Synthesis of Zirconia Micro-Nanoflakes with Highly Exposed (001) Facets and Their Crystal Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis Procedure
3. Results and Discussion
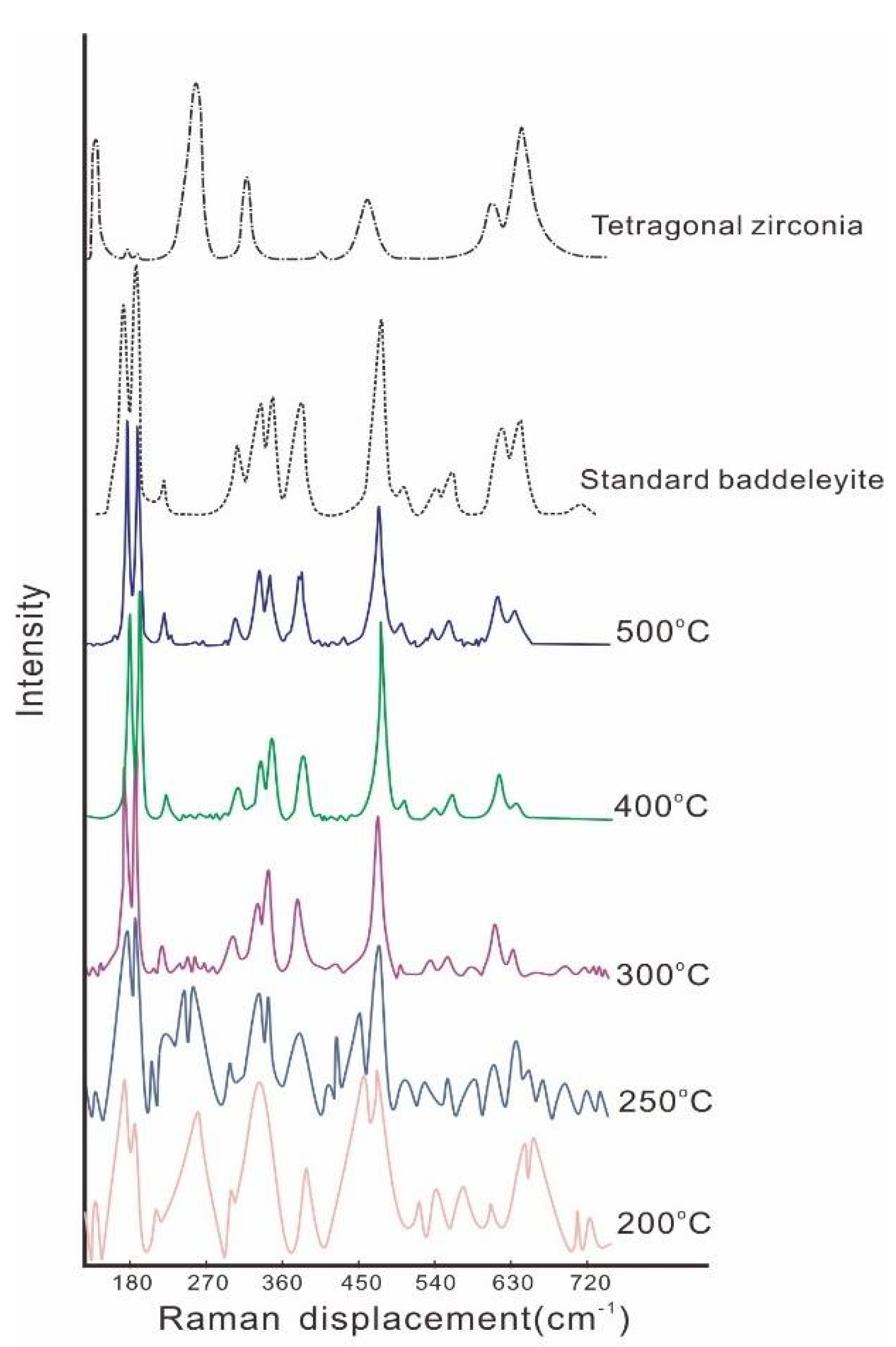
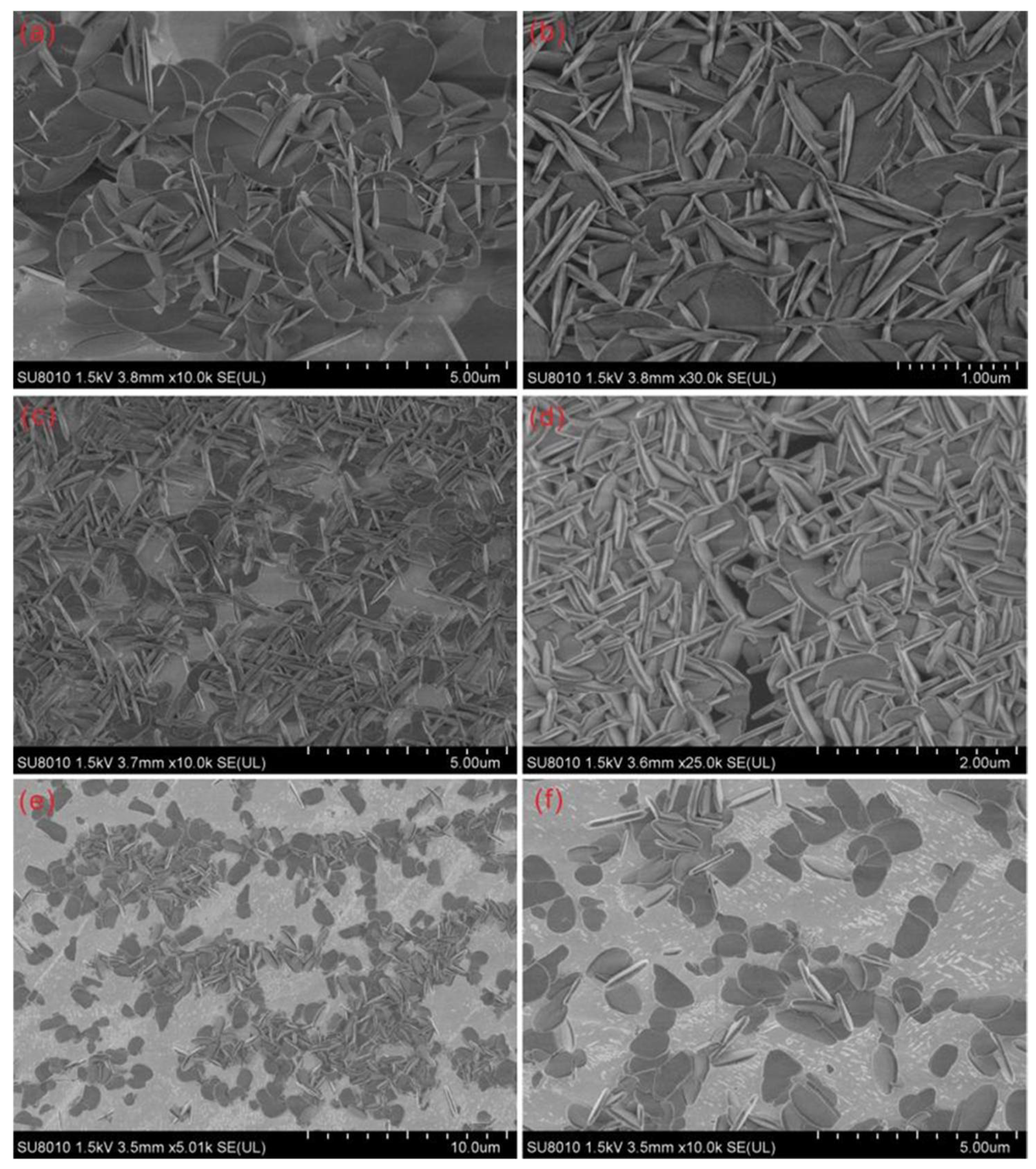
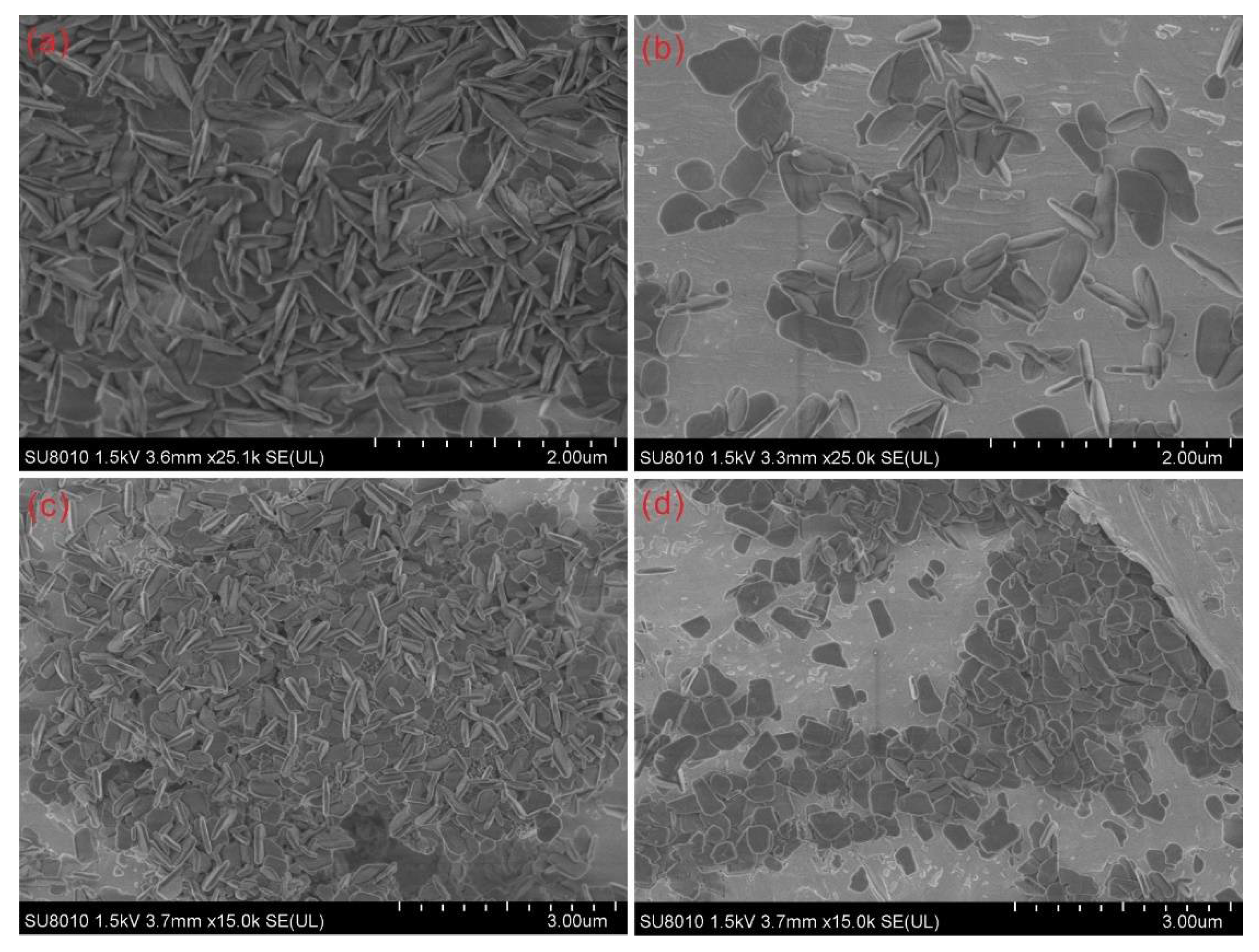
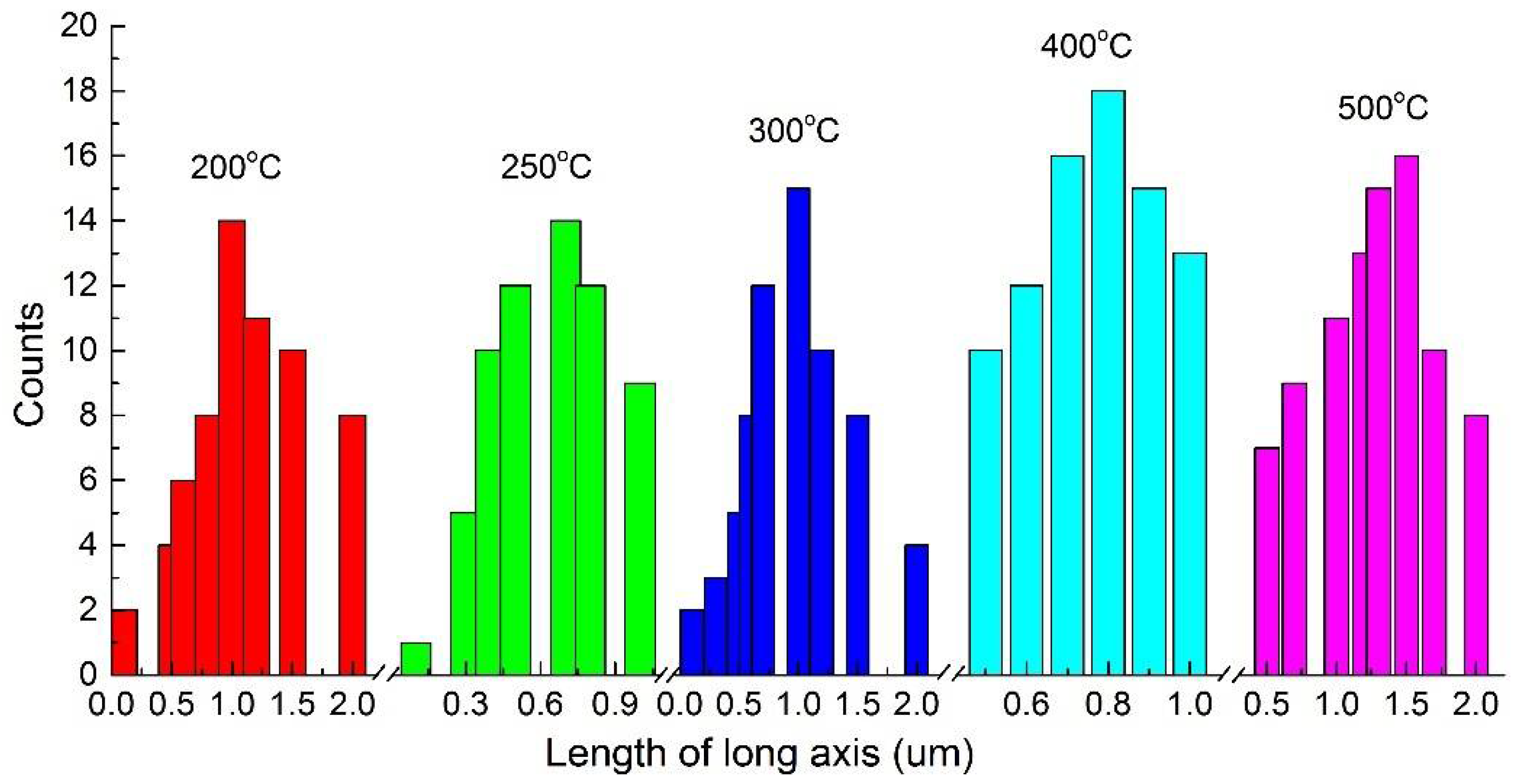
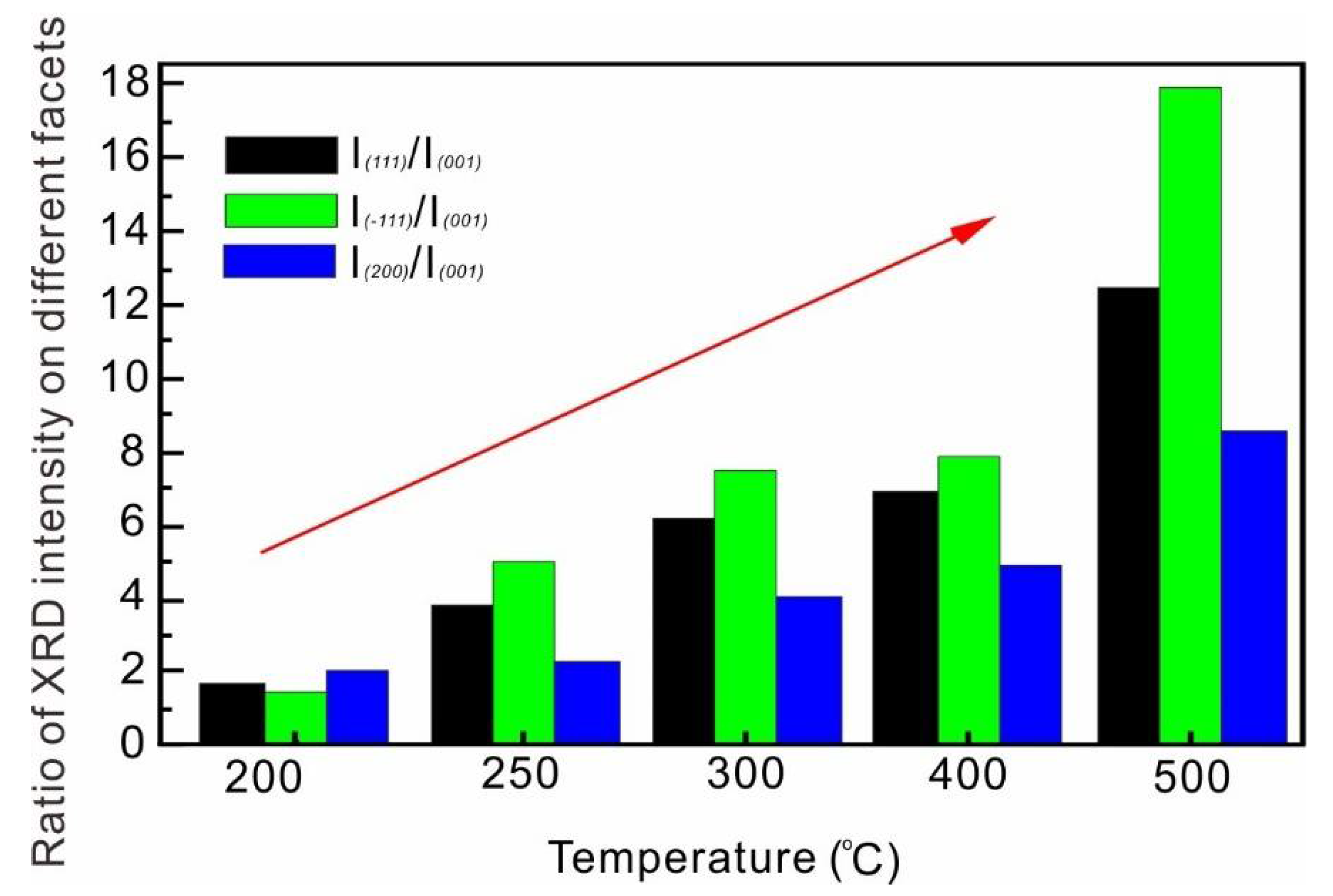
3.1. Temperature Dependence on the Crystal Form and the Morphology of the Synthesized Zirconia
3.2. Effect of Initial Solutions’ Concentration on the Morphology of the Synthesized Zirconia
3.3. Growth Mechanism of the Synthesized Zirconia

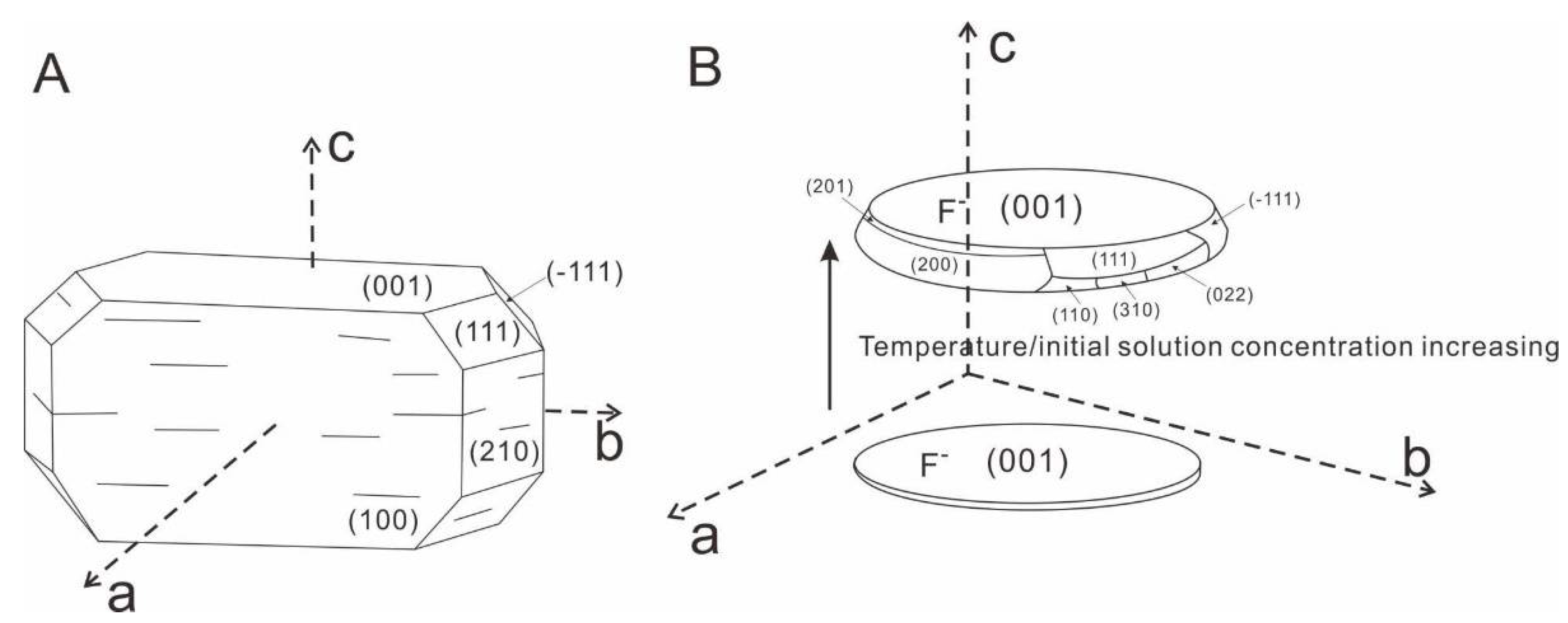
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P. Preparation and Characterization of Zirconia Ceramic Hollow Shells; Harbin Institute of Technology: Harbin, China, 2009. [Google Scholar]
- Chang, J.P.; Lin, Y.S.; Chu, K. Rapid thermal chemical vapor deposition of zirconium oxide for metal-oxide-semiconductor field effect transistor application. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Proc. Meas. Phenom. 2001, 19, 1782–1787. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.Y.; Zhu, G.D.; Dai, Y.; Wang, Y.B.; Liu, J.Y. Electrospun Zirconia Nanofibre-modified Glassy Carbon Electrode for Determination of Methyl Parathion. Chin. J. Anal. Chem. 2018, 46, 1982–1989, (In Chinese with English). [Google Scholar]
