The Progress of Additive Engineering for CH3NH3PbI3 Photo-Active Layer in the Context of Perovskite Solar Cells
Abstract
:1. Introduction
2. Organic Additives
2.1. N Donor Atom-Based Additives
2.1.1. Amine Additives
2.1.2. Cyano/Nitrile Additives
2.2. O Donor Atom-Based Additives
2.2.1. Carbonyl and Amide Additives
2.2.2. Sulfonyl Additives
2.2.3. Carboxylic/Acid Additives
2.2.4. Carboxylate/Acetate Additives
2.2.5. Ester- and Ether-Based Additives
2.2.6. Hydroxyl/Alcohol
2.2.7. Oxygen-Based Multifunctional Group Containing Additives
2.3. S Donor Atom-Based Additives
2.3.1. Sulfide and Organosulfur Additives
2.3.2. Thiocyanates
2.4. Alkane Additives
2.5. Quantum Dot-Based Additives
3. Inorganic Additives
3.1. Alkali Metals Additives
3.2. Transition Metals Additives
3.3. Other Metals Additives
3.4. Non-Metal Inorganic Salts
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, G.M.; Al-Jassim, M.; Metzger, W.K.; Glunz, S.W.; Verlinden, P.; Xiong, G.; Mansfield, L.M.; Stanbery, B.J.; Zhu, K.; Yan, Y.; et al. The 2020 photovoltaic technologies roadmap. J. Phys. D Appl. Phys. 2020, 53, 493001. [Google Scholar] [CrossRef]
- Pham, H.D.; Yang, T.C.; Jain, S.M.; Wilson, G.J.; Sonar, P. Development of Dopant-Free Organic Hole Transporting Materials for Perovskite Solar Cells. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.; Wang, L.; Pang, S. Review of Stability Enhancement for Formamidinium-Based Perovskites. Sol. RRL 2019, 3, 1900215. [Google Scholar] [CrossRef]
- Roy, P.; Sinha, N.K.; Tiwari, S.; Khare, A. A review on perovskite solar cells: Evolution of architecture, fabrication techniques, commercialization issues and status. Sol. Energy 2020, 198, 665–688. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Ke, J.C.-R.; Walton, A.S.; Lewis, D.J.; Tedstone, A.; O’Brien, P.; Thomas, A.G.; Flavell, W.R. In situ investigation of degradation at organometal halide perovskite surfaces by X-ray photoelectron spectroscopy at realistic water vapour pressure. Chem. Commun. 2017, 53, 5231–5234. [Google Scholar] [CrossRef] [Green Version]
- Abdelmageed, G.; Jewell, L.; Hellier, K.; Seymour, L.; Luo, B.; Bridges, F.; Zhang, J.Z.; Carter, S. Mechanisms for light induced degradation in MAPbI3 perovskite thin films and solar cells. Appl. Phys. Lett. 2016, 109, 233905. [Google Scholar] [CrossRef]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, U.; Kaur, M.; Kumar, M.; Kumar, A. Factors affecting the stability of perovskite solar cells: A comprehensive review. J. Photonics Energy 2019, 9, 021001. [Google Scholar] [CrossRef]
- Manser, J.S.; Saidaminov, M.; Christians, J.; Bakr, O.M.; Kamat, P.V. Making and Breaking of Lead Halide Perovskites. Acc. Chem. Res. 2016, 49, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.; Zheng, C.; Gao, D.; Huang, W. Advancements in the stability of perovskite solar cells: Degradation mechanisms and improvement approaches. RSC Adv. 2016, 6, 38079–38091. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem. Int. Ed. 2015, 54, 8208–8212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.-K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.-W.; Lee, J.S.; Kim, S.; Ihm, K.; et al. Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells using In-situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akbulatov, A.F.; Frolova, L.A.; Dremova, N.N.; Zhidkov, I.S.; Martynenko, V.M.; Tsarev, S.A.; Luchkin, S.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; et al. Light or Heat: What Is Killing Lead Halide Perovskites under Solar Cell Operation Conditions? J. Phys. Chem. Lett. 2020, 11, 333–339. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Ham, S.; Cheng, D.; Wynn, T.A.; Jung, H.S.; Meng, Y.S. Advanced Characterization Techniques for Overcoming Challenges of Perovskite Solar Cell Materials. Adv. Energy Mater. 2020, 11, 1–26. [Google Scholar] [CrossRef]
- Latini, A.; Gigli, G.; Ciccioli, A. A study on the nature of the thermal decomposition of methylammonium lead iodide perovskite, CH3NH3PbI3: An attempt to rationalise contradictory experimental results. Sustain. Energy Fuels 2017, 1, 1351–1357. [Google Scholar] [CrossRef]
- Brunetti, B.; Cavallo, C.; Ciccioli, A.; Gigli, G.; Latini, A. Erratum: Corrigendum: On the Thermal and Thermodynamic (In)Stability of Methylammonium Lead Halide Perovskites. Sci. Rep. 2017, 7, 46867. [Google Scholar] [CrossRef] [PubMed]
- Akbulatov, A.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal degradation of CH3NH3PbI3 perovskite into NH3 and CH3I gases observed by coupled thermogravimetry–mass spectrometry analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Cao, Z.; Yuan, Y.; Chee, M.O.L.; Xie, L.; Wang, A.; Xiang, Y.; Li, T.; Dong, P.; Ding, L.; et al. Coordination modulated crystallization and defect passivation in high quality perovskite film for efficient solar cells. Coord. Chem. Rev. 2020, 420, 213408. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive Engineering for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Azam, M.; Liu, K.; Sun, Y.; Wang, Z.; Liang, G.; Qu, S.; Fan, P.; Wang, Z. Recent advances in defect passivation of perovskite active layer via additive engineering: A review. J. Phys. D Appl. Phys. 2020, 53, 183002. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P.K. A review of aspects of additive engineering in perovskite solar cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Liao, K.; Yang, J.-A.; Li, C.; Li, T.S.; Hao, F. Off-Stoichiometric Methylammonium Iodide Passivated Large-Grain Perovskite Film in Ambient Air for Efficient Inverted Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 39882–39889. [Google Scholar] [CrossRef]
- Dänekamp, B.; Droseros, N.; Palazon, F.; Sessolo, M.; Banerji, N.; Bolink, H.J. Efficient Photo- and Electroluminescence by Trap States Passivation in Vacuum-Deposited Hybrid Perovskite Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 36187–36193. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Shao, F.; Wang, Y.; Xu, T.; Wang, D.; Huang, F. Enhanced Performance of Perovskite CH3NH3PbI3 Solar Cell by Using CH3NH3I as Additive in Sequential Deposition. ACS Appl. Mater. Interfaces 2015, 7, 12937–12942. [Google Scholar] [CrossRef]
- Yang, Y.; Song, J.; Zhao, Y.; Zhu, L.; Gu, X.; Gu, Y.; Che, M.; Qiang, Y. Ammonium-iodide-salt additives induced photovoltaic performance enhancement in one-step solution process for perovskite solar cells. J. Alloys. Compd. 2016, 684, 84–90. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Z.; Hu, Y.; Sheng, Y.; Jiang, P.; Han, H.; Zhang, J. Printed hole-conductor-free mesoscopic perovskite solar cells with excellent long-term stability using PEAI as an additive. J. Energy Chem. 2018, 27, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, S.; Zeng, Q.; Wang, R.; Qin, W.; Cao, H.; Yang, L.; Li, L.; Yin, S.; Zhang, F. Enhanced performance and stability of inverted planar perovskite solar cells by incorporating 1,6-diaminohexane dihydrochloride additive. Sol. Energy Mater. Sol. Cells 2018, 188, 140–148. [Google Scholar] [CrossRef]
- Laskar, A.R.; Luo, W.; Ghimire, N.; Chowdhury, A.H.; Bahrami, B.; Gurung, A.; Reza, K.M.; Pathak, R.; Bobba, R.S.; Lamsal, B.S.; et al. Phenylhydrazinium Iodide for Surface Passivation and Defects Suppression in Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Du, C.; Wang, S.; Miao, X.; Sun, W.; Zhu, Y.; Wang, C.; Ma, R. Polyvinylpyrrolidone as additive for perovskite solar cells with water and isopropanol as solvents. Beilstein J. Nanotechnol. 2019, 10, 2374–2382. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Liu, Z.; Zhou, Z.; Zhu, H.; Zhou, Y.; Huang, C.; Wang, Z.; Xu, H.; Jin, Y.; Fan, B.; et al. Reproducible One-Step Fabrication of Compact MAPbI3−xClx Thin Films Derived from Mixed-Lead-Halide Precursors. Chem. Mater. 2014, 26, 7145–7150. [Google Scholar] [CrossRef]
- Yu, H.; Wang, F.; Xie, F.; Li, W.; Chen, J.; Zhao, N. The Role of Chlorine in the Formation Process of “CH3NH3PbI3−xClx” Perovskite. Adv. Funct. Mater. 2014, 24, 7102–7108. [Google Scholar] [CrossRef]
- Yefang, J.; Ru, D.; Xuediao, C.; Jiangshan, F.; Zhike, L.; Shengzhong, L. Effect of Amphiphilic Quaternary Ammonium Salt Additive on Performance and Stability of Perovskite Solar Cells. J. Chin. Univ. 2019, 40, 1697–1705. [Google Scholar] [CrossRef]
- Mateen, M.; Arain, Z.; Liu, X.; Iqbal, A.; Ren, Y.; Zhang, X.; Liu, C.; Chen, Q.; Ma, S.; Ding, Y.; et al. Boosting optoelectronic performance of MAPbI3 perovskite solar cells via ethylammonium chloride additive engineering. Sci. China Mater. 2020, 63, 2477–2486. [Google Scholar] [CrossRef]
- Yao, J.; Wang, H.; Wang, P.; Gurney, R.S.; Intaniwet, A.; Ruankham, P.; Choopun, S.; Liu, D.; Wang, T. Trap passivation and efficiency improvement of perovskite solar cells by a guanidinium additive. Mater. Chem. Front. 2019, 3, 1357–1364. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Ye, F.; Zhang, H.; Chen, M.; Cai, J.; Li, D.; Liu, D.; Wang, T. Contrasting Effects of Organic Chloride Additives on Performance of Direct and Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 37833–37841. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, L.; Wang, L.; Gao, X.; Zhou, H. The investigation of an amidine-based additive in the perovskite films and solar cells. J. Semicond. 2017, 38, 14001. [Google Scholar] [CrossRef]
