1. Introduction
Ferrite ceramics have been widely used for a variety of microwave applications [
1]. In these applications, some devices with complex shapes are needed. Therefore, reliable joining technologies have become necessary and are vital method for breaking the restrictions of the manufacturing processes for ferrite ceramics.
Brazing technology employing glass as a braze has been becoming a potential candidate. Most glass present good mechanical properties and excellent wettability on the surfaces of various ceramics [
2,
3,
4,
5,
6,
7,
8]. In order to protect devices from excessive high temperatures, a relatively low joining temperature is desired in ferrite connection.
In our previous work [
9], 50Bi
2O
3-30B
2O
3-20ZnO glass was employed to braze sapphire ceramics successfully. However, the crystallization behavior of 50Bi
2O
3-30B
2O
3-20ZnO glass has not been studied deeply. Crystallization is crucial to the brazing process of ferrite to ferrite and the properties of corresponding joints [
10]; therefore, we aim to investigate the crystallization behavior of 50Bi
2O
3-30B
2O
3-20ZnO glass specifically. Consequently, an attempt to obtain a reliable ferrite/ferrite joint brazed by 50Bi
2O
3-30B
2O
3-2ZnO glass at the crystallization temperature is conducted.
2. Materials and Methods
The conventional melt-quench method was employed in order to prepare 50Bi
2O
3-30B
2O
3-20ZnO (mol.%) glass [
9]. Raw Bi
2O
3, H
3BO
3 and ZnO powders (AR grade, ≥99 wt.% purity, Aldrich Industrial Corporation, Shanghai, China) were mechanically well-mixed in an alumina crucible (99.99 wt.% purity) for 20 min and subsequently dried in an oven at 150 °C for 30 min. Then, the powders were placed into muffle furnace (KSL1400, Hefei Kejing Materials Technology Co., Ltd, Hefei, China) from room temperature to the melting temperature (1000 °C) for 2 h at 10 °C/min. Subsequently, the melts were poured into cold water in order to acquire glass particles, followed by ball-milling in an agate mortar to produce glass powders.
The morphologies of the glass and the joints were observed by scanning electron microscopy (SEM, Helios NanoLab 600i, FEI Co., Ltd., Hillsboro, OR, USA). X-ray diffraction (XRD, Empyrean-100, Malvern Panalytical Co., Ltd., Almelo, The Neitherlands) with Cu/Kα was conducted to identify the species of the crystals in the glass during heat treatment. Differential scanning calorimeter (DSC, STA449F3, NETZSCH Co., Ltd., Gebrüder-Netzsch-Straße, Germany) was employed to investigate the characteristic temperatures of the glass.
The ferrites used in the present work were of ultra-high purity (99.99 wt.%), and the dimensions were 8 × 8 × 3 and 12 × 8 × 3 mm
3, respectively.
Table 1 shows the chemical compositions of the ferrite substrate used in the current work. Prior to the brazing process, the ferrite plates were burnished by SiC sand papers and polished using 1 μm diamond paste. The cut ceramics were grinded by the sandpapers of 240, 400, 600 and 1000# in this order and then polished with a 1 μm diamond paste. Subsequently, the ceramics were ultrasonically washed in alcohol for 20 min. Meanwhile, the glass powders were mixed with terpineol in a weight ratio of 1:2 in order to produce the glass slurry.
Prior to the joining process, the glass powders and the ferrite plates were fabricated into a sandwich structure. After that the sandwich structure was then subsequently placed in a stainless mold under certain pressures. The assembly was heated to 551 °C and held for 20 min, then left to furnace cooling down to room temperature. The microstructure of joints was characterized via SEM.
3. Results and Discussion
3.1. Crystallization Behavior of 50Bi2O3-30B2O3-20ZnO Glass
The DCS curve for the glass has been presented in our previous work [
9]. The characteristic temperatures of the glass such as the glass transition (T
g), crystallization temperature (T
c), crystallization peak temperature (T
p) and crystallization melting temperature (T
m) were identified (see ref. [
9]).
In order to investigate the crystallization behavior of the glass and to confirm the species of the crystals, heat treatments were carried out at the crystallization peak temperatures for 20 min, which were 477 and 551 °C, respectively.
