in China. Resour. Conserv. Recycl. 2017, 126, 86–98. [Google Scholar] [CrossRef]
- Kisku, N.; Joshi, H.; Ansari, M.; Panda, S.K.; Nayak, S.; Dutta, S.C. A critical review and assessment for usage of recycled aggregate as sustainable construction material. Constr. Build. Mater. 2017, 131, 721–740. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. Time-dependent and long-term mechanical properties of concretes incorporating different grades of coarse recycled concrete aggregates. Eng. Struct. 2018, 157, 224–234. [Google Scholar] [CrossRef]
- McGinnis, M.J.; Davis, M.; de la Rosa, A.; Weldon, B.D.; Kurama, Y.C. Strength and stiffness of concrete with recycled concrete aggregates. Constr. Build. Mater. 2017, 154, 258–269. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Fan, Y.; Huang, X. An overview of study on recycled aggregate concrete in China (1996–2011). Constr. Build. Mater. 2012, 31, 364–383. [Google Scholar] [CrossRef]
- Evangelista, L.; de Brito, J. Durability performance of concrete made with fine recycled concrete aggregates. Cem. Concr. Compos. 2010, 32, 9–14. [Google Scholar] [CrossRef]
- Tangchirapat, W.; Khamklai, S.; Jaturapitakkul, C. Use of ground palm oil fuel ash to improve strength, sulfate resistance, and water permeability of concrete containing high amount of recycled concrete aggregates. Mater. Des. 2012, 41, 150–157. [Google Scholar] [CrossRef]
- Guo, H. Characteristics of Carbonated Recycled Concrete Aggregate and Its Effect on the Microstructure and Properties of Recycled Aggregate Concrete. Ph.D. Thesis, Henan Polytechnic University, Jiaozuo, China, 2019. [Google Scholar]
- Xuan, D.; Zhan, B.; Poon, C.H. Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cem. Concr. Compos. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Da Silva Neto, G.A.; de Oliveira, J.P.V.; Salles, P.V.; de Vasconcelos Barros, R.T.; Paulino, M.T.; Dos Santos, W.J. Influence of Heterogeneity, Typology, and Contaminants of Recycled Aggregates on the Properties of Concrete. Open Constr. Build. Technol. J. 2021, 14, 382–399. [Google Scholar] [CrossRef]
- Adessina, A.; Ben Fraj, A.; Barthélémy, J.; Chateau, C.; Garnier, D. Experimental and micromechanical investigation on the mechanical and durability properties of recycled aggregates concrete. Cem. Concr. Res. 2019, 126, 105900. [Google Scholar] [CrossRef]
- Bai, L. Experimental Study on Compressive Strength and Microscopic Properties of Recycled Concrete with Reinforced Coarse Aggregate. Master’s Thesis, Xi’an University of Architecture and Technology, Xian, China, 2019. [Google Scholar]
- Pepe, M.; Toledo Filho, R.D.; Koenders, E.A.B.; Martinelli, E. Alternative processing procedures for recycled aggregates in structural concrete. Constr. Build. Mater. 2014, 69, 124–132. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Kim, K.; Kim, J. Quality improvement of recycled aggregates using the acid treatment method and the strength characteristics of the resulting mortar. J. Mater. Cycles Waste 2017, 19, 968–976. [Google Scholar] [CrossRef]
- Menard, Y.; Bru, K.; Touze, S.; Lemoign, A.; Poirier, J.E.; Ruffie, G.; Bonnaudin, F.; Von Der Weid, F. Innovative process routes for a high-quality concrete recycling. Waste Manag. 2013, 33, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, G.; Savva, P.; Petrou, M.F. Enhancing mechanical and durability properties of recycled aggregate concrete. Constr. Build. Mater. 2018, 158, 228–235. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Combined influence of recycled concrete aggregates and high contents of fly ash on concrete properties. Constr. Build. Mater. 2017, 157, 554–572. [Google Scholar] [CrossRef]