- Bae, S.; Jo, J.W.; Lee, P.; Ko, M.J. Controlling the Morphology of Organic–Inorganic Hybrid Perovskites through Dual Additive-Mediated Crystallization for Solar Cell Applications. ACS Appl. Mater. Interfaces 2019, 11, 17452–17458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, N.; Feng, L.; Deng, X. Effects of Organic Cation Additives on the Fast Growth of Perovskite Thin Films for Efficient Planar Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 24703–24711. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hong, Z.; Li, G.; Chen, Q.; Zhou, H.; Yang, Y. One-step, low-temperature deposited perovskite solar cell utilizing small molecule additive. J. Photonics Energy 2015, 5, 057405. [Google Scholar] [CrossRef]
- Mangrulkar, M.; Luchkin, S.Y.; Boldyreva, A.G.; Troshin, P.A.; Stevenson, K.J. Influence of pyridine-based ligands on photostability of MAPbI3 thin films. Mendeleev Commun. 2021, 31, 319–322. [Google Scholar] [CrossRef]
- Frolova, L.A.; Davlethanov, A.I.; Dremova, N.N.; Zhidkov, I.S.; Akbulatov, A.F.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P. Efficient and Stable MAPbI3-Based Perovskite Solar Cells Using Polyvinylcarbazole Passivation. J. Phys. Chem. Lett. 2020, 11, 6772–6778. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, W.; Chen, M.; Li, B.; Jia, L.; Wang, M.; Yang, S. Pyridine-functionalized fullerene additive enabling coordination interactions with CH3NH3PbI3 perovskite towards highly efficient bulk heterojunction solar cells. J. Mater. Chem. A 2019, 7, 2754–2763. [Google Scholar] [CrossRef]
- Fu, S.; Li, X.; Wan, L.; Wu, Y.; Zhang, W.; Wang, Y.; Bao, Q.; Fang, J. Efficient Passivation with Lead Pyridine-2-Carboxylic for High-Performance and Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Cheng, F.; Jing, X.; Chen, R.; Cao, J.; Yan, J.; Wu, Y.; Huang, X.; Wu, B.; Zheng, N. N-Methyl-2-pyrrolidone as an excellent coordinative additive with a wide operating range for fabricating high-quality perovskite films. Inorg. Chem. Front. 2019, 6, 2458–2463. [Google Scholar] [CrossRef]
- Lee, S.; Tang, M.-C.; Munir, R.; Barrit, D.; Kim, Y.-J.; Kang, R.; Yun, J.-M.; Smilgies, D.-M.; Amassian, A.; Kim, D.-Y. In situ study of the film formation mechanism of organic–inorganic hybrid perovskite solar cells: Controlling the solvate phase using an additive system. J. Mater. Chem. A 2020, 8, 7695–7703. [Google Scholar] [CrossRef]
- Xia, R.; Fei, Z.; Drigo, N.; Bobbink, F.D.; Huang, Z.; Jasiūnas, R.; Franckevičius, M.; Gulbinas, V.; Mensi, M.; Fang, X.; et al. Retarding Thermal Degradation in Hybrid Perovskites by Ionic Liquid Additives. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Luo, C.; Li, G.; Chen, L.; Dong, J.; Yu, M.; Xu, C.; Yao, Y.; Wang, M.; Song, Q.; Zhang, S. Passivation of defects in inverted perovskite solar cells using an imidazolium-based ionic liquid. Sustain. Energy Fuels 2020, 4, 3971–3978. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, T.; Bai, L.; Chen, X.; Chen, Z.; Xu, X.; Song, D.; Xu, X.; Li, B.; Yang, Y. Enhanced thermal stability of inverted perovskite solar cells by interface modification and additive strategy. RSC Adv. 2020, 10, 18400–18406. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Q.; Li, J.; Zhao, C.; Guo, X.; Zhao, Y.; Jiu, T. Grain boundary passivation with triazine-graphdiyne to improve perovskite solar cell performance. Sci. China Mater. 2020, 63, 2465–2476. [Google Scholar] [CrossRef]
- Liao, J.-F.; Wu, W.-Q.; Zhong, J.-X.; Jiang, Y.; Wang, L.; Kuang, D.-B. Enhanced efficacy of defect passivation and charge extraction for efficient perovskite photovoltaics with a small open circuit voltage loss. J. Mater. Chem. A 2019, 7, 9025–9033. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Wang, Z.-K.; Li, M.; Zhang, C.-C.; Ye, Q.-Q.; Hu, K.-H.; Lu, D.-Z.; Fang, P.; Liao, L.-S. Passivated Perovskite Crystallization via g-C3N4 for High-Performance Solar Cells. Adv. Funct. Mater. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Hu, L.; Liu, T.; Sun, L.; Xiong, S.; Qin, F.; Jiang, X.; Jiang, Y.; Zhou, Y. Suppressing generation of iodine impurity via an amidine additive in perovskite solar cells. Chem. Commun. 2018, 54, 4704–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Chen, Y.; Liu, Z.; Chen, Q.; Wang, X.; Zhou, H. The Additive Coordination Effect on Hybrids Perovskite Crystallization and High-Performance Solar Cell. Adv. Mater. 2016, 28, 9862–9868. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Bo-Xin, Z.; Wei, J.; Qing-Hong, Z.; Hua-Qing, X. Polymer PVP Additive for Improving Stability of Perovskite Solar Cells. J. Inorg. Mater. 2019, 34, 96–102. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Z.; Shang, Y.; Cui, C.; Fu, P.; Jiang, X.; Wang, F.; Xu, K.; Yin, D.; Qu, D.; et al. Multi-functional organic molecules for surface passivation of perovskite. J. Photochem. Photobiol. A Chem. 2018, 355, 42–47. [Google Scholar] [CrossRef]
- Zhi, L.; Li, Y.; Cao, X.; Li, Y.; Cui, X.; Ci, L.; Wei, J. Perovskite Solar Cells Fabricated by Using an Environmental Friendly Aprotic Polar Additive of 1,3-Dimethyl-2-imidazolidinone. Nanoscale Res. Lett. 2017, 12, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Wang, L.; Ding, X.; Zhao, E.; Yang, S.; Zhao, Y.; Li, Y.; Wang, S.; Ma, T. Incredible PCE enhancement induced by damaged perovskite layers: Deeply understanding the working principle of additives in bulk heterojunction perovskite solar cells. J. Mater. Chem. A 2018, 6, 4365–4373. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yerramilli, A.; Chen, Y.; Fang, D.; Shen, Y.; Alford, T. Enhanced power conversion efficiency and preferential orientation of the MAPbI3 perovskite solar cells by introduction of urea as additive. Org. Electron. 2019, 73, 130–136. [Google Scholar] [CrossRef]
- Han, L.; Cong, S.; Yang, H.; Lou, Y.; Wang, H.; Huang, J.; Zhu, J.; Wu, Y.; Chen, Q.; Zhang, B.; et al. Environmental-Friendly Urea Additive Induced Large Perovskite Grains for High Performance Inverted Solar Cells. Sol. RRL 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Wen, X.; Cai, Q.; Shen, G.; Xu, X.; Dong, P.; Du, Y.; Dong, H.; Mu, C. Enhanced crystallization of solution-processed perovskite using urea as an additive for large-grain MAPbI3 perovskite solar cells. Nanotechnology 2021, 32, 30LT02. [Google Scholar] [CrossRef]
- Lee, J.-W.; Bae, S.-H.; Hsieh, Y.-T.; De Marco, N.; Wang, M.; Sun, P.; Yang, Y. A Bifunctional Lewis Base Additive for Microscopic Homogeneity in Perovskite Solar Cells. Chem 2017, 3, 290–302. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Ma, Z.; Ma, J.; Ma, R.; Wang, C. High-performance perovskite solar cells with large grain-size obtained by the synergy of urea and dimethyl sulfoxide. Appl. Surf. Sci. 2019, 467–468, 708–714. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Wu, J.; Wang, Q.; Ming, Y.; Zhang, D.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; et al. Amide Additives Induced a Fermi Level Shift To Improve the Performance of Hole-Conductor-Free, Printable Mesoscopic Perovskite Solar Cells. J. Phys. Chem. Lett. 2019, 10, 6865–6872. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Chen, J.; Cai, M.; Yang, Y.; Liu, X.; Tao, Y.; Guli, M.; Ding, Y.; Dai, S. Thermally stable perovskite solar cells with efficiency over 21% via a bifunctional additive. J. Mater. Chem. A 2020, 8, 7205–7213. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, H.; Wang, J.; Chen, L.; Bian, L.; An, Z.; Qian, Z.; Ren, G.; Wu, J.; Nüesch, F.; et al. All-in-One Deposition to Synergistically Manipulate Perovskite Growth for High-Performance Solar Cell. Research 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Qin, C.; Matsushima, T.; Fujihara, T.; Adachi, C. Multifunctional Benzoquinone Additive for Efficient and Stable Planar Perovskite Solar Cells. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Yu, W.; Yu, S.; Zhang, J.; Liang, W.; Wang, X.; Guo, X.; Li, C. Two-in-one additive-engineering strategy for improved air stability of planar perovskite solar cells. Nano Energy 2018, 45, 229–235. [Google Scholar] [CrossRef]
- Kuo, D.-W.; Liu, G.-Z.; Lee, R.-H. Star-shaped molecule with planar triazine core and perylene diimide branches as an n-type additive for bulk-heterojunction perovskite solar cells. Dye. Pigment. 2019, 170, 107562. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhang, K.; Huang, P.; Li, D.; Yuan, L.; Cao, T.; Sun, Z.; Li, Z.; Chen, Q.; et al. 3,4-Dihydroxybenzhydrazide as an additive to improve the morphology of perovskite films for efficient and stable perovskite solar cells. Org. Electron. 2019, 66, 47–52. [Google Scholar] [CrossRef]