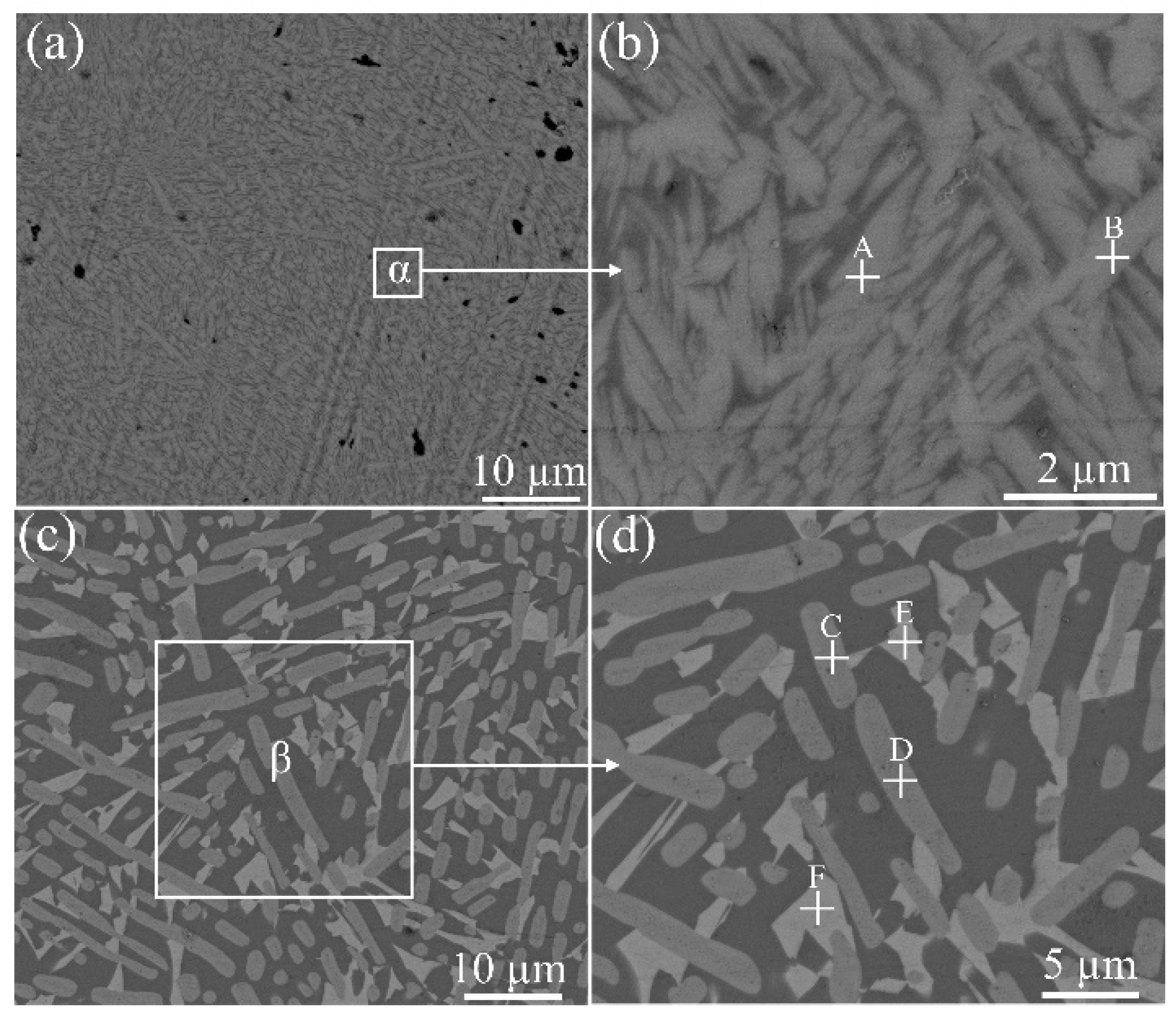
Figure 1a,b shows the SEM morphology of the glass after heat treatment at 477 °C, while
Figure 1c,d shows the morphology of the glass after treating at 551 °C. It can be observed that a lot of crystals were formed in the glass. In order to confirm these crystallization phases in the glass, XRD analysis was carried out.