- He, W.Y.; Liu, J.R.; Cao, Z.Z.; Li, C.H.; Gao, Y.F. Preparation and characterization of monodisperse zirconia spherical nanometer powder via lamellar liquid crystal template method. Chin. J. Chem. Eng. 2015, 23, 1721–1727. [Google Scholar] [CrossRef]
- Wijayanti, R.B.; Rosmayanti, I.; Wahyudi, K.; Maryani, E.; Hernawan, H.; Septawendar, R. Preparation of magnesia partially stabilized zirconia nanomaterials from zirconium hydroxide and magnesium carbonate precursors using PEG as a template. Crystals 2021, 11, 635. [Google Scholar] [CrossRef]
- Gao, Q.W.; Jiang, W.H.; Liu, J.M.; Feng, G.; Chen, T.; Miao, L.F. Preparation of Zirconia Nanorods by Molten Salt Method. Chin. J. Inorg. Chem. 2017, 33, 1555–1560, (In Chinese with English Abstract). [Google Scholar]
- Kang, J.; Ji, W.L.; Bu, J.L.; Chen, Y.; Wei, H.Y.; Lv, D.F.; Cui, Y.; Chen, Y.J.; Wei, Y.N.; Gong, L.P.; et al. Study on process of electrospinning preparation of zirconia hollow microspheres. Refract. Mater. 2018, 53, 170–175, (In Chinese with English Abstract). [Google Scholar]
- Gao, Q.W.; Liu, J.M.; Jiang, W.H.; Feng, G.; Chen, T.; Miao, L.F. Effect of Solvothermal Temperature on the Preparation of Zirconia Whiskers. J. Ceram. 2018, 39, 154–158, (In Chinese with English Abstract). [Google Scholar]
- Kwon, J.H.; Choi, J.H.; Bae, J.H.; Park, J. Hysteresis reduction for organic thin film transistors with multiple stacked functional zirconia polymeric films. Crystals 2019, 9, 634. [Google Scholar] [CrossRef]