- Xiong, S.; Song, J.; Yang, J.; Xu, J.; Zhang, M.; Ma, R.; Li, D.; Liu, X.; Liu, F.; Duan, C.; et al. Defect-Passivation Using Organic Dyes for Enhanced Efficiency and Stability of Perovskite Solar Cells. Sol. RRL 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.; Song, Y. Efficient flexible perovskite solar cells based on a polymer additive. Flex. Print. Electron. 2020, 5, 014001. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Wang, Y.; Fu, S.; Wan, L.; Liu, S.; Zhang, W.; Song, W.; Fang, J. Sulfonyl-based non-fullerene electron acceptor-assisted grain boundary passivation for efficient and stable perovskite solar cells. J. Mater. Chem. A 2019, 7, 19881–19888. [Google Scholar] [CrossRef]
- Cao, X.B.; Li, C.; Zhi, L.L.; Li, Y.H.; Cui, X.; Yao, Y.W.; Ci, L.J.; Wei, J.Q. Fabrication of high quality perovskite films by modulating the Pb–O bonds in Lewis acid–base adducts. J. Mater. Chem. A 2017, 5, 8416–8422. [Google Scholar] [CrossRef]
- Cao, X.; Zhi, L.; Li, Y.; Cui, X.; Ci, L.; Ding, K.; Wei, J. Enhanced performance of perovskite solar cells by strengthening a self-embedded solvent annealing effect in perovskite precursor films. RSC Adv. 2017, 7, 49144–49150. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, R.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.; Guo, P.; Zhou, N.; Marks, T.J.; Chang, R.P.H.; Kanatzidis, M.G. Solvent-Mediated Crystallization of CH3NH3SnI3 Films for Heterojunction Depleted Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 11445–11452. [Google Scholar] [CrossRef]
- Cao, X.; Zhi, L.; Li, Y.; Fang, F.; Cui, X.; Yao, Y.; Ci, L.; Ding, K.; Wei, J. Control of the morphology of PbI 2 films for efficient perovskite solar cells by strong Lewis base additives. J. Mater. Chem. C 2017, 5, 7458–7464. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, X.; Zhu, J.; Hayat, T.; Alsaedi, A.; Li, Z.; Xu, X.; Ding, Y.; Yang, S.; Kong, M.; et al. A Bi-functional additive for linking PI 2 and decreasing defects in organo-halide perovskites. J. Alloys Compd. 2018, 758, 171–176. [Google Scholar] [CrossRef]
- Foley, B.J.; Girard, J.; Sorenson, B.A.; Chen, A.Z.; Niezgoda, J.S.; Alpert, M.; Harper, A.; Smilgies, D.-M.; Clancy, P.; Saidi, W.A.; et al. Controlling nucleation, growth, and orientation of metal halide perovskite thin films with rationally selected additives. J. Mater. Chem. A 2017, 5, 113–123. [Google Scholar] [CrossRef]
- Gutwald, M.; Rolston, N.; Printz, A.D.; Zhao, O.; Elmaraghi, H.; Ding, Y.; Zhang, J.; Dauskardt, R.H. Perspectives on intrinsic toughening strategies and passivation of perovskite films with organic additives. Sol. Energy Mater. Sol. Cells 2020, 209, 110433. [Google Scholar] [CrossRef]
- Lin, C.-T.; De Rossi, F.; Kim, J.; Baker, J.; Ngiam, J.; Xu, B.; Pont, S.; Aristidou, N.; Haque, S.A.; Watson, T.; et al. Evidence for surface defect passivation as the origin of the remarkable photostability of unencapsulated perovskite solar cells employing aminovaleric acid as a processing additive. J. Mater. Chem. A 2019, 7, 3006–3011. [Google Scholar] [CrossRef] [Green Version]
- Santhosh, N.; Sitaaraman, S.; Pounraj, P.; Govindaraj, R.; Pandian, M.S.; Ramasamy, P. Fabrication of hole-transport-free perovskite solar cells using 5-ammonium valeric acid iodide as additive and carbon as counter electrode. Mater. Lett. 2019, 236, 706–709. [Google Scholar] [CrossRef]
- Li, N.; Xu, F.; Qiu, Z.; Liu, J.; Wan, X.; Zhu, X.; Yu, H.; Li, C.; Liu, Y.; Cao, B. Sealing the domain boundaries and defects passivation by Poly(acrylic acid) for scalable blading of efficient perovskite solar cells. J. Power Sources 2019, 426, 188–196. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Zhou, P.; Li, S.; Ren, J.; He, H.; Wang, J.; Wang, N.; Guo, S. Efficient Bifacial Passivation with Crosslinked Thioctic Acid for High-Performance Methylammonium Lead Iodide Perovskite Solar Cells. Adv. Mater. 2020, 32, 1905661. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Q.; Deng, X.; Zheng, J.; Ou-Yang, W.; Chen, X.; Huang, S. Enhanced efficiency and stability of carbon based perovskite solar cells using terephthalic acid additive. Electrochim. Acta 2017, 258, 1262–1272. [Google Scholar] [CrossRef]
- Su, L.; Xiao, Y.; Han, G.; Lu, L.; Li, H.; Zhu, M. Performance enhancement of perovskite solar cells using trimesic acid additive in the two-step solution method. J. Power Sources 2019, 426, 11–15. [Google Scholar] [CrossRef]
- Su, L.; Xiao, Y.; Lu, L.; Han, G.; Zhu, M. Enhanced stability and solar cell performance via π-conjugated Lewis base passivation of organic inorganic lead halide perovskites. Org. Electron. 2020, 77, 105519. [Google Scholar] [CrossRef]
- Yao, X.; Zheng, L.; Zhang, X.; Xu, W.; Hu, W.; Gong, X. Efficient Perovskite Solar Cells through Suppressed Nonradiative Charge Carrier Recombination by a Processing Additive. ACS Appl. Mater. Interfaces 2019, 11, 40163–40171. [Google Scholar] [CrossRef]
- Pockett, A.; Raptis, D.; Meroni, S.M.P.; Baker, J.; Watson, T.M.; Carnie, M. Origin of Exceptionally Slow Light Soaking Effect in Mesoporous Carbon Perovskite Solar Cells with AVA Additive. J. Phys. Chem. C 2019, 123, 11414–11421. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Luo, J.; Malik, H.A.; Zhao, B.; Wan, Z.; Jia, C. A functional sulfonic additive for high efficiency and low hysteresis perovskite solar cells. J. Power Sources 2017, 359, 577–584. [Google Scholar] [CrossRef]
- Gao, C.; Dong, H.; Bao, X.; Zhang, Y.; Saparbaev, A.; Yu, L.; Wen, S.; Yang, R.; Dong, L. Additive engineering to improve the efficiency and stability of inverted planar perovskite solar cells. J. Mater. Chem. C 2018, 6, 8234–8241. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Siffalovic, P.; Chen, L.-C.; Cao, G.; Tian, J. Monolayer-like hybrid halide perovskite films prepared by additive engineering without antisolvents for solar cells. J. Mater. Chem. A 2018, 6, 15386–15394. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, F.; Chen, H.; Yang, X.; Su, H.; Cai, M.; Zhou, Z.; Noda, T.; Han, L. Thermally Stable MAPbI3Perovskite Solar Cells with Efficiency of 19.19% and Area over 1 cm2achieved by Additive Engineering. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, P.; Zhou, W.; Wei, X.; Chen, T.; Yang, S. Acetate Salts as Nonhalogen Additives To Improve Perovskite Film Morphology for High-Efficiency Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 15333–15340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, W.; Wei, X.; Zhang, L.; Yang, Z.; Wei, Z.; Shen, T.; Si, H.; Qi, J. Promoted performance of carbon based perovskite solar cells by environmentally friendly additives of CH3COONH4 and Zn(CH3COO)2. J. Alloys Compd. 2019, 802, 694–703. [Google Scholar] [CrossRef]
- Tang, G.; You, P.; Tai, Q.; Wu, R.; Yan, F. Performance Enhancement of Perovskite Solar Cells Induced by Lead Acetate as an Additive. Sol. RRL 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Fu, S.; Song, C.; Wan, L.; Zhang, W.; Li, X.; Yang, W.; Song, W.; Fang, J. Barium acetate as an additive for high performance perovskite solar cells. J. Mater. Chem. C 2019, 7, 11411–11418. [Google Scholar] [CrossRef]
- Guan, Y.; Mei, A.; Rong, Y.; Duan, M.; Hou, X.; Hu, Y.; Han, H. Fullerene derivative as an additive for highly efficient printable mesoscopic perovskite solar cells. Org. Electron. 2018, 62, 653–659. [Google Scholar] [CrossRef]
- Hu, L.; Li, S.; Zhang, L.; Liu, Y.; Zhang, C.; Wu, Y.; Sun, Q.; Cui, Y.; Zhu, F.; Hao, Y.; et al. Unravelling the role of C60 derivatives as additives into active layers for achieving high-efficiency planar perovskite solar cells. Carbon 2020, 167, 160–168. [Google Scholar] [CrossRef]
- Chen, N.; Yi, X.; Zhuang, J.; Wei, Y.; Zhang, Y.; Wang, F.; Cao, S.; Li, C.; Wang, J. An Efficient Trap Passivator for Perovskite Solar Cells: Poly(propylene glycol) bis(2-aminopropyl ether). Nano-Micro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Hsu, H.-L.; Jiang, B.-H.; Chung, C.-L.; Yu, Y.-Y.; Jeng, R.-J.; Chen, C.-P. Commercially available jeffamine additives for p–i–n perovskite solar cells. Nanotechnol. 2020, 31, 274002. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, S.; Qu, T.; Zhang, Y.; He, X.; Guo, X.; Zhao, Q.; Braun, S.; Chen, J.; Xu, J.; et al. Extremely Low-Cost and Green Cellulose Passivating Perovskites for Stable and High-Performance Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 13491–13498. [Google Scholar] [CrossRef]
- Guan, M.; Zhang, Q.; Wang, F.; Liu, H.; Zhao, J.; Jia, C.; Chen, Y. Employing tetraethyl orthosilicate additive to enhance trap passivation of planar perovskite solar cells. Electrochim. Acta 2019, 293, 174–183. [Google Scholar] [CrossRef]
- De Carvalho, B.A.; Kavadiya, S.; Huang, S.; Niedzwiedzki, D.; Biswas, P. Highly Stable Perovskite Solar Cells Fabricated Under Humid Ambient Conditions. IEEE J. Photovoltaics 2017, 7, 532–538. [Google Scholar] [CrossRef]