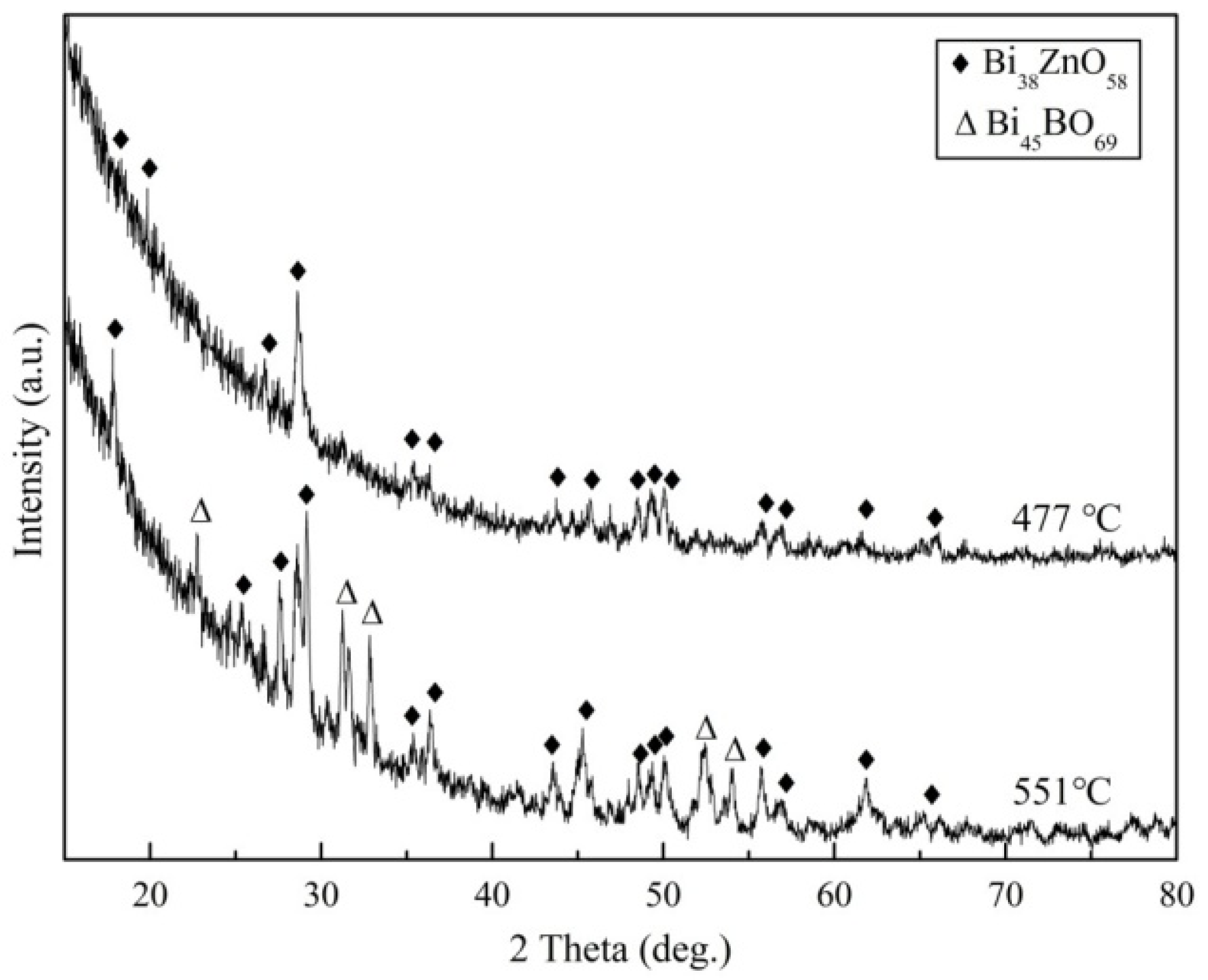
Figure 2 shows the XRD patterns of the 50Bi
2O
3-30B
2O
3-20ZnO glass heat treated at 477 and 551 °C, respectively. The results showed that the phase of Bi38ZnO58 (JCPDS File No. 42-0183) was formed in the glass when heat-treated at 477 °C, while that of Bi
38ZnO
58 and Bi
45BO
69 (JCPDS File No. 42-0194) formed when the glass was heat-treated at 551 °C.