- Zhu, J.F.; Xiao, D.; Li, C.Y.; Huang, J.F.; Yong, X.; Cao, L.Y.; Fei, J. Microwave hydrothermal synthesis and crystallization mechanism of tetragonal phase zirconia nanocrystalline. J. Shaanxi Univ. Sci. Technol. (Nat. Sci. Ed.) 2015, 33, 60–68. [Google Scholar]
- Wu, J.; Lu, Y.F.; Liu, Y.; Yu, Z.Y.; Xin, B.F. Fabrication and Characterization of ZrO2 Nanofibers with Controllable Crystalline Phase by a Halid-free Approach. Chem. J. Chin. Univ. Chin. Ed. 2015, 36, 1403–1408, (In Chinese with English Abstract). [Google Scholar]
- Pan, L.; Xu, H.B.; Sun, Y.Y.; Zhao, J.P.; Li, Y. Preparation of three-dimensional photonic crystals of zirconia by electrodeposition in a colloidal crystals template. Crystals 2016, 6, 76. [Google Scholar] [CrossRef]
- Jayakumar, S.; Ananthapadmanabhan, P.V.; Perumal, K.; Thiyagarajan, T.K.; Mishra, S.C.; Su, L.T.; Tok, A.; Guo, J. Characterization of nano-crystalline ZrO2 synthesized via reactive plasma processing. Mater. Sci. Eng. B 2011, 176, 894–899. [Google Scholar] [CrossRef]
- Noh, H.J.; Seo, D.S.; Kim, H.; Lee, J.K. Synthesis and crystallization of anisotropic shaped ZrO2 nanocrystalline powders by hydrothermal process. Mater. Lett. 2003, 57, 2425–2431. [Google Scholar] [CrossRef]
- Binner, J.; Vaidhyanathan, B. Processing of bulk nanostructured ceramics. J. Eur. Ceram. Soc. 2008, 28, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Gaudon, M.; Djurado, E.; Menzler, N.H. Morphology and sintering behaviour of yttria stabilised zirconium (8-YSZ) powders synthesised by spray pyrolysis. Ceram. Int. 2004, 30, 2295–2303. [Google Scholar] [CrossRef]