- Isa, M.J.A.; Sulistianto, J.; Kevin, L.; Poespawati, N.R. Optimization of Perovskite Solar Cell Performance using Optimized Level of Tetraethyl Orthosilicate Concentration. In Proceedings of the 2019 11th International Conference on Information Technology and Electrical Engineering (ICITEE), Pattaya, Thailand, 10–11 October 2019; Volume 7. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kulkarni, A.; Jena, A.K.; Ikegami, M.; Miyasaka, T. Tetrahydrofuran as an Oxygen Donor Additive to Enhance Stability and Reproducibility of Perovskite Solar Cells Fabricated in High Relative Humidity (50%) Atmosphere. Energy Technol. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, W.; Xia, R. Antisolvent diethyl ether as additive to enhance the performance of perovskite solar cells. Thin Solid Film. 2018, 663, 9–13. [Google Scholar] [CrossRef]
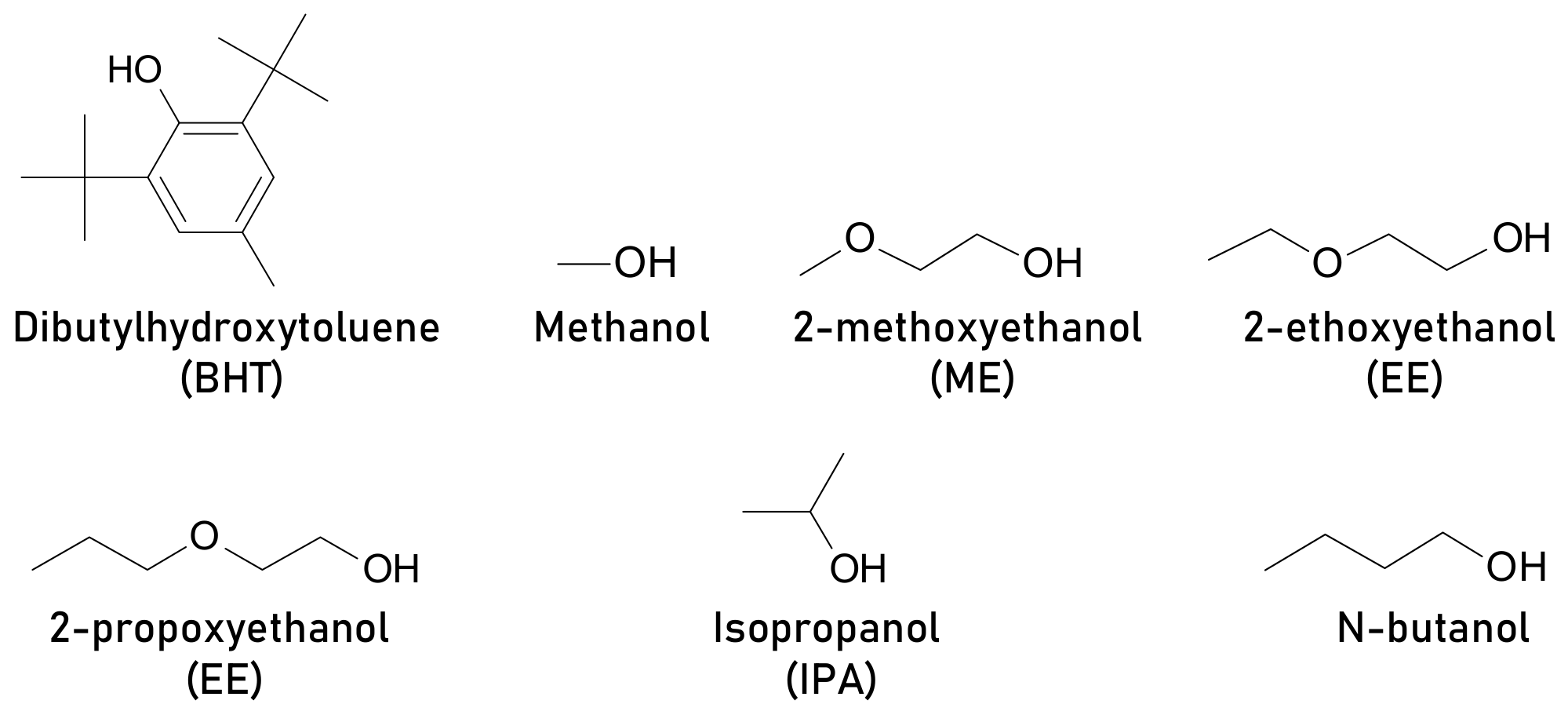
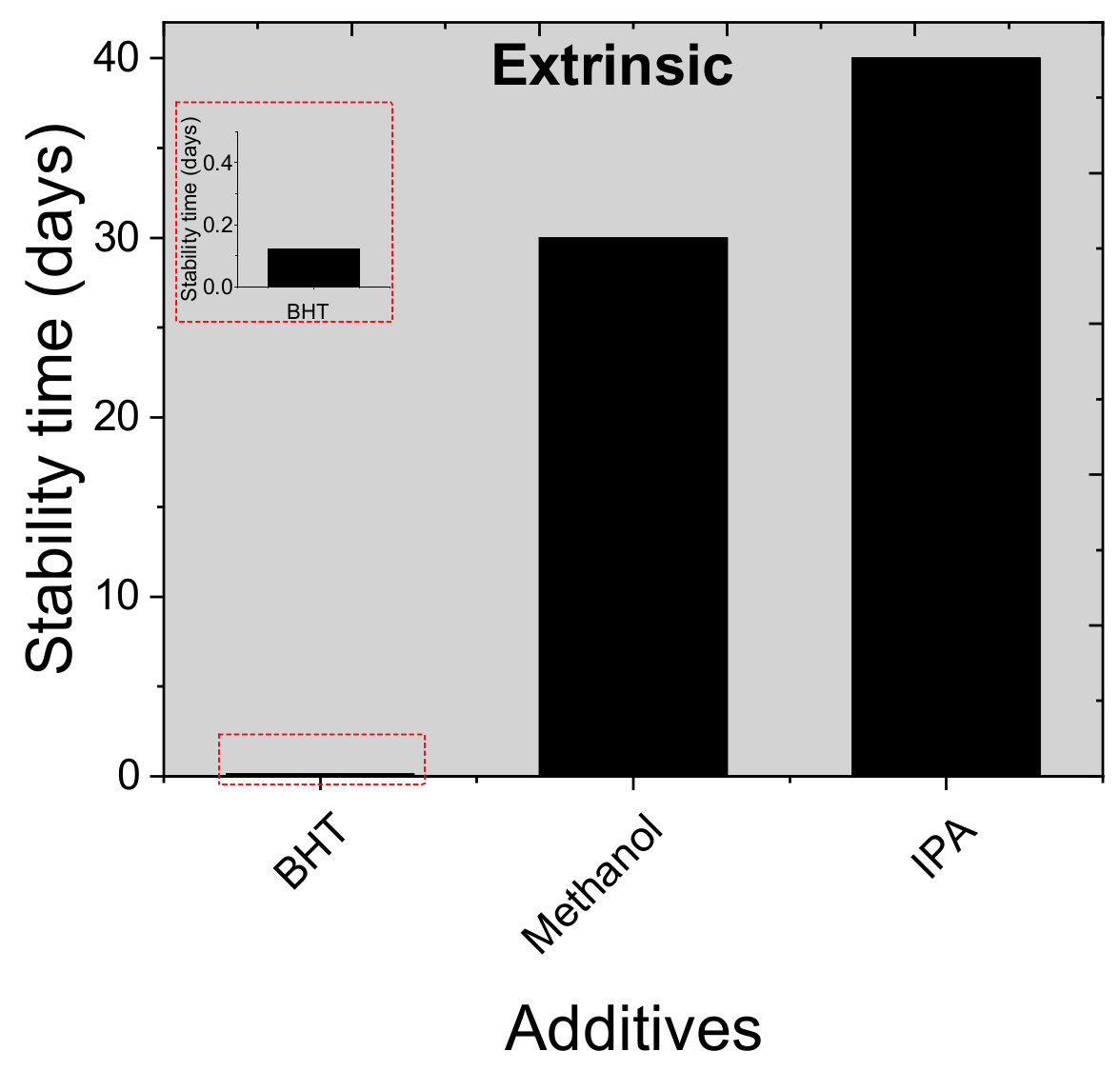
- Kumar, S.; Choi, Y.; Kang, S.-H.; Oh, N.K.; Lee, J.; Seo, J.; Jeong, M.; Kwon, H.W.; Seok, S.I.; Yang, C.; et al. Multifaceted Role of a Dibutylhydroxytoluene Processing Additive in Enhancing the Efficiency and Stability of Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 38828–38837. [Google Scholar] [CrossRef]
- Feng, M.; You, S.; Cheng, N.; Du, J. High quality perovskite film solar cell using methanol as additive with 19.5% power conversion efficiency. Electrochim. Acta 2019, 293, 356–363. [Google Scholar] [CrossRef]
- You, S.; Bi, S.; Qiushuang, J.; Jia, Q.; Yuan, Y.; Xia, Y.; Xiao, Z.; Sun, Z.; Liu, J.; Sun, S.; et al. Additive-Enhanced Crystallization of Solution Process for Planar Perovskite Solar Cells with Efficiency Exceeding 19 %. Chem. A Eur. J. 2017, 23, 18140–18145. [Google Scholar] [CrossRef] [PubMed]
- Ugur, E.; Sheikh, A.D.; Munir, R.; Khan, J.I.; Barrit, D.; Amassian, A.; Laquai, F. Improved Morphology and Efficiency of n–i–p Planar Perovskite Solar Cells by Processing with Glycol Ether Additives. ACS Energy Lett. 2017, 2, 1960–1968. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, H.; Liu, S.; Zheng, B.; Xue, Y.; Liu, X.; Gao, C. Tuning PbI2layers by n-butanol additive for improving CH3NH3PbI3light harvesters of perovskite solar cells. RSC Adv. 2016, 6, 89609–89613. [Google Scholar] [CrossRef]
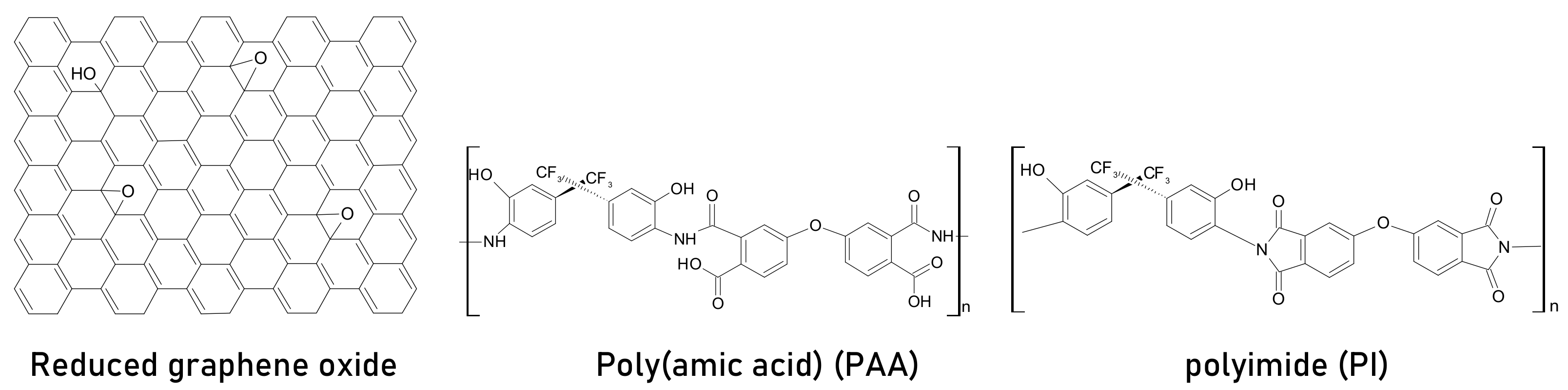
- Balis, N.; Zaky, A.A.; Athanasekou, C.; Silva, A.; Sakellis, E.; Vasilopoulou, M.; Stergiopoulos, T.; Kontos, A.G.; Falaras, P. Investigating the role of reduced graphene oxide as a universal additive in planar perovskite solar cells. J. Photochem. Photobiol. A Chem. 2020, 386, 112141. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Tseng, C.; Chien, W.-C.; Hsu, H.-L.; Chen, C.-P. Photovoltaic Performance Enhancement of Perovskite Solar Cells Using Polyimide and Polyamic Acid as Additives. J. Phys. Chem. C 2019, 123, 23826–23833. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, X.; Yang, Z.; Zhang, X.; Niu, J.; Wang, Z.; Zuo, S.; Priya, S.; Liu, F.; Yang, D. Record Efficiency Stable Flexible Perovskite Solar Cell Using Effective Additive Assistant Strategy. Adv. Mater. 2018, 30, 1801418. [Google Scholar] [CrossRef]
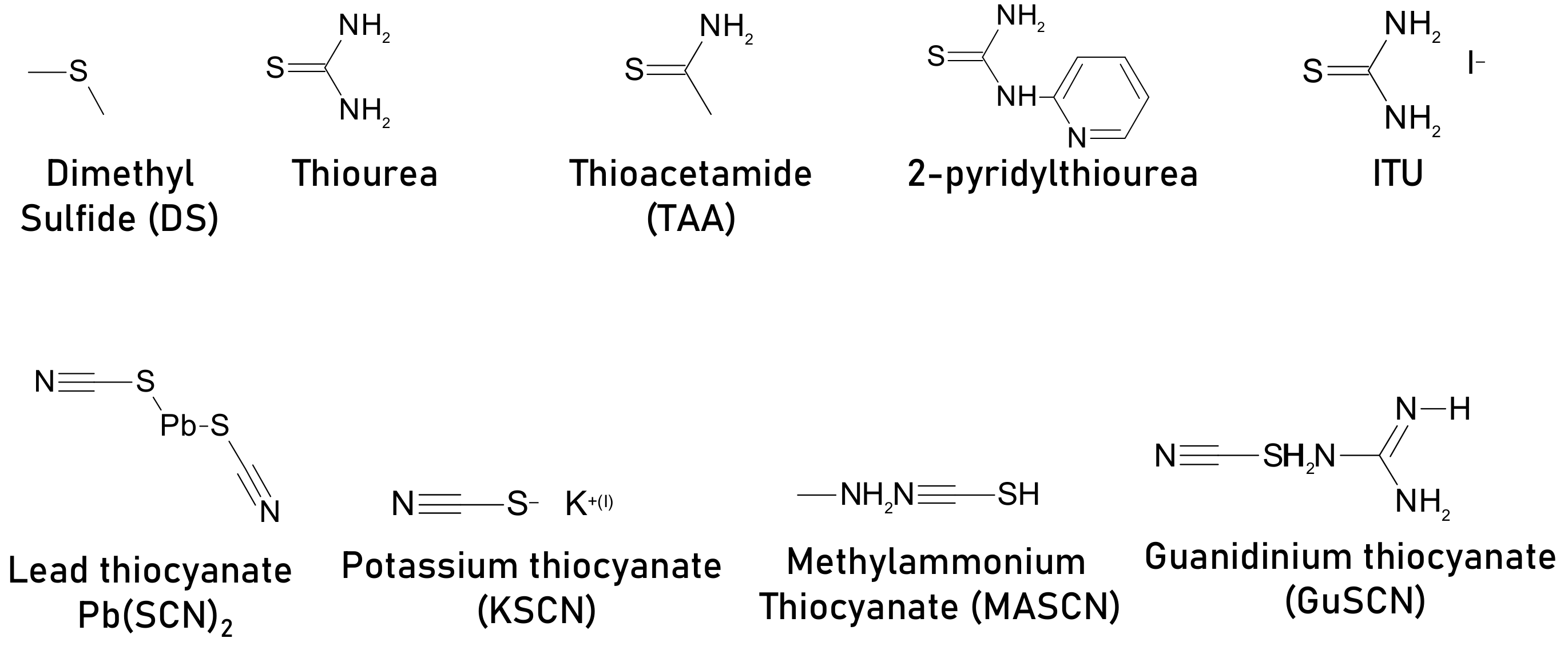
- Hsieh, C.-M.; Liao, Y.-S.; Lin, Y.-R.; Chen, C.-P.; Tsai, C.-M.; Diau, E.W.-G.; Chuang, S.-C. Low-temperature, simple and efficient preparation of perovskite solar cells using Lewis bases urea and thiourea as additives: Stimulating large grain growth and providing a PCE up to 18.8%. RSC Adv. 2018, 8, 19610–19615. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Xie, D.; Lin, P.; Hu, H.; Che, S.; Xiao, K.; Wang, P.; Xu, L.; Yang, D.; Yu, X. Thioacetamide additive assisted crystallization of solution-processed perovskite films for high performance planar heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2020, 208, 110435. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, F.; Liu, H.; Li, X.; Xiao, Y.; Wang, S. Tuning the crystal growth of perovskite thin-films by adding the 2-pyridylthiourea additive for highly efficient and stable solar cells prepared in ambient air. J. Mater. Chem. A 2017, 5, 13448–13456. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Liu, Y.; Chen, C.; Bai, X.; Yang, L.; Shi, Z.; Yu, W.W.; Dai, Q.; Zhang, Y. Dual Functions of Crystallization Control and Defect Passivation Enabled by an Ionic Compensation Strategy for Stable and High-Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 12, 3631–3641. [Google Scholar] [CrossRef]
- Ke, W.; Xiao, C.; Wang, C.; Saparov, B.; Duan, H.-S.; Zhao, D.; Xiao, Z.; Schulz, P.; Harvey, S.P.; Liao, W.; et al. Employing Lead Thiocyanate Additive to Reduce the Hysteresis and Boost the Fill Factor of Planar Perovskite Solar Cells. Adv. Mater. 2016, 28, 5214–5221. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jeon, T.; Park, H.I.; Lee, J.M.; Nam, S.A.; Kim, S.O. Effective control of crystal grain size in CH3NH3PbI3 perovskite solar cells with a pseudohalide Pb(SCN)2additive. CrystEngComm 2016, 18, 6090–6095. [Google Scholar] [CrossRef]
- Zhang, R.; Li, M.; Huan, Y.; Xi, J.; Zhang, S.; Cheng, X.; Wu, H.; Peng, W.; Bai, Z.; Yan, X. A potassium thiocyanate additive for hysteresis elimination in highly efficient perovskite solar cells. Inorg. Chem. Front. 2019, 6, 434–442. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, M.; Xia, Y.; Wei, Q.; Wang, Z.; Cheng, Y.; Chen, Y.; Huang, W. Synergistic effect of anions and cations in additives for highly efficient and stable perovskite solar cells. J. Mater. Chem. A 2018, 6, 9264–9270. [Google Scholar] [CrossRef]
- Han, Q.; Bai, Y.; Liu, J.; Du, K.; Li, T.; Ji, D.; Zhou, Y.; Cao, C.; Shin, D.; Ding, J.; et al. Additive engineering for high-performance room-temperature-processed perovskite absorbers with micron-size grains and microsecond-range carrier lifetimes. Energy Environ. Sci. 2017, 10, 2365–2371. [Google Scholar] [CrossRef]
- Cheng, N.; Li, W.; Zhang, M.; Wu, H.; Sun, S.; Zhao, Z.; Xiao, Z.; Sun, Z.; Zi, W.; Fang, L. Enhance the performance and stability of methylammonium lead iodide perovskite solar cells with guanidinium thiocyanate additive. Curr. Appl. Phys. 2019, 19, 25–30. [Google Scholar] [CrossRef]
- Zou, J.; Liu, W.; Deng, W.; Lei, G.; Zeng, S.; Xiong, J.; Gu, H.; Hu, Z.; Wang, X.; Li, J. An efficient guanidinium isothiocyanate additive for improving the photovoltaic performances and thermal stability of perovskite solar cells. Electrochim. Acta 2018, 291, 297–303. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Anaya, M.; Lozano, G.; Tress, W.; Domanski, K.; Saliba, M.; Matsui, T.; Jacobsson, J.; Calvo, M.; Abate, A.; et al. Unbroken Perovskite: Interplay of Morphology, Electro-optical Properties, and Ionic Movement. Adv. Mater. 2016, 28, 5031–5037. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Lin, B.; Zhu, Y.; Zhang, K.; Yuan, N.-Y.; Ding, J.-N.; Fang, B. PVDF-HFP additive for visible-light-semitransparent perovskite films yielding enhanced photovoltaic performance. Sol. Energy Mater. Sol. Cells 2017, 170, 178–186. [Google Scholar] [CrossRef]
- Sun, C.; Guo, Y.; Fang, B.; Yang, J.; Qin, B.; Duan, H.; Chen, Y.; Li, H.; Liu, H. Enhanced Photovoltaic Performance of Perovskite Solar Cells Using Polymer P(VDF-TrFE) as a Processed Additive. J. Phys. Chem. C 2016, 120, 12980–12988. [Google Scholar] [CrossRef]
- Eze, V.O.; Seike, Y.; Mori, T. Synergistic Effect of Additive and Solvent Vapor Annealing on the Enhancement of MAPbI3 Perovskite Solar Cells Fabricated in Ambient Air. ACS Appl. Mater. Interfaces 2020, 12, 46837–46845. [Google Scholar] [CrossRef] [PubMed]
- AnkiReddy, K.; Ghahremani, A.H.; Martin, B.; Gupta, G.; Druffel, T. Rapid thermal annealing of CH3NH3PbI3 perovskite thin films by intense pulsed light with aid of diiodomethane additive. J. Mater. Chem. A 2018, 6, 9378–9383. [Google Scholar] [CrossRef]
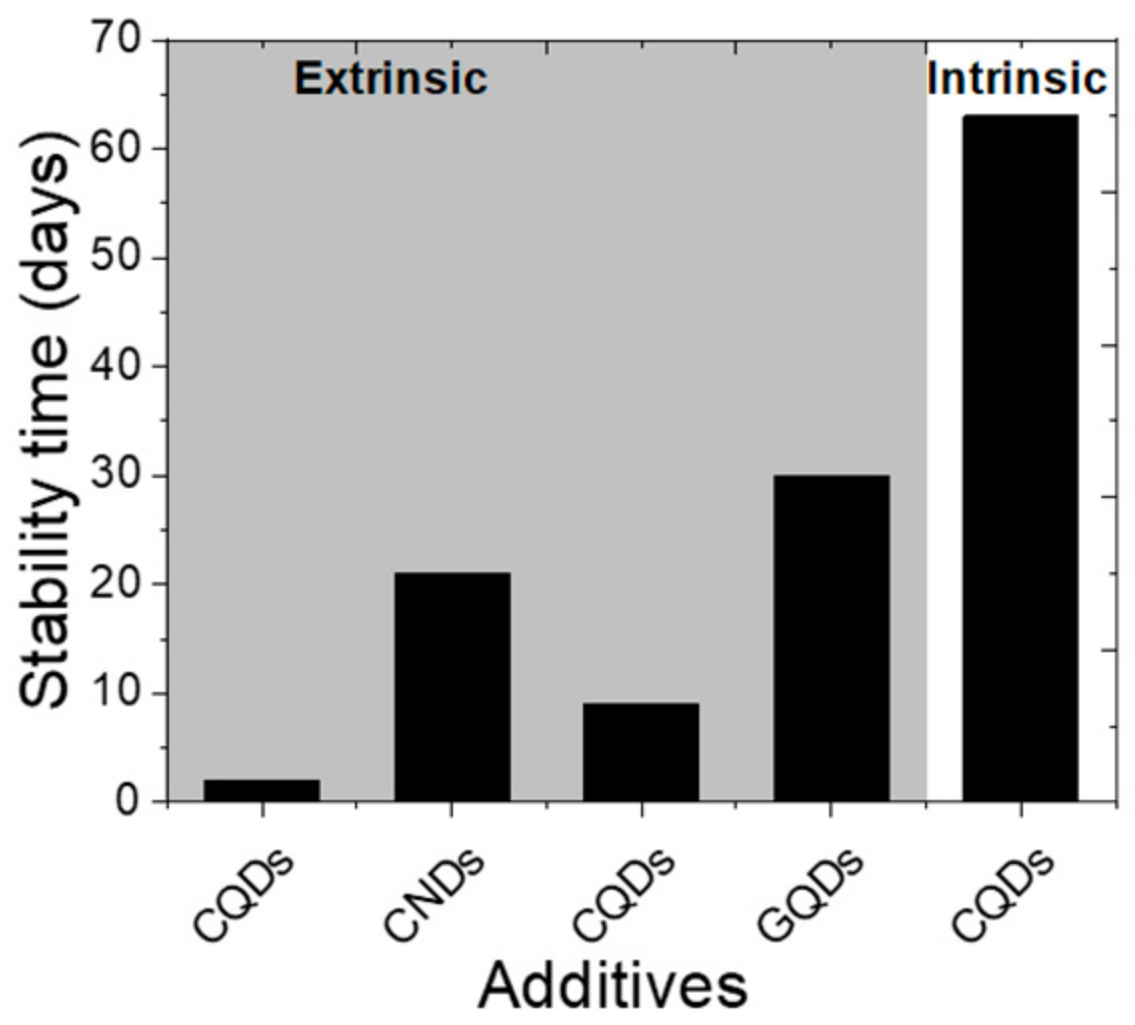
- Ma, Y.; Zhang, H.; Zhang, Y.; Hu, R.; Jiang, M.; Zhang, R.; Lv, H.; Tian, J.; Chu, L.; Zhang, J.; et al. Enhancing the Performance of Inverted Perovskite Solar Cells via Grain Boundary Passivation with Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2018, 11, 3044–3052. [Google Scholar] [CrossRef]
- Kırbıyık, Ç.; Toprak, A.; Başlak, C.; Kuş, M.; Ersöz, M. Nitrogen-doped CQDs to enhance the power conversion efficiency of perovskite solar cells via surface passivation. J. Alloys Compd. 2020, 832, 154897. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, F.; Zhang, B.; Zhou, S.; Zhang, J.; Bai, Y.; Fan, L.; Hayat, T.; Alsaedi, A.; Tan, Z. Passivation of the grain boundaries of CH3NH3PbI3 using carbon quantum dots for highly efficient perovskite solar cells with excellent environmental stability. Nanoscale 2019, 11, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-L.; Hsiao, H.-T.; Juang, T.-Y.; Jiang, B.-H.; Chen, S.-C.; Jeng, R.-J.; Chen, C.-P. Carbon Nanodot Additives Realize High-Performance Air-Stable p-i-n Perovskite Solar Cells Providing Efficiencies of up to 20.2%. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Liu, C.; Gao, F.; Shen, L.; Guo, W. Efficient perovskite solar cells enabled by ion-modulated grain boundary passivation with a fill factor exceeding 84%. J. Mater. Chem. A 2019, 7, 22359–22365. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, S.; Zhang, H.; Li, L.; Fu, Z. Surface passivation with nitrogen-doped carbon dots for improved perovskite solar cell performance. J. Mater. Sci. 2018, 53, 9180–9190. [Google Scholar] [CrossRef]