3.2. Microstructure and Mechanical Property of the Ferrite Joint Brazed at Crystallization Temperature Zone
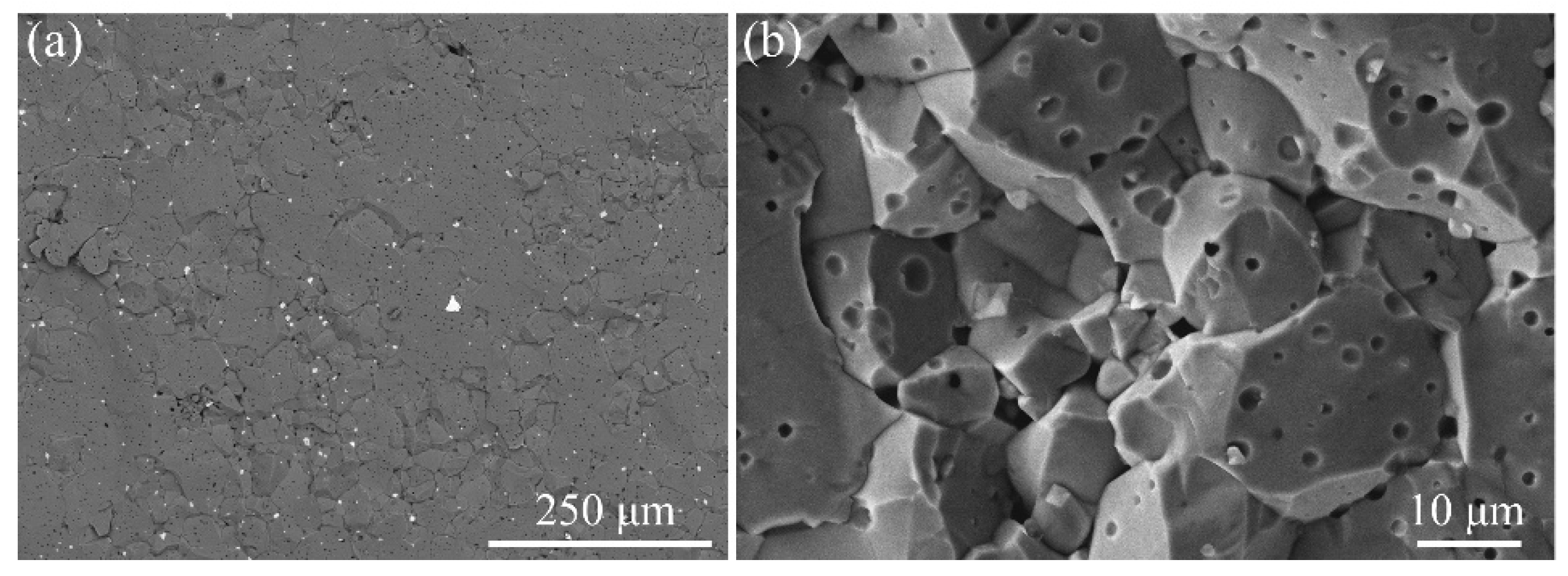
The microstructure of the joint brazed at 551 °C for 20 min is shown in
Figure 3. The results showed that the ferrite was successfully bonded to themselves using the 50Bi
2O
3-30B
2O
3-20ZnO glass. No obvious defects, such as cracks and voids, were detected in the joint domain.
Figure 4b shows the magnified morphology of the interface (selected areas of α), and intimate contact could be observed. In the figure, no other products could be observed except crystallization phases, indicating no chemical reactions between the glass and ferrite under these joining parameters.
The shear test performed on the ferrite joint showed the joint processed an average strength of 76.6 MPa.
Figure 4 shows the fracture surfaces of a joint after shear test. It can be observed that the fracture was completely brittle and took place at the ferrite side, indicating that the interfacial bonding strength between the ferrite and glass is stronger than that of the ferrite ceramic itself. The ideal shear strength of the joint might be due to the formation of the crystals in the joint domain.
4. Conclusions
In the present work, 50Bi2O3-30B2O3-20ZnO (mol.%) glass braze was used to join ferrite ceramics. The following conclusions could be drawn:
(1) The glass has two crystallization peaks during the heating process. Bi38ZnO58 and Bi45BO69 crystals were formed during the brazing process.