- Soo, M.T.; Prastqmo, N.; Matsuda, A.; Kawamura, G.; Muto, H.; Noor, A.; Lockman, Z.; Cheong, K.Y. Elaboration and characterization of sol–gel derived ZrO2 thin films treated with hot water. Appl. Surf. Sci. 2012, 258, 5250–5258. [Google Scholar] [CrossRef]
- Garg, N.; Mittal, V.K.; Bera, S.; Dasgupta, A.; Sankaralingam, V. Preparation and characterization of tetragonal dominant nanocrystalline ZrO2 obtained via direct precipitation. Ceram. Int. 2012, 38, 2507–2512. [Google Scholar] [CrossRef]
- Chang, Y.; Li, X.B. Preparation of ZrO2 spherical nanometer powders. J. Cent. South. Univ. (Sci. Technol.) 2007, 38, 46–50, (In Chinese with English Abstract). [Google Scholar]
- Silva Junior, E.; Antonio, S.G.; Longo, E. Synthesis and structural evolution of partially and fully stabilized ZrO2 from a versatile method aided by microwave power. Ceram. Int. 2018, 44, 3517–3522. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.X.; Lin, J.W.; Zhan, Y.H.; Zhang, Z.B.; Chu, M. Adsorption of Phosphate from Aqueous Solution on Hydrous Zirconium Oxides Precipitated at Different pH Values. Enviorn. Sci. 2017, 38, 1936–1946. [Google Scholar] [CrossRef]
- Ji, Y.; Han, L.Z.; Zheng, W.Q.; Wang, Q.; Yang, H.Z. Influence of pH values on different zirconia systems. Chin. J. Tissue Eng. Res. 2017, 21, 4131–4136. [Google Scholar]