- Fang, X.; Ding, J.; Yuan, N.; Sun, P.; Lv, M.; Ding, G.; Zhu, C. Graphene quantum dot incorporated perovskite films: Passivating grain boundaries and facilitating electron extraction. Phys. Chem. Chem. Phys. 2017, 19, 6057–6063. [Google Scholar] [CrossRef] [PubMed]
- Brakkee, R.; Williams, R.M. Minimizing Defect States in Lead Halide Perovskite Solar Cell Materials. Appl. Sci. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Soe, C.M.M.; Stoumpos, C.C.; Harutyunyan, B.; Manley, E.F.; Chen, L.X.; Bedzyk, M.J.; Marks, T.J.; Kanatzidis, M.G. Room Temperature Phase Transition in Methylammonium Lead Iodide Perovskite Thin Films Induced by Hydrohalic Acid Additives. ChemSusChem 2016, 9, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, N.; Li, G.; Qian, X.; Zhao, Y. A controllable fabrication of grain boundary PbI2 nanoplates passivated lead halide perovskites for high performance solar cells. Nano Energy 2016, 26, 50–56. [Google Scholar] [CrossRef]
- Wen, Y.; Tang, Y.-G.; Yan, G.-Q. Large grain size CH3NH3PbI3 film for perovskite solar cells with hydroic acid additive. AIP Adv. 2018, 8, 095226. [Google Scholar] [CrossRef] [Green Version]
- Abdi-Jalebi, M.; Dar, M.I.; Sadhanala, A.; Senanayak, S.P.; Franckevicius, M.; Arora, N.; Hu, Y.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Grätzel, M.; et al. Impact of Monovalent Cation Halide Additives on the Structural and Optoelectronic Properties of CH3NH3PbI3 Perovskite. Adv. Energy Mater. 2016, 6, 1502472. [Google Scholar] [CrossRef] [Green Version]
- Boopathi, K.M.; Mohan, R.; Huang, T.-Y.; Budiawan, W.; Lin, M.-Y.; Lee, C.-H.; Ho, K.-C.; Chu, C.-W. Synergistic improvements in stability and performance of lead iodide perovskite solar cells incorporating salt additives. J. Mater. Chem. A 2016, 4, 1591–1597. [Google Scholar] [CrossRef]
- Park, I.J.; Seo, S.; Park, M.A.; Lee, S.; Kim, D.H.; Zhu, K.; Shin, H.; Kim, J.Y. Effect of Rubidium Incorporation on the Structural, Electrical, and Photovoltaic Properties of Methylammonium Lead Iodide-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 41898–41905. [Google Scholar] [CrossRef]
- Chan, S.-H.; Wu, M.-C.; Lee, K.-M.; Chen, W.-C.; Lin, T.-H.; Su, W.-F. Enhancing perovskite solar cell performance and stability by doping barium in methylammonium lead halide. J. Mater. Chem. A 2017, 5, 18044–18052. [Google Scholar] [CrossRef]
- Chen, C.; Xu, Y.; Wu, S.; Zhang, S.; Yang, Z.; Zhang, W.; Zhu, H.; Xiong, Z.; Chen, W.; Chen, W. CaI2: A more effective passivator of perovskite films than PbI2 for high efficiency and long-term stability of perovskite solar cells. J. Mater. Chem. A 2018, 6, 7903–7912. [Google Scholar] [CrossRef]
- Mabrouk, S.; Bahrami, B.; Gurung, A.; Reza, K.M.; Adhikari, N.; Dubey, A.; Pathak, R.; Yang, S.; Qiao, Q. Higher efficiency perovskite solar cells using additives of LiI, LiTFSI and BMImI in the PbI2 precursor. Sustain. Energy Fuels 2017, 1, 2162–2171. [Google Scholar] [CrossRef]
- Duan, B.; Ren, Y.; Xu, Y.; Chen, W.; Ye, Q.; Huang, Y.; Zhu, J.; Dai, S. Identification and characterization of a new intermediate to obtain high quality perovskite films with hydrogen halides as additives. Inorg. Chem. Front. 2017, 4, 473–480. [Google Scholar] [CrossRef]
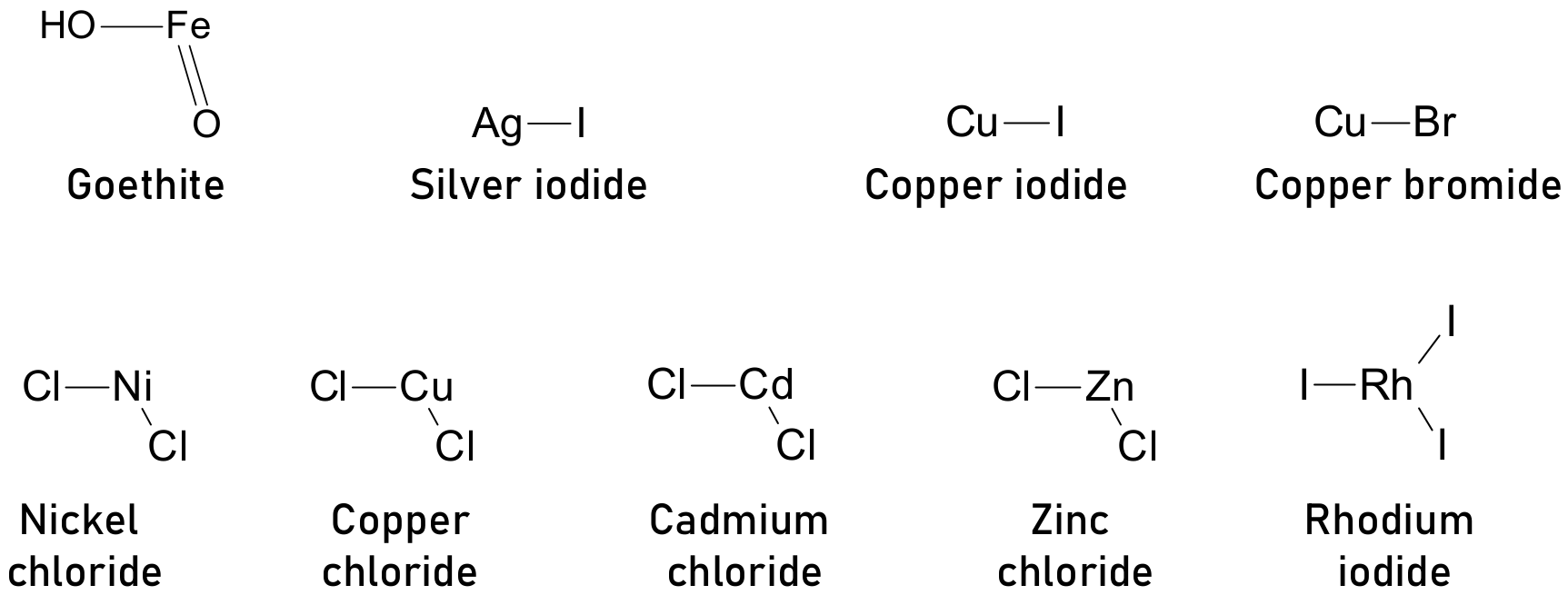
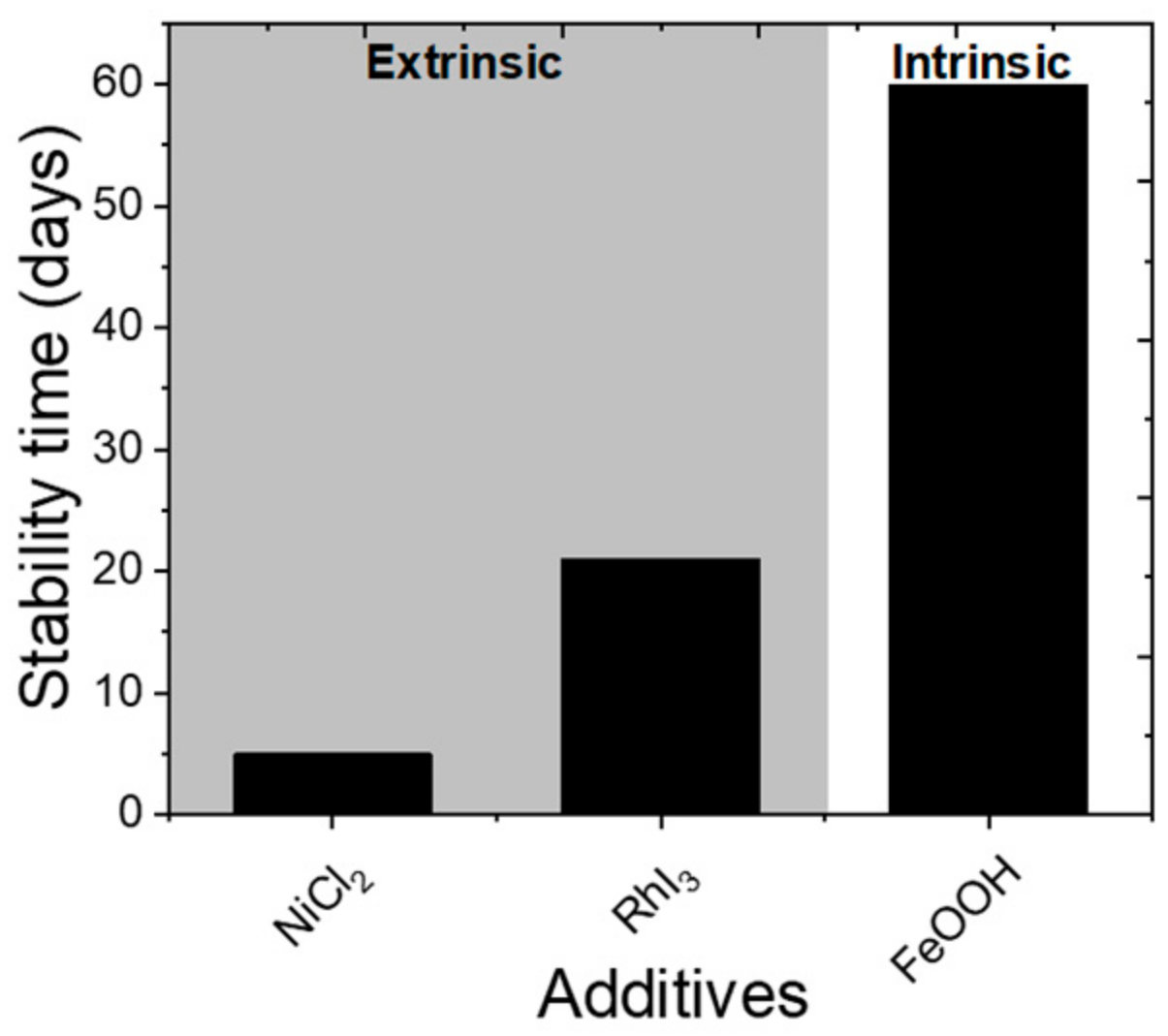
- Chen, H.; Luo, Q.; Liu, T.; Ren, J.; Li, S.; Tai, M.; Lin, H.; He, H.; Wang, J.; Wang, N. Goethite Quantum Dots as Multifunctional Additives for Highly Efficient and Stable Perovskite Solar Cells. Small 2019, 15, e1904372. [Google Scholar] [CrossRef]
- Gong, X.; Guan, L.; Pan, H.; Sun, Q.; Zhao, X.; Li, H.; Pan, H.; Shen, Y.; Shao, Y.; Sun, L.; et al. Highly Efficient Perovskite Solar Cells via Nickel Passivation. Adv. Funct. Mater. 2018, 28, 1804286. [Google Scholar] [CrossRef]
- Kayesh, E.; Matsuishi, K.; Chowdhury, T.H.; Kaneko, R.; Noda, T.; Islam, A. Enhanced Photovoltaic Performance of Perovskite Solar Cells by Copper Chloride (CuCl2) as an Additive in Single Solvent Perovskite Precursor. Electron. Mater. Lett. 2018, 14, 712–717. [Google Scholar] [CrossRef]
- Almutawah, Z.S.; Watthage, S.C.; Song, Z.; Ahangharnejhad, R.H.; Subedi, K.K.; Shrestha, N.; Phillips, A.B.; Yan, Y.; Ellingson, R.J.; Heben, M.J. Enhanced Grain Size and Crystallinity in CH3NH3PbI3 Perovskite Films by Metal Additives to the Single-Step Solution Fabrication Process. MRS Adv. 2018, 3, 3237–3242. [Google Scholar] [CrossRef] [Green Version]
- Watthage, S.C.; Song, Z.; Shrestha, N.; Phillips, A.B.; Liyanage, G.K.; Roland, P.J.; Ellingson, R.J.; Heben, M.J. Impact of Divalent Metal Additives on the Structural and Optoelectronic Properties of CH3NH3PbI3 Perovskite Prepared by the Two-Step Solution Process. MRS Adv. 2017, 2, 1183–1188. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Ji, S.; Hua, H.; Ma, Y.; Hu, R.; Zhang, J.; Chu, L.; Li, X.; Huang, W. Perfection of Perovskite Grain Boundary Passivation by Rhodium Incorporation for Efficient and Stable Solar Cells. Nano-Micro Lett. 2020, 12, 119. [Google Scholar] [CrossRef]
- Carmona, C.R.; Gratia, P.; Zimmermann, I.; Grancini, G.; Gao, P.; Graetzel, M.; Nazeeruddin, M.K. High efficiency methylammonium lead triiodide perovskite solar cells: The relevance of non-stoichiometric precursors. Energy Environ. Sci. 2015, 8, 3550–3556. [Google Scholar] [CrossRef]
- Rafizadeh, S.; Wienands, K.; Schulze, P.S.C.; Bett, A.J.; Andreani, L.C.; Hermle, M.; Glunz, S.W.; Goldschmidt, J.C. Efficiency Enhancement and Hysteresis Mitigation by Manipulation of Grain Growth Conditions in Hybrid Evaporated–Spin-coated Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yerramilli, A.; Shen, Y.; Zhao, Z.; Alford, T. Effect of excessive Pb content in the precursor solutions on the properties of the lead acetate derived CH3NH3PbI3 perovskite solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 478–484. [Google Scholar] [CrossRef]
- Yerramilli, A.; Chen, Y.; Sanni, D.; Asare, J.; Theodore, N.D.; Alford, T. Impact of excess lead on the stability and photo-induced degradation of lead halide perovskite solar cells. Org. Electron. 2018, 59, 107–112. [Google Scholar] [CrossRef]
- Barbé, J.; Newman, M.; Lilliu, S.; Kumar, V.; Lee, H.K.H.; Charbonneau, C.; Rodenburg, C.; Lidzey, D.G.; Tsoi, W.C. Localized effect of PbI2 excess in perovskite solar cells probed by high-resolution chemical–optoelectronic mapping. J. Mater. Chem. A 2018, 6, 23010–23018. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Shao, Z.; Fan, Y.; Liu, R.; Wang, L.; Pang, S. Controlled surface decomposition derived passivation and energy-level alignment behaviors for high performance perovskite solar cells. J. Mater. Chem. A 2018, 6, 9397–9401. [Google Scholar] [CrossRef]
- Meier, T.; Gujar, T.P.; Schönleber, A.; Olthof, S.; Meerholz, K.; van Smaalen, S.; Panzer, F.; Thelakkat, M.; Köhler, A. Impact of excess PbI2 on the structure and the temperature dependent optical properties of methylammonium lead iodide perovskites. J. Mater. Chem. C 2018, 6, 7512–7519. [Google Scholar] [CrossRef]
- Gujar, T.P.; Unger, T.; Schönleber, A.; Fried, M.; Panzer, F.; van Smaalen, S.; Köhler, A.; Thelakkat, M. The role of PbI2 in CH3NH3PbI3 perovskite stability, solar cell parameters and device degradation. Phys. Chem. Chem. Phys. 2018, 20, 605–614. [Google Scholar] [CrossRef]
- Liu, F.; Dong, Q.; Wong, M.K.; Djurišić, A.B.; Ng, A.; Ren, Z.; Shen, Q.; Surya, C.; Chan, W.K.; Wang, J.; et al. Is Excess PbI2 Beneficial for Perovskite Solar Cell Performance? Adv. Energy Mater. 2016, 6, 1502206. [Google Scholar] [CrossRef]
- Mangrulkar, M.; Luchkin, S.Y.; Akbulatov, A.F.; Zhidkov, I.; Kurmaev, E.Z.; Troshin, P.A.; Stevenson, K.J. Rationalizing the effect of overstoichiometric PbI2 on the stability of perovskite solar cells in the context of precursor solution formulation. Synth. Met. 2021, 278, 116823. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, X.; Wei, D.; Li, M.; Fu, P.; Xie, B.; Song, D.; Li, Y. DMSO-based PbI2 precursor with PbCl2 additive for highly efficient perovskite solar cells fabricated at low temperature. RSC Adv. 2015, 5, 104606–104611. [Google Scholar] [CrossRef]
- Jiang, F.; Rong, Y.; Liu, H.; Liu, T.; Mao, L.; Meng, W.; Qin, F.; Jiang, Y.; Luo, B.; Xiong, S.; et al. Synergistic Effect of PbI2 Passivation and Chlorine Inclusion Yielding High Open-Circuit Voltage Exceeding 1.15 V in Both Mesoscopic and Inverted Planar CH3NH3PbI3(Cl)-Based Perovskite Solar Cells. Adv. Funct. Mater. 2016, 26, 8119–8127. [Google Scholar] [CrossRef]