(2) Ferrite ceramics were successfully brazed to themselves by the 50Bi2O3-30B2O3-20ZnO glass at 551 °C for 20 min. The main products in the joint domain were Bi38ZnO58 and Bi45BO69 crystals, which was mainly due to the crystallization behavior in the glass.
(3) The average shear strength for the brazed joints was 76.6 MPa under the brazing condition of 551 °C for 20 min. The results demonstrated that the fracture occurred at the ferrite side.
Author Contributions
Conceptualization, W.G.; methodology, W.G.; software, W.G.; validation, W.G.; formal analysis, W.G.; investigation, W.G.; resources, W.G.; data curation, W.G.; writing—original draft preparation, W.G.; writing—review and editing, W.G.; visualization, W.G.; supervision, L.F. and P.H.; project administration, W.G.; funding acquisition, W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Supported by State Key Laboratory of Advanced Welding and Joining, Harbin Institute of Technology (Grant No. AWJ-22M10); Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2021JQ-103). The authors also gratefully acknowledge financial support from the China Scholarship Council (CSC) (Contract No. 201906295008).
Acknowledgments
Thanks Jinglong Li, and Jiangtao Xiong for offering the great help to me in the present work and in the past three years. Your encouragement and inspiration keep me fighting. Thanks.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, W.; Lin, T.; He, P. Joining ferrite at a relatively low temperature using 43SnO-15ZnO-35P2O5-7SiO2 phosphate glass braze. Mater. Lett. 2021, 292, 129656. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.; Mao, Y.; Zhang, L.X.; Feng, J.C. Joining of SiC ceramics using CaO-Al2O3-SiO2 (CAS) glass ceramics. J. Eur. Ceram. Soc. 2020, 40, 267–275. [Google Scholar] [CrossRef]
- Zhu, W.W.; Jiang, H.F.; Sun, S.C.; Sun, S.Q.; Liu, Y.D. Effect of TiO2 content on the crystallization behavior and properties of CaO-Al2O3-SiO2 glass ceramic fillers for high temperature joining application. J. Alloys Compd. 2018, 732, 141–148. [Google Scholar] [CrossRef]
- Lin, Y.J.; Tu, S.H. Joining of Mullite Ceramics with Yttrium Aluminosilicate Glass Interlayers. Ceram. Int. 2009, 35, 1311–1315. [Google Scholar] [CrossRef]
- Lee, H.L.; Nam, S.W.; Hahn, B.S. Joining of Silicon Carbide using MgO-Al2O3-SiO2 Filler. J. Mater. Sci. 1998, 33, 5007–5014. [Google Scholar] [CrossRef]
- Faga, M.G.; Guicciardi, S.; Esposito, L.; Bellosi, A.; Pezzotti, G. Alumina/Alumina and Alumina/Zirconia/Alumina-Zirconia Joints through Glass Interlayer, Microstructure, Mechanical Properties and Residual Stresses. Adv. Mater. 2015, 7, 535–540. [Google Scholar] [CrossRef]
- Guo, W.; Fu, L.; He, P.; Lin, T.S.; Wan, M.L.; Hou, J.; Wu, Y.H.; Liu, X.C.; Shen, Z.K. Air-brazed Al2O3 joint with a novel bismuth glass. Ceram. Int. 2019, 45, 15213–15222. [Google Scholar] [CrossRef]
- Xu, M.J.; Liu, B.S.; Zhao, Y.Q.; Wang, Z.M.; Dong, Z.B. Direct joining of thermoplastic ABS to aluminium alloy 6061-T6 using friction lap welding. Sci. Technol. Weld. Join. 2020, 25, 391–397. [Google Scholar] [CrossRef]
- Guo, W.; Lin, T.S.; He, P.; Sekulic, D.P.; Sun, Z.; Lin, P.P.; Shan, X.; Feng, G.J.; Wu, B.Z.; Wang, M.C. Microstructure and characterization of interfacial phases of sapphire/sapphire joint bonded using Bi2O3-B2O3-ZnO glass. J. Eur. Ceram. Soc. 2017, 37, 1073–1081. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, S.; Gao, H.; Lu, Q.; Lin, T.; He, P.; Geng, H. Characterization of multiple-filled skutterudites with high thermoelectric performance. J. Alloys Compd. 2020, 814, 152272. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).