- Spaeth, V.; Djerbi Tegguer, A. Improvement of recycled concrete aggregate properties by polymer treatments. Int. J. Sustain. Built Environ. 2013, 2, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Poon, C.S.; Xiao, J.; Xuan, D. Effect of carbonated recycled coarse aggregate on the dynamic compressive behavior of recycled aggregate concrete. Constr. Build. Mater. 2017, 151, 52–62. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Qian, X.; Chen, P.; Xu, Y.; Guo, J. An environmentally friendly method to improve the quality of recycled concrete aggregates. Constr. Build. Mater. 2017, 144, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Liu, S.; Gao, X.; Wang, R. Evaluation of effect of microbial induced steuvite precipition strerngthing calcareous sand in seawater environment. J. Civ. Environ. Eng. 2020, 42. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Chu, J.; He, J. Microbially Induced Calcite Precipitation for Seepage Control in Sandy Soil. Geomicrobiol. J. 2019, 36, 366–375. [Google Scholar] [CrossRef]
- Crawford, R.L.; Burbank, M.B.; Weaver, T.J.; Williams, B.C. In Situ Precipitation of Calcium Carbonate (CaCO3) by Indigenous Microorganisms to Improve Mechanical Properties of a Geomaterial. U.S. Patent 8,420,362, 16 April 2013. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Liu, S.; Yu, S.; Zeng, W.; Peng, X.; Cai, Y.; Tu, B. Repair effect of tabia cracks with microbially induced carbonate precipitation. Chin. J. Rock Mech. Eng. 2020, 39, 191–204. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.; Shin, Y.; So, J. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springer Plus 2016, 5, 250. [Google Scholar] [CrossRef] [Green Version]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Férard, C. Significance, mechanisms and environmental implications of microbial biomineralization. Comptes Rendus Geosci. 2011, 343, 160–167. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- García-González, J.; Rodríguez-Robles, D.; Wang, J.; De Belie, N.; Morán-del Pozo, J.M.; Guerra-Romero, M.I.; Juan-Valdés, A. Quality improvement of mixed and ceramic recycled aggregates by biodeposition of calcium carbonate. Constr. Build. Mater. 2017, 154, 1015–1023. [Google Scholar] [CrossRef]
- Dick, J.; De Windt, W.; De Graef, B.; Saveyn, H.; Van der Meeren, P.; De Belie, N.; Verstraete, W. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation 2006, 17, 357–367. [Google Scholar] [CrossRef]
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Gebru, K.A.; Kidanemariam, T.G.; Gebretinsae, H.K. Bio-cement production using microbially induced calcite precipitation (MICP) method: A review. Chem. Eng. Sci. 2021, 238, 116610. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, F.; Mahmoud, M.A.; Sabet, V. Zeta Potential and Surface Area of Calcium Carbonate as Related to Phosphate Sorption1. Soil Sci. Soc. Am. J. 1985, 49, 1137–1142. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W.P. Effect of particle size distribution on the bio-cementation of coarse aggregates. Acta Geotech. 2018, 13, 1019–1025. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of recycled concrete aggregate by calcium carbonate biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Zhan, M.; Pan, G.; Wang, Y.; Fu, M.; Lu, X. Recycled aggregate mortar enhanced by microbial calcite precipitation. Mag. Concr. Res. 2020, 72, 622–633. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Influence of Calcium Sources on Microbially Induced Calcium Carbonate Precipitation by Bacillus sp. CR2. Appl. Biochem. Biotechnol. 2014, 173, 307–317. [Google Scholar] [CrossRef]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol. Eng. 2010, 36, 99–111. [Google Scholar] [CrossRef]
- De Muynck, W.; Cox, K.; Belie, N.D.; Verstraete, W. Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr Build. Mater. 2008, 22, 875–885. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. J. Ind. Microbiol. Biot. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Omoregie, A.L.; Ngu, L.H.; Ong, D.E.L.; Nissom, P.M. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotechnol. 2019, 17, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Qiu, J.; Tng, D.Q.S.; Yang, E. Surface treatment of recycled concrete aggregates through microbial carbonate precipitation. Constr. Build. Mater. 2014, 57, 144–150. [Google Scholar] [CrossRef]