- Ngo, T.T.; Masi, S.; Méndez, P.F.; Kazes, M.; Oron, D.; Seró, I.M. PbS quantum dots as additives in methylammonium halide perovskite solar cells: The effect of quantum dot capping. Nanoscale Adv. 2019, 1, 4109–4118. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dong, W.; Fang, X.; Zhang, Q.; Zhou, S.; Deng, Z.; Tao, R.; Shao, J.; Xia, R.; Song, C.; et al. Credible evidence for the passivation effect of remnant PbI2 in CH3NH3PbI3 films in improving the performance of perovskite solar cells. Nanoscale 2016, 8, 6600–6608. [Google Scholar] [CrossRef] [PubMed]
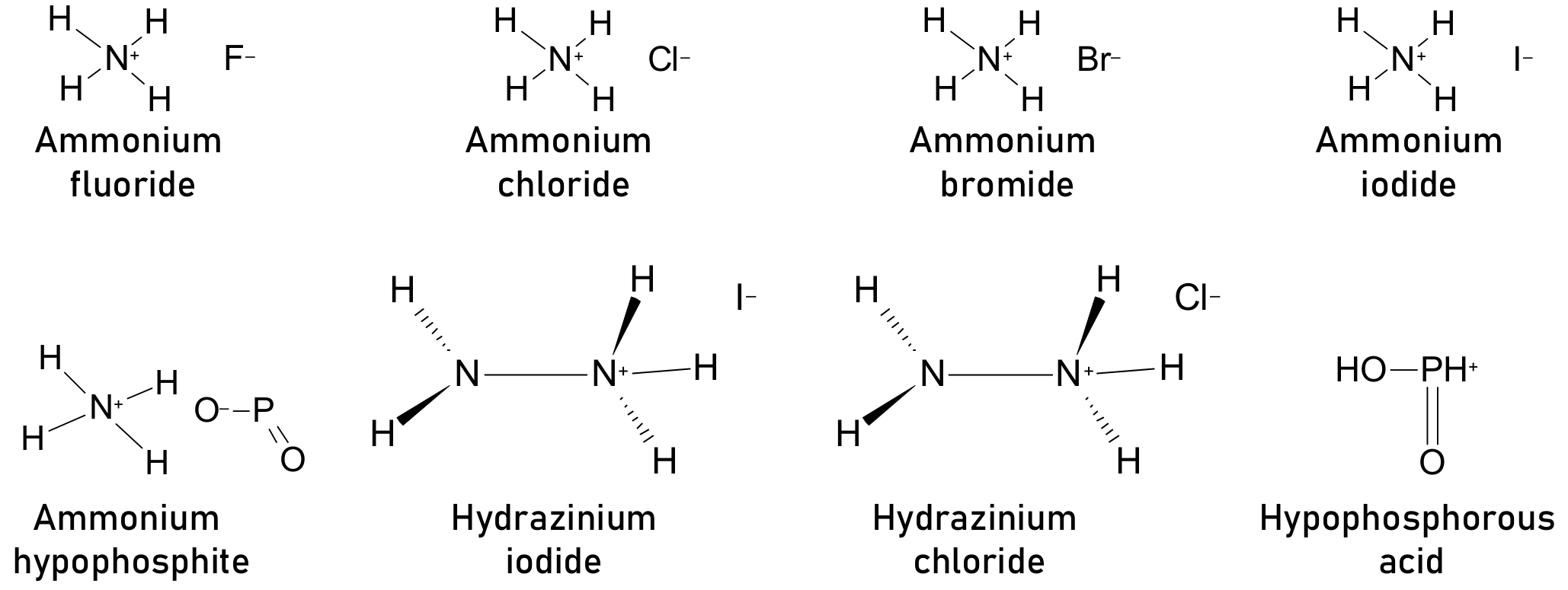
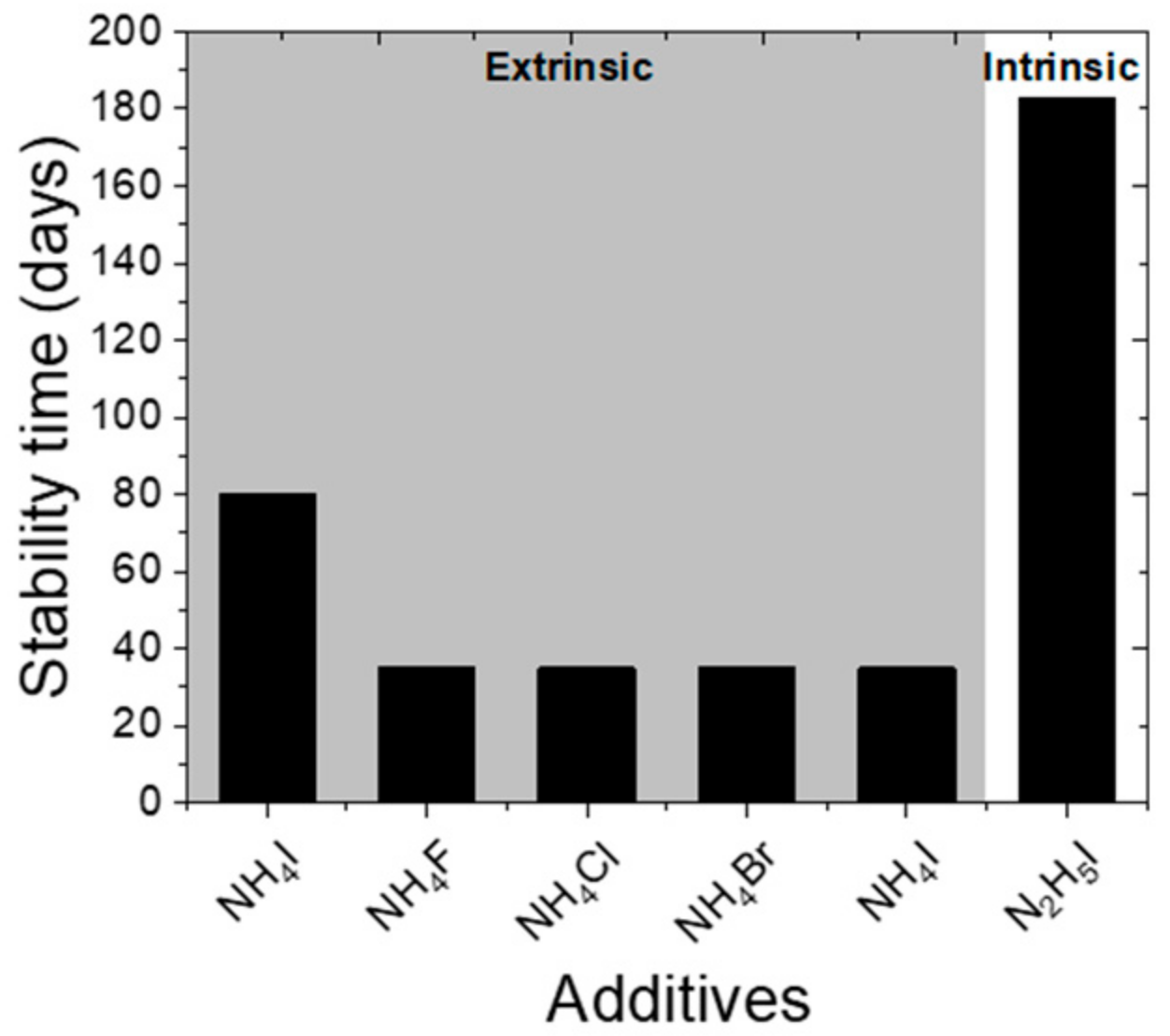
- Pathipati, S.R.; Shah, M.N. Performance enhancement of perovskite solar cells using NH4I additive in a solution processing method. Sol. Energy 2018, 162, 8–13. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. An 80.11% FF record achieved for perovskite solar cells by using the NH4Cl additive. Nanoscale 2014, 6, 9935–9938. [Google Scholar] [CrossRef]
- Jahandar, M.; Khan, N.; Jahankhan, M.; Song, C.E.; Lee, H.K.; Lee, S.K.; Shin, W.S.; Lee, J.-C.; Im, S.H.; Moon, S.-J. High-performance CH3NH3PbI3 inverted planar perovskite solar cells via ammonium halide additives. J. Ind. Eng. Chem. 2019, 80, 265–272. [Google Scholar] [CrossRef]
- Zhang, W.; Pathak, S.; Sakai, N.; Stergiopoulos, T.; Nayak, P.K.; Noel, N.K.; Haghighirad, A.A.; Burlakov, V.M.; Dequilettes, D.W.; Sadhanala, A.; et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun. 2015, 6, 10030. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Wang, D.; Dong, Q.; Wang, Q.; Wei, W.; Dai, J.; Zeng, X.; Huang, J. Unraveling the hidden function of a stabilizer in a precursor in improving hybrid perovskite film morphology for high efficiency solar cells. Energy Environ. Sci. 2016, 9, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Lei, G.; Tao, C.; Zhang, J.; Liu, X.; Xu, X.; Lai, W.-Y.; Gao, F.; Huang, W. Precisely Controlling the Grain Sizes with an Ammonium Hypophosphite Additive for High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1802320. [Google Scholar] [CrossRef]
- Mangrulkar, M.; Boldyreva, A.G.; Lipovskikh, S.A.; Troshin, P.A.; Stevenson, K.J. Influence of hydrazinium iodide on the intrinsic photostability of MAPbI3 thin films and solar cells. J. Mater. Res. 2021, 36, 1846–1854. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, S.; Lu, H.; Zhang, H.; Wang, P.; Cui, X.; Zhang, Y.; Liu, Q.; Wang, J.; Zhan, Y.; et al. Hydrazinium Salt as Additive To Improve Film Morphology and Carrier Lifetime for High-Efficiency Planar-Heterojunction Perovskite Solar Cells via One-Step Method. ACS Appl. Mater. Interfaces 2017, 9, 36810–36816. [Google Scholar] [CrossRef] [PubMed]
- Abzieher, T.; Mathies, F.; Hetterich, M.; Welle, A.; Gerthsen, D.; Lemmer, U.; Paetzold, U.W.; Powalla, M. Additive-Assisted Crystallization Dynamics in Two-Step Fabrication of Perovskite Solar Cells. Phys. Status Solidi Appl. Mater. Sci. 2017, 214, 1–9. [Google Scholar] [CrossRef]
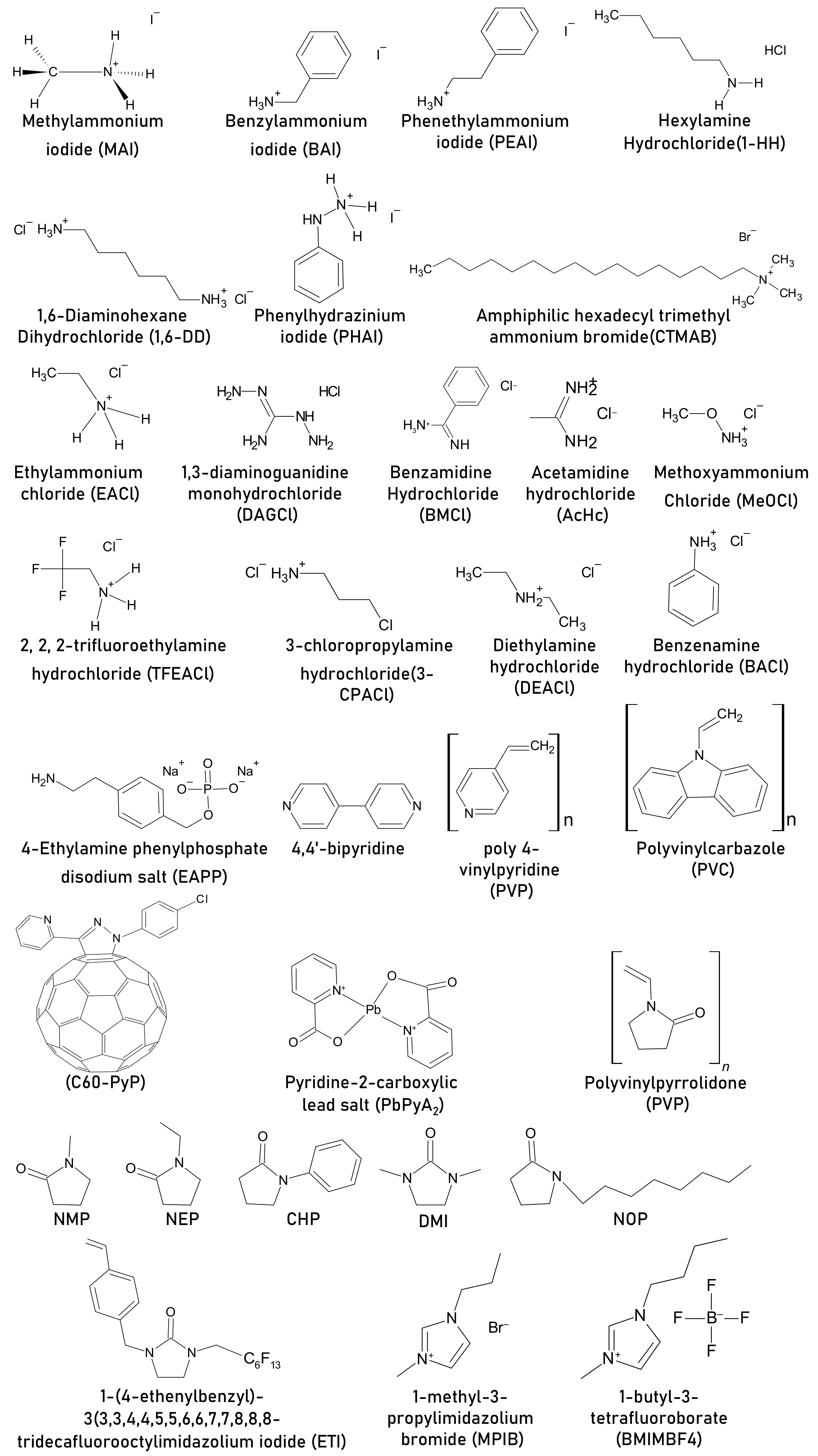
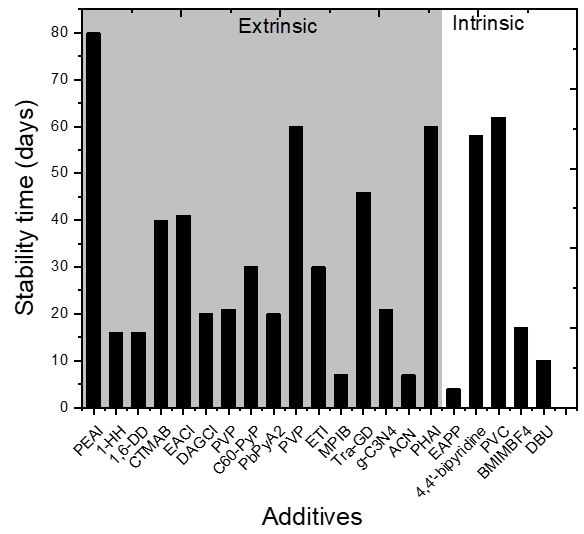
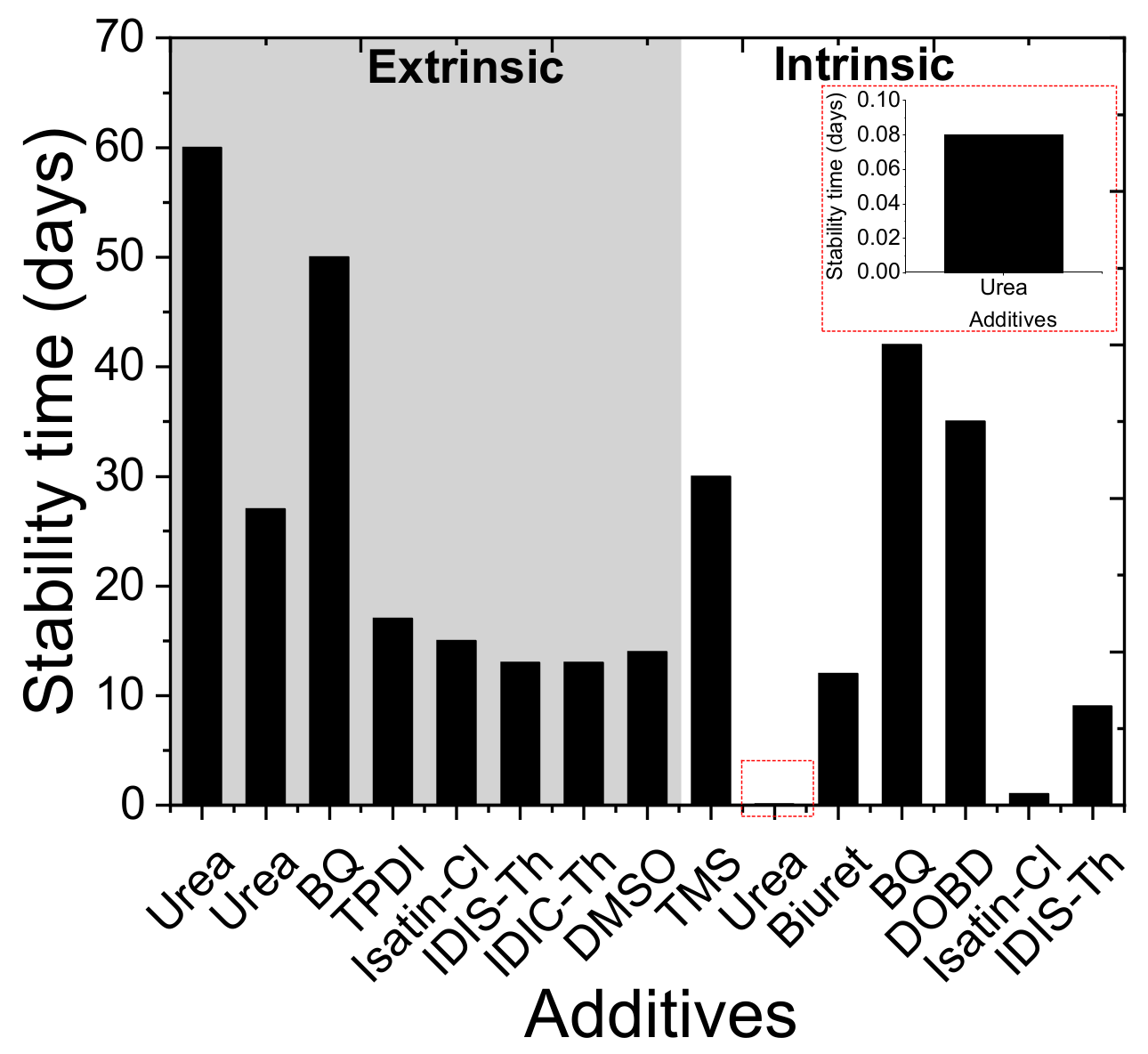



| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| excess MAI | FTO/SnO2/CH3NH3PbI3/PCBM/Ag | 15.14 | N/A | 17.24 | molar ratio PbI2: MAI = 1:1.05 | N/A | N/A | Hot casting, improved crystallinity, decrease in defect density, increased PL lifetime | [29] |
| excess MAI | ITO/PCBM/CH3NH3PbI3/HTL/Au | N/A | N/A | N/A | molar ratio MAI: PbI2 = 3:1 | N/A | N/A | Vacuum deposition, increased PL lifetime, reduced trap states | [30] |
| excess MAI | FTO/TiO2/CH3NH3PbI3/Spiro-OmATAD/Au | 11.13 | N/A | 13.37 | 0.2 mM | N/A | N/A | Sequential deposition, good quality perovskite film | [31] |
| benzylammonium iodide (BAI) | FTO/TiO2/CH3NH3PbI3/HTL/metal | 6.83 | N/A | 9.05 | molar ratio BAI:MAI = 0.2 | N/A | N/A | Better light harvesting property and low charge recombination | [32] |
| phenethylammonium iodide (PEAI) | FTO/ c-TiO2/m-TiO2/mp- ZrO2/CH3NH3PbI3/Carbon | 6.3 | Retained ~77% PCE after 80 days | 8.60 | molar ratio PEAI: MAI = 1:20 | Retained 90% PCE after 80 days | air, light 100 mW cm−2 | Better contact with TiO2, longer exciton lifetime, good quality of perovskite film | [33] |
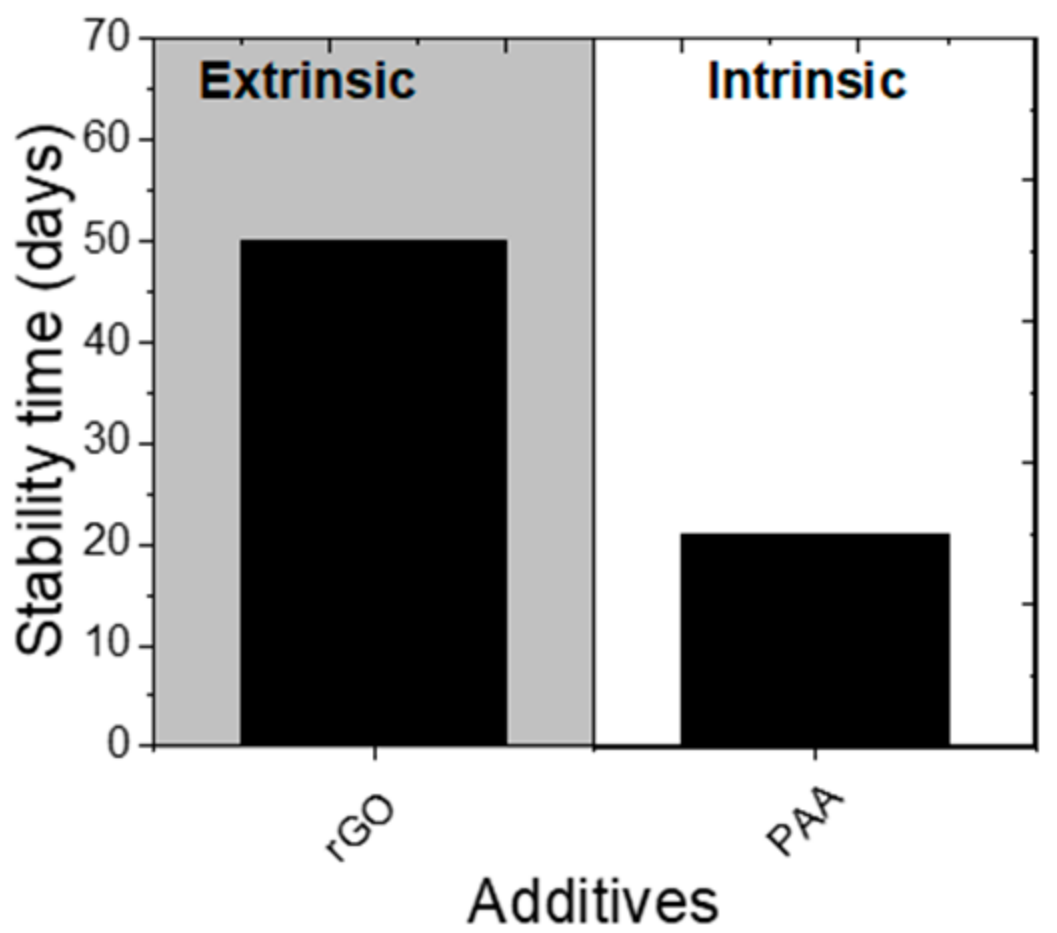
| hexylamine hydrochloride (1-HH) | Glass/ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/BCP/Ag | 14.37 | Retained 43% PCE from initial after 16 days | 15.70 | 0.05 wt% | Retained ~85% PCE from initial after 16 days | Unencapsulated, ambient, RT, air, RH = 10–20% | Increases grain size and passivate defects, NH3+ group could form N-H…I. hydrogen bond with the I- of the [PbI6]4- passivating “A” vacancy; hydrophobic hexane alkyl chain protects against moisture | [34] |
| 1,6-diaminohexane dihydrochloride (1,6-DD) | 17.00 | Retained 90% PCE from initial after 16 days | |||||||
| phenylhydrazinium iodide (PHAI) | FTO/PEDOT:PSS/CH3NH3PbI3/PCBM/Rhoda mine/Ag | 14.63 | (a) Retained ~53% PCE after 60 days (b) died in 20 days | 17.2 | 10 mg mL−1 | (a) Retained ~90% PCE after 60 days (b) Retained ~85% PCE after 20 days | (a) unencapsulated, N2, dark, RH = 20%, T = 26 °C (b) unencapsulated, ambient room environment, dark, 30 ± 5% RH, 24 ± 2 °C | PbI2 and PHAI complex/intermediate formation results in passivation against vacancy defects, hydrophobic phenyl rings acts as a barrier against moisture | [35] |
| amphiphilic hexadecyl trimethyl ammonium bromide(CTMAB) | Glass/FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 17.05 | Retained 70% PCE after 40 days | 18.03 | 10 mg CTMAB in 1 mL DMSO. Used 20 uL of this to add in 1 mL precursor | Retained 95% PCE after 40 days | Non-encapsulated, RH~40%, T = 25 °C, dark | Improved crystallinity and morphology | [39] |
| ethylammonium chloride (EACl) | Glass/FTO/c-TiO2/meso-TiO2/CH3NH3PbI3/HTL/Au | 17.35 | Retained ~30% of the original PCE after 1000 h | 20.3 | 2.5%, molar % | Retained ~89% of the original PCE after 1000 h | encapsulated | Improves morphology, grain boundary passivation | [40] |
| 1,3-diaminoguanidine monohydrochloride (DAGCl) | Glass/ ITO/PolyTPD/CH3NH3PbI3/PC61BM/ZrAcac/Ag | 19.1 | Retained 70% PCE after 20 days | 20.3 | 0.6%, wt% of MAI | Retained 80% PCE after 20 days | Non-encapsulated, ambient, RH ~ 50% | Increased grain size, reduced trap density, DAG cation can bond with I− via hydrogen | [41] |
| benzamidine hydrochloride (BMCl) | ITO/TiO2/PC61BM/CH3NH3PbI3/PTAA/MoO3/Ag | 17.8 | N/A | 18.4 | N/A | N/A | N/A | Enhance work function of perovskite film improves efficiency | [42] |
| acetamidine salt (AcHc) | ITO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.45 | N/A | 16.54 | molar ratio MAI:AaHc = 1:0.08 | N/A | N/A | Smooth film, full and uniform coverage, large grain size, improve carrier lifetime, | [43] |
| methoxyammonium chloride (MeOCl) | FTO/compact-TiO2 layer/mesoporous-TiO2 layer/CH3NH3PbI3/Spiro-MeOTAD/Ag | 17.15 | N/A | 19.71 | molar ratio PbI2:MeOCl =1:0.10 | N/A | N/A | Improvement in grain size and crystallinity | [44] |
| 2, 2, 2- trifluoroethylamine hydrochloride (TFEACl) | Glass/ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/Al | N/A | N/A | 4.98 | molar ratio of additive:MAI:PbI2 = 0.4:1:1 | N/A | N/A | Compact smooth high quality film | [45] |
| benzenamine hydrochloride (BACl) | 11.07 | ||||||||
| 3-chloropropylamine hydrochloride (3-CPACl) | 8.21 | ||||||||
| diethylamine hydrochloride (DEACl) | 9.89 | ||||||||
| 4-ethylamine Phenylphosphate disodium salt (EAPP) | FTO/C-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 18.83 | (a) Dead in 6 h (b) Remained 60% from initial PCE after 100 h | 17.61 | 1 mol% | (a) Remained 90% from initial PCE after 12 h (a) Remained ~99% from initial PCE after 100 h | (a) ATM condition, RH = 80%, unencapsulated, (b) inert, N2, unencapsulated, | Phenylethylamine group protects against moisture and improves ambient stability, phosphate sodium prevents the formation of (CH3NH3)4PbI6.H2O | [62] |
| 4,4’-bipyridine | Glass/CH3NH3PbI3 | N/A | Died in ~900 h | N/A | 5 wt% | Remained active for 1400 h | inert, unencapsulated, light 70-80 mW cm−2, 50-60 °C | Forms complex with PbI2, thus slows down the formation of metallic lead | [47] |
| poly 4-vinylpyridine (PVP) | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 6.09 | Remained 1.55% PCE as of final PCE (absolute value) after 3 weeks | 13.07 | 0.4 wt% | Remained 6.6% PCE as of final PCE (absolute value) after 3 weeks. | Air, 50% RH, non-encapsulated | Inhibits carrier recombination, reduced defects | [61] |
| Polyvinylcarbazole (PVC) | ITO/PTAA/CH3NH3PbI3/PCBM/Al | 17.4 | Died in 1500 h | 18.7 | 1 mass% | Retained ~70% of the initial efficiency after light soaking for 1500 h, | light 50 ± 3 mW cm−2, 65 ± 2 °C, inert nitrogen, | Defect passivation due to interaction with lone pair of electrons from N atom with Pb2+ | [48] |