- Wang, H.Y.; Guo, Z.S.; Xing, G.E.; Song, X.Q. Study on Preparing Nano-sized ZrO2 by Different Techniques. J. Synth. Cryst. 2006, 35, 753–756. [Google Scholar]
- Wang, J.J. Research progress of photocatalytic performance of nanometer zirconia. Light Ind. Stand. Qual. 2017, 2, 68–69. (In Chinese) [Google Scholar]
- Li, W.; Liu, F.H.; Wu, D.W. Preparation of Flexible Yttria-stabilized Zirconia Nanofibers. Guangzhou Chem. Ind. 2018, 46, 45–51, (In Chinese with English Abstract). [Google Scholar]
- Xu, G.F.; Song, H.Z.; Yang, D.L. The Hdrothermal Preparation of Stable Zirconia Nano Powder. Surf. Technol. 2017, 46, 95–100. [Google Scholar] [CrossRef]
- Du, P.; Zhang, J.F.; Zhao, J.L.; Wang, X.X.; Zhang, L.B. Study on decolourization and degradation of methyl rrange by stir-ultrasonic ZrO2 nanotubes. J. Synth. Cryst. 2015, 44, 2518–2523, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.X.; Zhao, J.L.; Guo, L.M.; Yu, X.; Tang, C.C. Fabrication of Zirconia Nanotube Arrays on Curved Surface of Zirconium Wire. Rare Met. Mat. Eng. 2012, 41, 35–37, (In Chinese with English Abstract). [Google Scholar]
- Li, J. Study on performance of nanometer zirconia-cerium oxide composites. Sci. Technol. Inf. 2014, 3, 51–79, (In Chinese with English Abstract). [Google Scholar]
- Guo, J.H.; Li, X.H.; Li, Z.W.; Du, Z.L.; Zhang, Z.J. Synthesis of sheet-like silica/zirconia nanocomposites by sol-gel process. Chem. Res. 2011, 22, 49–54. [Google Scholar]