- Zeng, W.; Zhao, Y.; Poon, C.S.; Feng, Z.; Lu, Z.; Shah, S.P. Using microbial carbonate precipitation to improve the properties of recycled aggregate. Constr. Build. Mater. 2019, 228, 116743. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yao, W. Coupled effects of carbonation and bio-deposition in concrete surface treatment. Cem. Concr. Compos. 2019, 104, 103358. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; González-Muñoz, M.T. Influence of Substrate Mineralogy on Bacterial Mineralization of Calcium Carbonate: Implications for Stone Conservation. Appl. Environ. Microb. 2012, 78, 4017–4029. [Google Scholar] [CrossRef] [Green Version]
- Tobler, D.J.; Cuthbert, M.O.; Greswell, R.B.; Riley, M.S.; Renshaw, J.C.; Handley-Sidhu, S.; Phoenix, V.R. Comparison of rates of ureolysis between Sporosarcina pasteurii and an indigenous groundwater community under conditions required to precipitate large volumes of calcite. Geochim. Cosmochim. Acta 2011, 75, 3290–3301. [Google Scholar] [CrossRef]
- Zhu, Y.; Rong, D.; Xu, P.; Chen, F.; Sun, W. Influence of Oxygen Supply Agent Concentration and Soaking Position on MICP Recycled Aggregate Properties. Mater. Rep. 2021, 35, 4074–4078. [Google Scholar] [CrossRef]
- Martin, D.; Dodds, K.; Ngwenya, B.T.; Butler, I.B.; Elphick, S.C. Inhibition of Sporosarcina pasteurii under Anoxic Conditions: Implications for Subsurface Carbonate Precipitation and Remediation via Ureolysis. Environ. Sci. Technol. 2012, 46, 8351–8355. [Google Scholar] [CrossRef]
- Zamarreño, D.V.; Inkpen, R.; May, E. Carbonate Crystals Precipitated by Freshwater Bacteria and Their Use as a Limestone Consolidant. Appl Environ. Microb. 2009, 75, 5981–5990. [Google Scholar] [CrossRef] [Green Version]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of Calcite (Calcium Carbonate) Crystals by Soil Bacteria is a General Phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Youn, H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Bhatt, A.; Patel, R. Role of Microbial Induced Calcite Precipitation in Sustainable Development. Sch. Res. Libr. 2018, 1, 7–17. [Google Scholar]
- Van Paassen, L.A. Ground Improvement by Microbially Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Meyers, M.A.; Chen, P.; Lin, A.Y.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- Schultze-Lam, S.; Fortin, D.; Davis, B.S.; Beveridge, T.J. Mineralization of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Beveridge, T.J. The bacterial surface: General considerations towards design and function. Can. J. Microbiol. 1988, 34, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Taylor, J.L.; Gresham, T.L.T.; Delwiche, M.E.; Colwell, F.S.; McLing, T.L.; Petzke, L.M.; Smith, R.W. Stimulation Of Microbial Urea Hydrolysis In Groundwater To Enhance Calcite Precipitation. Environ. Sci. Technol. 2008, 42, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Chahal, N.K. Effect of ureolytic bacteria on concrete properties. Constr. Build. Mater. 2011, 25, 3791–3801. [Google Scholar] [CrossRef]
- Wang, J.; Vandevyvere, B.; Vanhessche, S.; Schoon, J.; Boon, N.; De Belie, N. Microbial carbonate precipitation for the improvement of quality of recycled aggregates. J. Clean Prod. 2017, 156, 355–366. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.M.; Schlangen, E. Selection of Nutrient Used in Biogenic Healing Agent for Cementitious Materials. Front. Mater. 2017, 4, 15. [Google Scholar] [CrossRef]
- Xu, J.; Yao, W. Non-ureolytic Microbiologically-induced Calium Carbonate Precipitation. J. Tongji Univ. Nat. Sci. 2013, 41, 1542–1546. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in COrHzO solutions between 0 and 90℃, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Qian, C.; Luo, M.; Pan, Q.; Li, R. Mechanism of Microbially Induced Calcite Precipitation in Self-healing Concrete. J. Chin. Ceram. Soc. 2013, 41, 620–626. [Google Scholar] [CrossRef]
- Tripp, B.C.; Smith, K.; Ferry, J.G. Carbonic Anhydrase: New Insights for an Ancient Enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef]