| pyridine-functionalized fullerene derivative (C60-PyP) | ITO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 17.61 | Retained 70% PCE after 30 days | 19.82 | 0.13 wt% | Retained 90% PCE after 30 days | 25 ℃, RH = 30%, dark, non-encapsulation, | Enlarged grain size, improved crystallization, interaction b/w N atom of the pyridine moiety within C60-PyP and Pb2+ ion within MAPbl3 leads to the passivation of trap states of perovskite layer, hydrophobic nature of C60-PyP molecule increases ambient stability | [49] |
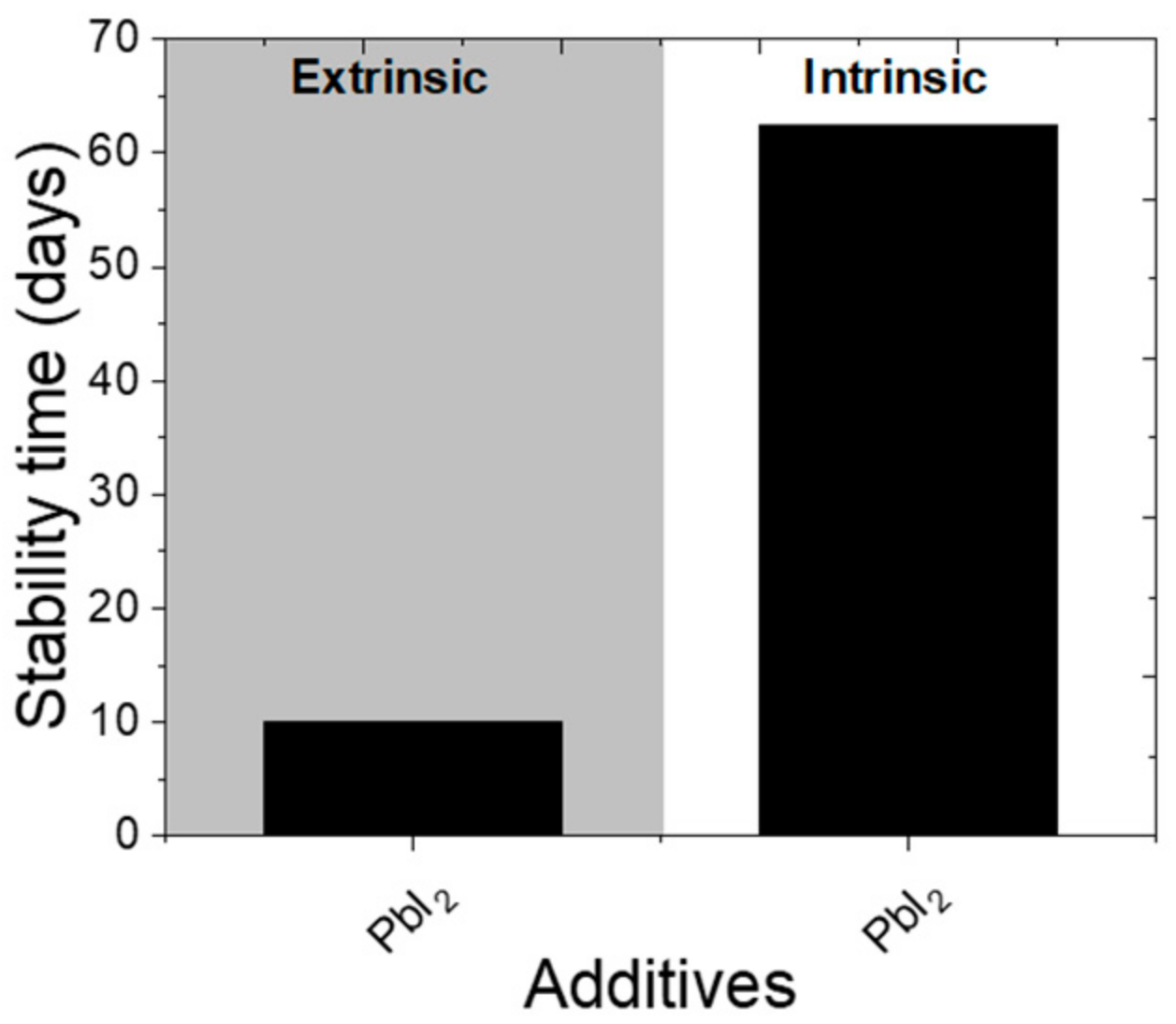
| pyridine-2-carboxylic lead salt (PbPyA2) | ITO/P3CT-N/CH3NH3PbI3/ (PCBM)/C60/(BCP)/Ag | 18.86 | (a) Retained 20% of PCE after 480 h (b) retained 20% of PCE after 540 h (c) retained 30% of PCE from initial after 480h | 19.96 | 4 mg mL−1 | (a) Retained 80% PCE after 480 h (b) retained 93% of initial PCE after 540 h (c) retained 90% PCE from initial after 480h | (a) 90 °C, RH = 40–60%, dark, not encapsulated, (b)MPP- tracking, non-encapsulated, white light-100 mW cm−2, inert, 25 °C, (c) Air, non-encapsulated, dark, RH = 40–60% | Controlled crystallization, passivation of grain boundaries, the interaction of pyridine and carboxylate to cations increases hydrophobicity | [50] |
| polyvinylpyrrolidone (PVP) | Ito/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.33 | Retained 76% from initial PCE after 400 h | 15.19 | 1 mg mL−1 | Retained 80% of the initial PCE after 60 days | encapsulated, ambient, RH = 10%, RT | Lewis base, the pyridine part (side chain) of the PVP polymer, can passivate the surface defects caused by misaligned lead ions and can fill the iodine vacancy traps on the surface of the perovskite film, C=O also stabilizes, allows not to degrade | [36] |
| NMP | FTO/ZnO-MgO-EA+ /mesoporous-TiO2/CH3NH3PbI3/spiro-OMeTAD/Au | 18.0 | N/A | 19.2 | molar ratio PbI2:NMP =1:2 | N/A | N/A | The same kind of intermediate, regardless of NMP ratio, results in excellent morphology | [51] |
| NMP | Glass/ITO/PEDOT:PSS/CH3NH3PbI3/PC61BM/Al | 1.50 | N/A | 7.03 | 60 μL in 1 mL precursor | N/A | N/A | Lewis acid base interaction | [52] |
| NEP | 10.04 | - | |||||||
| CHP | 12.87 | Suppression of solvate formation | |||||||
| NOP | 2.79 | Lewis acid–base interaction | |||||||
| DMI | FTO/compact-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 10.72 | N/A | 14.54 | 10 vol% | N/A | N/A | Pb-O bond formation due to Lewis adduct between DMI and Pb | [63] |
| 1-(4-ethenylbenzyl)- 3-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctylimidazolium iodide (ETI) | FTO/C-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 19.2 | (a) Retained 49% PCE from initial 700 h (b) Retained 60% PCE from initial 700 h | 19.5 | 1 mol% | (a)Retained 85% PCE from initial 700 h (b) Retained 80% PCE from initial 700 h | (a) MPPT inert, 60 °C, light 100 mW cm−2, unencapsulated, (b) RH = 40%,RT, dark, air | Enables the full transformation into precursor, suppresses thermal decomposition pathway and, provides outstanding hydrophobicity within the active material | [53] |
| 1-methyl-3- propylimidazolium bromide (MPIB) | ITO/PEDOT:PSS/CH3NH3PbI3/PC61BM/BCP/Ag. | 15.9 | retain ~50% of its original PCE after 150 h | 18.2 | 0.5 mg mL−1 | retain 78% of its original PCE after 150 h | atmospheric environment, RT, | Passivation of the uncoordinated Pb2+ to reduce the defects in the perovskite film due to the lone-pair electron in its cation group, and beneficial to promote crystal growth to improve film quality | [54] |
| 1-butyl-3- tetrafluoroborate (BMIMBF4) | ITO/NiOx/CH3NH3PbI3/C60/Ag | 18.13 | retaining 30% of their initial PCE after thermal ageing of 400 h at 85 °C | 18.07 | 0.4 mol% | retaining 80% of their initial PCE after thermal ageing of 400 h at 85 °C | 85 °C, unencapsulated | Thermal stability by effective suppression of perovskite decomposition | [55] |
| Spiro-OMeTAD | FTO/Den TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.52 | N/A | 17.77 | 0.01 wt% | N/A | N/A | Facilitates charge transport | [64] |
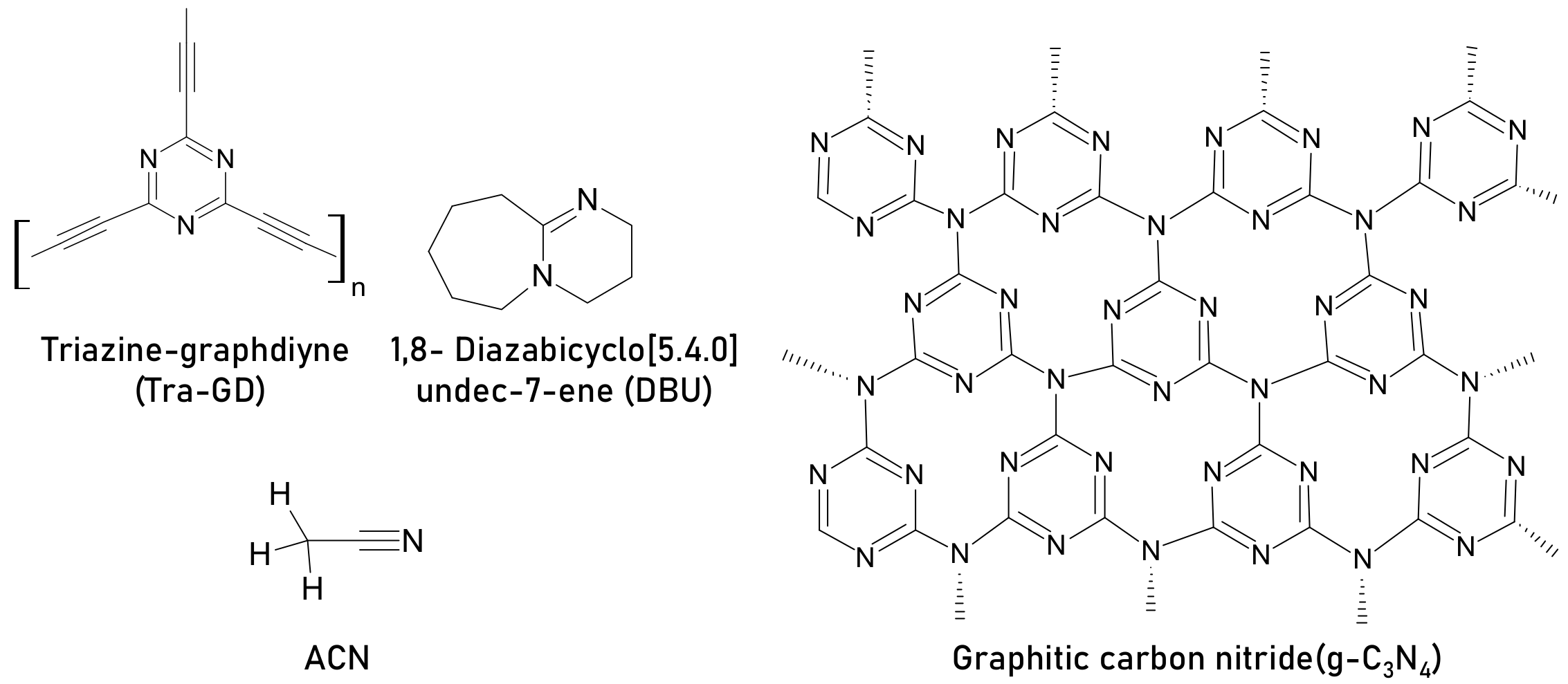
| triazine-graphdiyne (Tra-GD), | ITO/P3CT-K/CH3NH3PbI3/PC61BM/ZnO/Al | 17.90 | Died in 1100 h | 20.33 | 2 mg mL−1 | Retain above 90% after 1100 h | Unencapsulated | Interacts with Pb2+ at grain boundaries and passivates grain boundaries and inhibits ion migration | [56] |
| graphitic carbon nitride(g-C3N4) | FTO/compact TiO2/CH3NH3PbI3/Spiro-MeOTAD/Au | 18 | Retained 46% PCE after 300 h | 21.6 | 0.1 wt% | Retained 90% PCE from initial after 500 h | Encapsulated, light 100 mW cm−2 | Improves grain size and crystallinity, passivates grain boundaries, C=N to interact with Pb2+ that forms compact tight bonding resulting in good morphology | [57] |
| graphitic carbon nitride (g-C3N4) | FTO/ compact TiO2/CH3NH3PbI3/Spiro-OMeTAD/MoO3/Ag | 16.22 ± 0.83 | N/A | 19.34 ± 0.63 | 0.4 mg mL−1 | N/A | N/A | Passivation, enhanced crystallinity, C=N to interact with Pb2+, increases the conductivity and carrier mobility | [58] |
| 1,8- Diazabicyclo[5.4.0]undec-7-ene (DBU) | Glass/ITO/NiOx/CH3NH3PbI3/PCBM/PEI/Ag | 15.98 | Retained 50% PCE from initial after 10 days | 18.13 | 3% weight ratio | Retained 80% PCE from initial after 10 days | unencapsulated, inert (N2,) mpp tracking | Iodine quencher, adduct with PbI2 (C=N interaction with Pb2+), reduced defects, high-quality perovskite film | [59] |
| ACN | ITO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.04 ± 0.48 | Remained ~20% of initial PCE after 150 h | 19.7 | molar ratio ACN: PbI2 = 0.5 | Remained ~60% PCE after 150 h | light 100 mW cm−2 RH = 60%–90%, in air without encapsulation | Morphology enhancement | [60] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
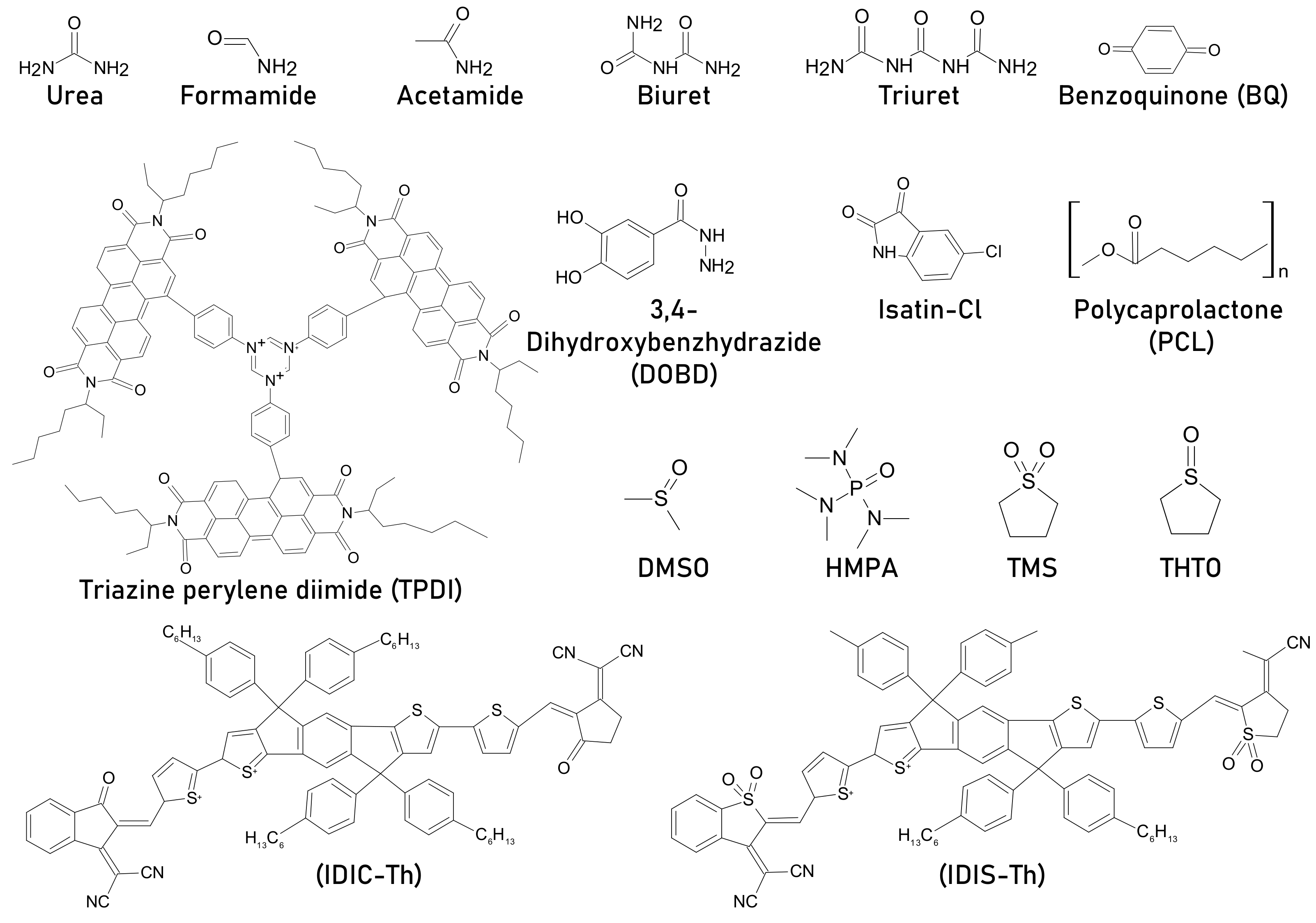
| urea | Glass/ITO/PEDOT:PSS/CH3NH3PbI3/PCMB/BCP/Al | 15.1 | Retained ~35% from original PCE after 2 h | 17 | molar ratio urea: lead acetate = 0.5 | Retained ~60% from original PCE after 2 h | light, 100 mW cm−2, 50–60 °C, inert, unencapsulated | Interaction of carbonyl group of urea with PbI2 results in crystalline film, resulting passivation and improvement in stability | [65] |
| urea | ITO/PEDOT:PSS/PbI2/MAI/PCBM/BPhen/Ag | 12.75 | N/A | 18.01 | 5 wt%, wt% of PbI2 | N/A | N/A | Double step spin coating, urea and PbI2 together form PbI2.O=C(NH2)2 complex/intermediate, resulting big flat grains | [66] |
| urea | FTO/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.48 | Remained 50% from original PCE after 60 days | 18.52 | 20 mol% | Remained 80% from original PCE after 60 days | air, 50% humidity, without any encapsulation | PbI2-urea adduct results increment in grain size and passivate grain boundaries | [67] |
| urea | ITO/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 16.80 | a) Retained 10% PCE as of final PCE after ~27 days b) retained nearly 90% PCE from initial after 60 min | 18.5 | 4 mol% | (a) Remained 12% PCE as of final PCE after ~27 days (b) retained nearly 100% PCE from initial after 60 min | mppt tracking (a) dark, ambient, (b) 1 sun, in-situ | Adduct formation, high crystallinity, good film, suppress non-radiative recombination and passivates grain boundaries | [68] |
| formamide | FTO/c-TiO2/ mp-TiO2//CH3NH3PbI3/ZrO2 layer mp-ZrO2/ carbon electrode | 14.26 | N/A | 15.21 | N/A | N/A | the amide additives shifted the Fermi level of the MAPbI3 perovskite from −4.36 eV to −4.63, −4.65, and −4.61 eV, respectively for formamide, acetamide and urea and suppressed non-radiative recombination. Reduced iodide defects vacancy | [70] | |
| acetamide | 15.57 | N/A | N/A | ||||||
| urea | 15.07 | N/A | N/A | ||||||
| biuret | FTO/TiO2/CH3NH3PbI3/PCBM/Ag | 18.26 | Maintains ~50%PCE after 12 days | 21.16 | 2 mol% | Preserves 94% of its initial efficiency after 12 day | 85 °C, N2, inert, non-encapsulated, | Increased grain size, reduced trap state, additive acts as Lewis base and interacted with uncoordinated Pb2+, improves the thermal stability | [71] |
| urea | Glass/ITO/NiOx/CH3NH3PbI3//PC61BM/Ag | 15 | N/A | 18.6 | 0.05 mmol | N/A | N/A | Passivate the defects and eliminate non-radiative charge recombination due to adduct with PbI2 | [72] |
| biuret | 20.1 | N/A | N/A | ||||||
| triuret | 19.2 | N/A | N/A | ||||||
| benzoquinone (BQ) | ITO/PEDOT:PSS/CH3NH3PbI3/ C60/BCP/Au | 10.7 | Remained ~50% PCE from initial after 900 h | 15.6 | 0.5 mol% | Remained ~80% PCE from initial after 1000 h | unencapsulated, light 1 sun, open circuit, without UV-filter | Reduces trap density | [73] |
| benzoquinone (BQ) | FTO/TiO2/CH3NH3PbI3/SpiroOMeTAD/Au | 17.37 | (a) Dead in 1200 h (b)Dead in ~800 h | 18.01 | 0.25% | (a) Retained 75% PCE from initial after 2400 h (b) Retained ~30% PCE from initial after 1200 h | unencapsulated, without UV-filter (a) RH ~ 20%, RT, (b) ambient air, RH = 40 - 70%, RT | Improves crystal quality, passivate grain boundary, reduce trap states, suppress PbI2 formation at GB | [74] |
| triazine perylene diimide (TPDI) | ITO/PEDOT:PSS/CH3NH3PbI3 /PC61BM/Ag | 8.32 | Retained 30% from initial PCE after 400 h | 10.84 | 1.2 mg mL−1 | Retained 60% from initial PCE after 400h | Non-encapsulated, ambient | Minimize grain boundary defects and enhance the coverage and crystal grain sizes | [75] |