- Yang, S.F. Study on liquid phase reaction self-assembly preparation and performance of ZrO2 films. Tianjin Chem. Ind. 2015, 29, 31–33. [Google Scholar]
- Yan, H.B.; Sun, W.D.; Liu, J.F.; Tu, X.L.; Ding, X. Thermodynamic properties of ruthenium (IV) chloride complex and the transport of ruthenium in magmatic-hydrothermal fluids. Ore Geol. Rev. 2021, 131, 104043. [Google Scholar] [CrossRef]
- He, J.J.; Ding, X.; Wang, Y.R.; Sun, W.D. The effects of precipitation-aging-re-dissolution and pressure on hydrolysis of fluorine-rich titanium complexes in hydrothermal fluids and its geological implications. Acta Pet. Sin. 2015, 31, 1870–1878, (In Chinese with English Abstract). [Google Scholar]
- He, J.J.; Ding, X.; Wang, Y.R.; Sun, W.D. The effect of temperature and concentration on hydrolysis of fluorine-rich titanium complexes in hydrothermal fluids: Constraints on titanium mobility in deep geological processes. Acta Pet. Sin. 2015, 31, 802–810, (In Chinese with English Abstract). [Google Scholar]
- Di, J.; Yan, H.B.; Liu, Z.Y.; Ding, X. Synthesis and Characterization of Anatase TiO2 Microspheres Self-Assembled by Ultrathin Nanosheets. Materials 2021, 14, 2870. [Google Scholar] [CrossRef]
- Yan, H.B.; He, J.J.; Liu, X.W.; Wang, H.B.; Liu, J.F.; Ding, X. Thermodynamic Investigation of the Hydrolysis Behavior of Fluorozirconate Complexes at 423.15–773.15 K and 100 MPa. J. Solut. Chem. 2020, 49, 836–848. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Ding, X.; Li, W.C. Diverse serpentinization and associated abiotic methanogenesis within multiple types of olivine-hosted fluid inclusions in orogenic peridotite from northern Tibet-Science Direct. Geochim. Cosmochim. Acta 2021, 296, 1–17. [Google Scholar] [CrossRef]
- Baes, J.C.F.; Mesmer, R.E. The thermodynamics of cation hydrolysis. Am. J. Sci. 1980, 281, 935–962. [Google Scholar] [CrossRef]
- Ding, X.; Harlov, D.E.; Chen, B.; Sun, W.D. Fluids, metals, and mineral/ore deposits. Geofluids 2018, 1452409. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.R.; Chou, I.M. Characteristics of hydrolysis of the complex Na2SnF6 in hydrothermal solutions—An experimental study. Chin. J. Geochem. 1987, 6, 372–382. [Google Scholar] [CrossRef]
- Lee, G.S.; Lee, Y.J.; Yoon, K.B. Layer-by layer assembly of zeolite crystals on glass with polyelectrolytes as ionic linkers. J. Am. Chem. Soc. 2001, 123, 9769–9779. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.Z.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystal with a large percentage of reactive facets. Nature 2008, 453, 638–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; He, J.J.; Liu, Z.Y. Experimental studies on crystal growth of anatase under hydrothermal conditions. Earth Sci. 2018, 43, 1763–1772. [Google Scholar]
- Bouvier, P.; Lucazeau, G. Raman spectra and vibrational analysis of nanometric tetragonal zirconia under high pressure. J. Phys. Chem. Solids 2000, 61, 569–578. [Google Scholar] [CrossRef]