- Zeng, X. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master in Engineering. Master’s Thesis, Huazhong University of Science & Technology, Wuhan, China, 2004. [Google Scholar]
- Ren, L.; Qian, C. Restoration of Cracks on Surface of Cement-Based Materials by Carbonic Anhydrase Microbiologically Precipition Calium Carbonate. J. Chin. Ceram. Soc. 2014, 42, 1389–1395. [Google Scholar] [CrossRef]
- Yang, Q. Experimental Study on the Sand Soil Solidification Using Carbonic Anhydrase Induced Precipitation. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2018. [Google Scholar]
- Erşan, Y.Ç.; Verbruggen, H.; De Graeve, I.; Verstraete, W.; De Belie, N.; Boon, N. Nitrate reducing CaCO3 precipitating bacteria survive in mortar and inhibit steel corrosion. Cem. Concr. Res. 2016, 83, 19–30. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A.D. Review on microbial induced calcite precipitation mechanisms leading to bacterial selection for microbial concrete. Constr. Build. Mater. 2019, 22, 67–75. [Google Scholar] [CrossRef]
- Wang, L.; Zou, K. Research progress of concrete microbial self-healing technology. Acta Silic. Sin. 2019, 47, 135–145. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Application of calcifying bacteria for remediation of stones and cultural heritages. Front. Microbiol. 2014, 5, 304. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L.; Newman, D.K. Geomicrobiology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Singh, L.P.; Bisht, V.; Aswathy, M.S.; Chaurasia, L.; Gupta, S. Studies on performance enhancement of recycled aggregate by incorporating bio and nano materials. Constr. Build. Mater. 2018, 181, 217–226. [Google Scholar] [CrossRef]
- Xu, P.; Chen, F.; Li, Q.; Ren, Y.; Wu, C.; Zhu, Y. Effect of Microbial Mineralization Deposition on Interfacial Transition Zone of Recycled Aggregate. Mater. Rep. 2020, 34, 6095–6099. [Google Scholar] [CrossRef]
- Feng, Z.; Zhao, Y.; Zeng, W.; Lu, Z.; Shah, S.P. Using microbial carbonate precipitation to improve the properties of recycled fine aggregate and mortar. Constr. Build. Mater. 2020, 230, 116949. [Google Scholar] [CrossRef]

| Classification of Aggregate | Strengthening Effect | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recycled Aggregates | Recycled Mortar and Recycled Concrete | ||||||||||
| Bibulous Rate (%) | Porosity (%) | Percentage Increase in Mass (%) | Compressive Strength (MPa) | Flexural Strength (MPa) | Percentage Reduction of Chloride Ion Permeability Coefficient (%) | ||||||
| Before | After | Before | After | Before | After | Before | After | ||||
| Coarse aggregate | 5–5.5 | 4.5–5 | 15 | 10 | 0.8 | 44–54 | 51–76 | - | 6.2–10.5 | [50,65,72] | |
| Fine aggregate | 24 | 16.5 | - | 2.7 | 29 | 38 | 2.65 | 3.6 | - | [49,82,83] | |
| 12 | 13.7 | 1.8 | 3.32 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Cui, B.; Ge, H.; Huang, Y.; Zhang, W.; Zhu, J. Reinforcement of Recycled Aggregate by Microbial-Induced Mineralization and Deposition of Calcium Carbonate—Influencing Factors, Mechanism and Effect of Reinforcement. Crystals 2021, 11, 887. https://doi.org/10.3390/cryst11080887
Feng C, Cui B, Ge H, Huang Y, Zhang W, Zhu J. Reinforcement of Recycled Aggregate by Microbial-Induced Mineralization and Deposition of Calcium Carbonate—Influencing Factors, Mechanism and Effect of Reinforcement. Crystals. 2021; 11(8):887. https://doi.org/10.3390/cryst11080887
Chicago/Turabian StyleFeng, Chunhua, Buwen Cui, Haidong Ge, Yihong Huang, Wenyan Zhang, and Jianping Zhu. 2021. "Reinforcement of Recycled Aggregate by Microbial-Induced Mineralization and Deposition of Calcium Carbonate—Influencing Factors, Mechanism and Effect of Reinforcement" Crystals 11, no. 8: 887. https://doi.org/10.3390/cryst11080887
APA StyleFeng, C., Cui, B., Ge, H., Huang, Y., Zhang, W., & Zhu, J. (2021). Reinforcement of Recycled Aggregate by Microbial-Induced Mineralization and Deposition of Calcium Carbonate—Influencing Factors, Mechanism and Effect of Reinforcement. Crystals, 11(8), 887. https://doi.org/10.3390/cryst11080887