| No. | Initial Concentration (mol/L) | Temperature (°C) | Pressure (MPa) | Time (h) | Thickness (nm) | Length (μm) |
|---|---|---|---|---|---|---|
| 1 | 0.02 | 200 | 100 | 12 | 1–50 | 0.1–2.0 |
| 2 | 0.01 | 250 | 80 | 14 | 10–50 | 0.1–1.0 |
| 3 | 0.02 | 250 | 100 | 12 | 10–50 | 0.1–1.0 |
| 4 | 0.02 | 300 | 120 | 12 | 20–80 | 0.1–2.0 |
| 5 | 0.02 | 400 | 100 | 12 | 50–100 | 0.5–1.0 |
| 6 | 0.01 | 400 | 80 | 12 | 20–80 | 0.5–1.0 |
| 7 | 0.01 | 500 | 80 | 12 | 50–80 | 0.5–1.0 |
| 8 | 0.02 | 500 | 80 | 12 | 50–100 | 0.5–2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Di, J.; Li, J.; Liu, Z.; Liu, J.; Ding, X. Synthesis of Zirconia Micro-Nanoflakes with Highly Exposed (001) Facets and Their Crystal Growth. Crystals 2021, 11, 871. https://doi.org/10.3390/cryst11080871
Yan H, Di J, Li J, Liu Z, Liu J, Ding X. Synthesis of Zirconia Micro-Nanoflakes with Highly Exposed (001) Facets and Their Crystal Growth. Crystals. 2021; 11(8):871. https://doi.org/10.3390/cryst11080871
Chicago/Turabian StyleYan, Haibo, Jian Di, Jiahao Li, Zhuoyu Liu, Junfeng Liu, and Xing Ding. 2021. "Synthesis of Zirconia Micro-Nanoflakes with Highly Exposed (001) Facets and Their Crystal Growth" Crystals 11, no. 8: 871. https://doi.org/10.3390/cryst11080871
APA StyleYan, H., Di, J., Li, J., Liu, Z., Liu, J., & Ding, X. (2021). Synthesis of Zirconia Micro-Nanoflakes with Highly Exposed (001) Facets and Their Crystal Growth. Crystals, 11(8), 871. https://doi.org/10.3390/cryst11080871