| 3,4-Dihydroxybenzhydrazide (DOBD) | ITO/PEDOT:PSS/CH3NH3PbI3/C6 0/BCP/Al | 14.47 | Retained 70%PCE from initial after 35 days | 17.58 | 3 mg mL−1 | Retained 85%PCE from initial after 35 days | Non-encapsulated, inert, dark | Increases grain size and decrease of grain boundaries, both of which facilitate charge transportation and suppress charge recombination, due to Lewis acid–base interaction between Pb2+ and C=O | [76] |
| Isatin-Cl | ITO/PTAA:F4TCNQ/CH3NH3PbI3/PCBM/Al | 18.13 | (a) Retained 60% PCE from initial after 350 h. (b)Retained 30% PCE from initial after 24 h | 20.18 | 0.0001 wt% | (a) Retained 95% PCE from initial after 350 h. (b) Retained 75% PCE from initial after 24 h | unencapsulated, (a) in ambient air, RH = 45%, room temperature, (b) in nitrogen atmosphere at 85 °C | The carbonyl groups and hydrogen-bond structures on Isatin-Cl passivate the defect states in the perovskite grain boundaries and improved charge transport, suppress charge recombination, hydrophobic ring attached to Isatin-Cl improves stability against humidity | [77] |
| polycaprolactone (PCL) | glass or PET/ITO/PEDOT:PSS/CH3NH3PbI3/PC61BM/BCP/Ag | 10.52 | Retained 32% of initial PCE (10.12%) after 300 bending cycles | 14.49 | 0.025 wt% | Retained 90% of initial PCE (10.12%) after 300 bending cycle | mechanical bending stability | Carbonyl (C=O) and Pb2+ bond helps the uniform coverage of perovskite, which avoids the defects and achieve the grain boundary regulation on flexible PSC | [78] |
| IDIS-Th | ITO/P3CT-N/CH3NH3PbI3/PC61BM/Bphen/Ag | 17.78 | (a)Retained 50% from initial PCE after 300h (b) Retained 78% from initial PCE after 200 h | 20.1 | 0.05 mg mL−1 | (a) Retained 80% from initial PCE after 300 h (b) Retained 85% from initial PCE after 200 h | (a) light 100 mW cm−2, ambient, RH = 30%, non-encapsulated, (b) 85 °C, inert, dark, | Passivate grain boundary, reduce charge recombination, adduct between additive and under coordinated Pb ions can effectively inhibit ion migration and moisture diffusion to enhance the stability of PSC devices | [79] |
| IDIC-Th | 18.78 | (a) Retained 80% from initial PCE after 300 h (b) Retained 78% from initial PCE after 200 h | |||||||
| NMP | FTO/TiO2/PbI2/MAI/Spiro-OMeTAD/Au | 12.25 | Died after 14 days | 14.66 | 30% NMP in 1 mL precursor | N/A | [84] | ||
| DMSO | 16.17 | 20% DMSO in 1 mL precursor | Retained 66% PCE from initial after 14 days | in air | Morphology control based on Lewis basicity-based, donor number and boiling point | ||||
| HMPA | 12.06 | 5% HMPA | N/A | ||||||
| tetramethylene sulfone (TMS) | FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 8.7 | Retained ~70% from initial PCE after30 days | 16.2 | molar ratio =PbI2:MAI:TMS= 2:2:1 | Retained 80% from initial PCE after30 days | RT, RH = 10 - 40%, un encapsulated, dark | Intermediate phase via cross-linking. PbI2 act as Lewis acid TMS with S=O group acts as Lewis base | [85] |
| tetrahydrothiophene oxide (THTO), | ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/Al | N/A | N/A | 12.1 | molar ratio THTO:Pb = 3:1 | N/A | N/A | Additive alters the nucleation and growth processes, lowered the free energy of the precursor by incorporating a sulfoxide (S=O), which strongly interacts with MAPbI3 precursors, allowing an unprecedented degree of control over the nucleation density and growth rate. | [86] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
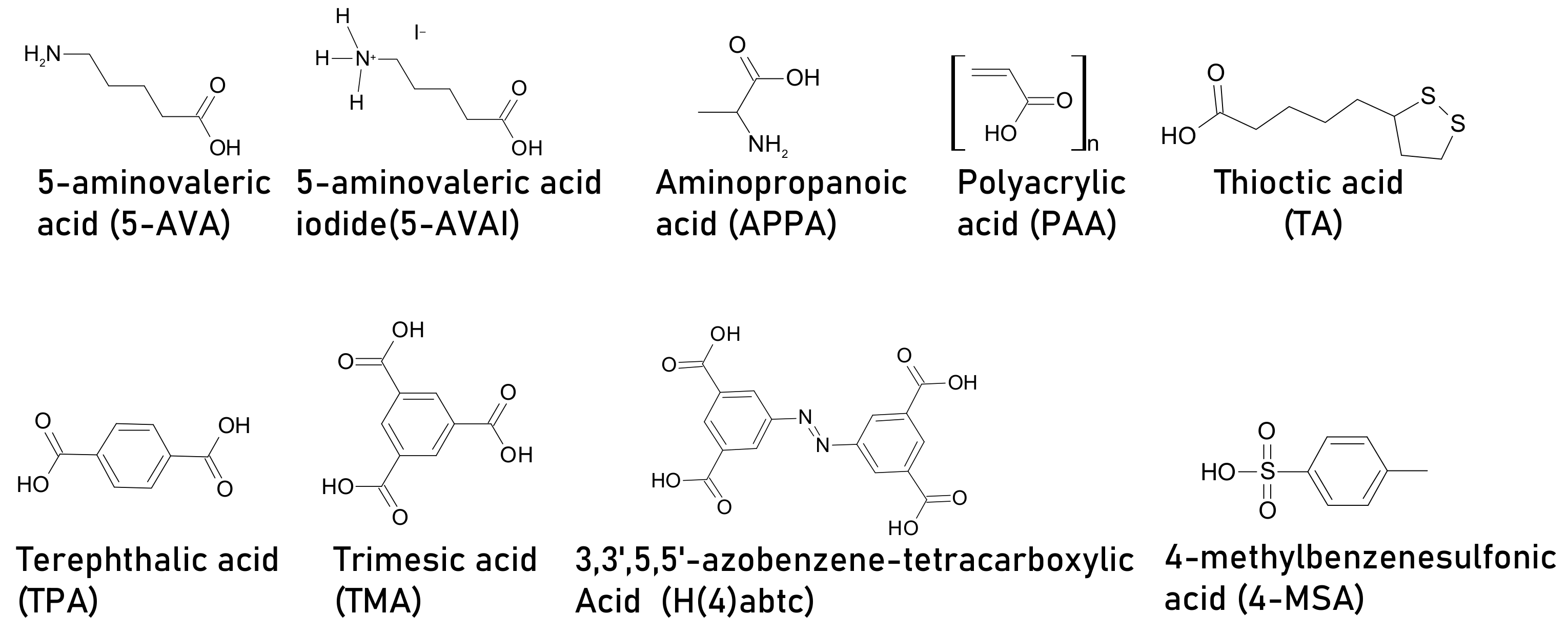
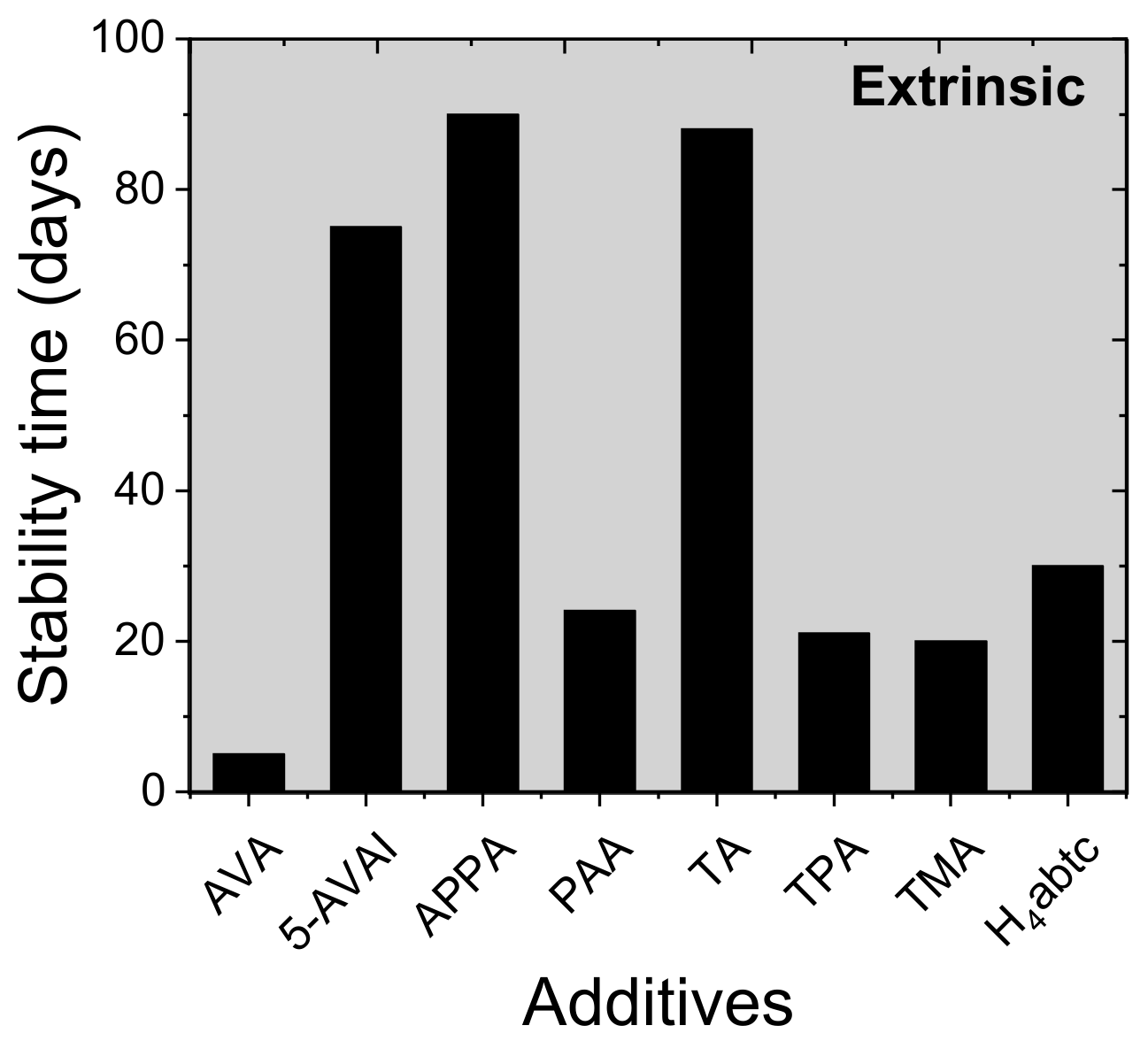
| 5-aminovaleric acid (5-AVA) | ITO/C60/CH3NH3PbI3/PTAA/Au | 15.4 | N/A | 13.7 | 5% wt | N/A | N/A | Larger grain size, improved crystallinity, improved mechanical robustness | [87] |
| amino valeric acid AVA | FTO/compact TiO2/mesoporous TiO2/mesoporous ZrO2/mesoporous carbon | 11.1 | Died in 110 h | 9.1 | molar ratio AVA: MAI=3% | Retained 50% PCE from initial after 110 h | RH = 15%, 1 Sun, non-encapsulated, | AVA located at grain boundaries is able to passivate surface defect sites, resulting in enhanced resistivity to oxygen-induced degradation. | [88] |
| 5-ammonium valeric acid iodide (5-AVAI) | FTO/TiO2/CH3NH3PbI3/ZrO2/Carbon | N/A | N/A | 6.68 | 0.072M of AVAI | 5.62% PCE as of final PCE after 75 days | ambient, | Big area 0.25 cm2, lower charge transport resistance | [89] |
| aminovaleric acid iodide (AVAI) | TiO2/ZrO2/CH3NH3PbI3/Spiro-OMeTAD/Au | ~7 | N/A | ~9 | AVA:PbI2= 3 mol% | N/A | N/A | Controls ion migration- | [96] |
| aminopropanoic acid (APPA) | ITO/PTAA/CH3NH3PbI3/ PC61BM/BCP/Ag, | 17.51 | Remained 6% as of absolute value of PCE after 90 days | 19.23 | weight ratio MAI:APPA= 0.56 | Remained 13% as of absolute value of PCE after90 days | unencapsulated, RH = 10% | Smaller grain size, smooth surface, suppress non-radiative charge recombination, resulting in enhanced JSC and VOC | [95] |
| Polyacrylic acid (PAA) | FTO/ NiOx:Zn/CH3NH3PbI3/ PC61BM/bathocuproine (BCP)/Ag | 10.3 | Retained 60% from initial PCE after 24 days | 14.9 | 5 mg mL−1 | Retained 80% from initial PCE after 24 days | RT, air, ambient, RH ~ 30 ± 5%, non-encapsulated | Using the doctor blade method, a smooth, uniform, and pin-hole free film of high electronic quality, passivate defects in large area devices (1 cm2) | [90] |
| thioctic acid (TA) | FTO/ c-TiO2/TiO2-Poly(TA)/CH3NH3PbI3/Spiro-OMeTAD/Au | 17.4 | (a) Died after 180 min (b) Died after 400 h c) N/A | 20.4 | 20 mg mL−1 | (a) Retained 98% of its original PCE after 450 min (b) 97% PCE after 2100 h (c)retained 92% PCE from initial after 600 h | (a) under UV irradiation (35.8 mW cm−2). (b) air, RH = 50 ± 10%, (c) mppt, inert,1.5 AMG | Carboxylic acid moieties binds at TiO2 surface. Then five-membered ring-containing the dynamic covalent disulfide is bonded to the other end of the molecule through the thermal-initiated ring-opening, and interacts with Pb, forming high-quality perovskite film due to the Lewis acid–base reaction between S and Pb2+. Extra passivation on TiO2 helps for efficient charge extraction and stability under UV illumination and ambient conditions | [91] |
| terephthalic acid (TPA) | FTO/compact-TiO2/CH3NH3PbI3/Carbon layer | 11.5 ±0.67 | (a)Retained 81% PCE from initial after 21 days (b) ~6% PCE as of final PCE after 700 h (c) retained 79% PCE from initial after 40 min | 14. 29 ± 0 | 8 mg mL−1 | (a) Retained 94% PCE from initial after 21 days (b) 10.7% PCE as of final PCE after 700 h (c) retained 90% PCE from initial after 40 min | (a) unencapsulated, air, RH = 35%, 25 °C, ambient, dark, (b) 60 °C (c) 365 nm UV illumination 250 mW cm−2 | (–COO−) from TPA can strongly attract Pb2+ ions crosslinking additive within perovskite, resulting in compact, dense improved morphology, the rigidity of the phenyl skeleton allows moisture and thermal resistance | [92] |
| trimesic acid (TMA) | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 14.27 | (a)Retained 49% from initial PCE after 10h (b) retained 46% from initial PCE after 20 days | 17.21 | 1.0 mol L−1 PbI2 precursor solution | (a) Retained 49% from initial PCE after 10 h (b) retained 71% from initial PCE after 20 days | (a) 100 °C, air, ambient, dark, non-encapsulated, (b)RT, air, RH ~ 30%, dark, non-encapsulated, | TMA with a benzene ring and three carboxyl groups maintained stability. The strong hydrogen bond between the hydroxyl group and iodide to suppress the loss of iodide ion, preventing the perovskite from decomposing. Moreover, the benzene ring with rigidity and the π-π bond effect, and the hydrophobic alkyl chains further protects the perovskite from reacting with water | [93] |
| 3,3’,5,5’-azobenzene-tetracarboxylic acid (H4abtc) | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 14.25 | (a) Remained 25% after 20 h (b) retained 66% of its initial PCE after 30 d | 17.67 | 2.0% (mass ratio with respect to PbI2 | (a) Remained 61% after 20 h (b) retained 84% of its initial PCE after 30 d | (a) 100 °C without encapsulation, air, light 100 mW cm−2 (AM 1.5), (b) RT, RH = 30%without encapsulation. | High-quality perovskite film, H4abtc can passivate grain boundaries by reacting with the lead cation, therefore leading to good thermal stability and anti-moisture of perovskite films due to rigidity of the benzene ring and azo bond | [94] |
| 4-methylbenzenesulfonic acid (4- MSA) | FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au. | 14.05 | N/A | 17.58 | 6 mg mL−1 | N/A | N/A | Improved performance, reduced hysterias, improve J-V characteristics, the sulfonic group is chemically bonded to mp-TiO2 and phenyl backbone has p-conjugated structure. Jsc and Voc of 4-MSA doped PSC are enhanced. | [97] |
| Additive in Active Layer | architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
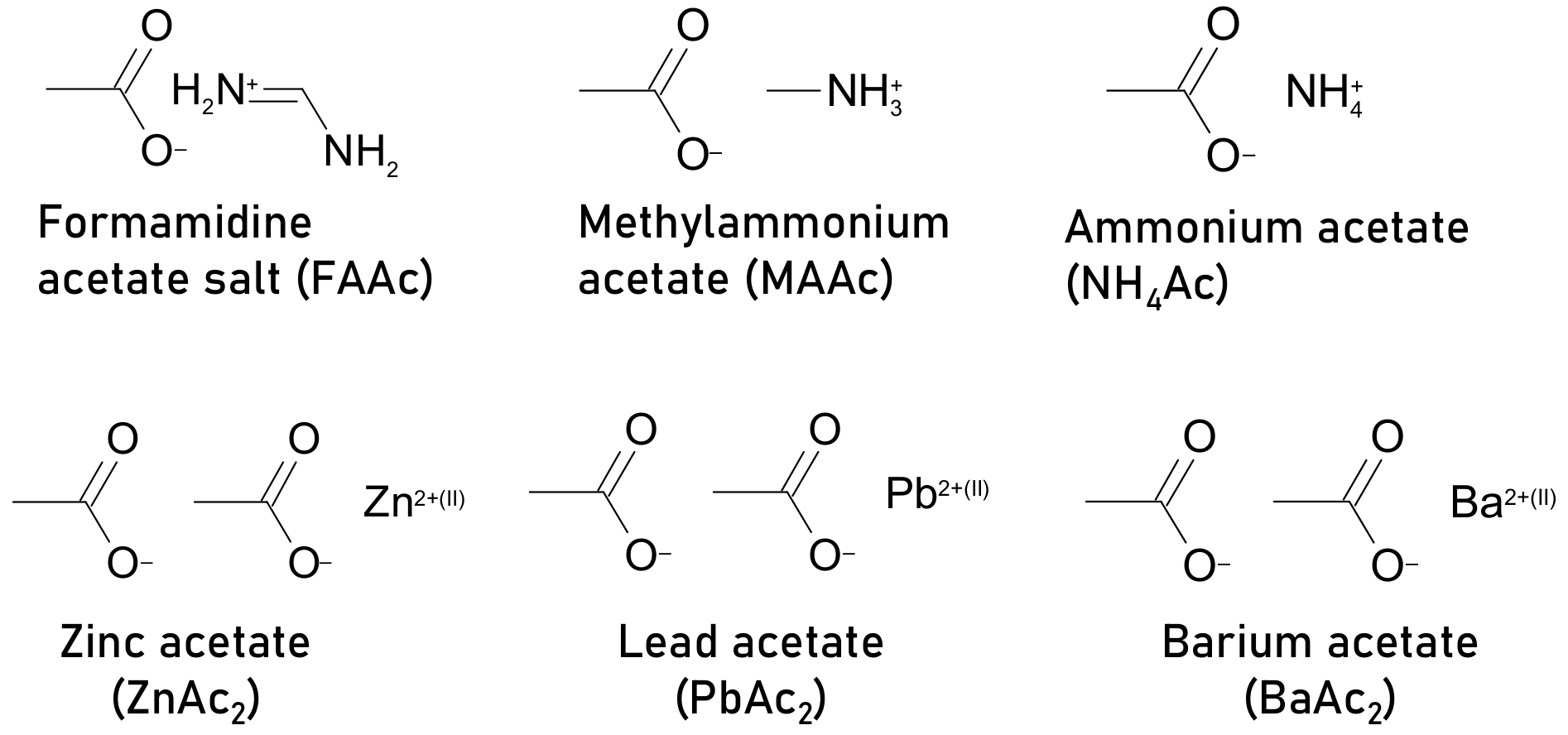
| formamidine acetate salt (FAAc) | ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/Al | 12.13 | (a) N/A (b) N/A | 16.59 | 5 mol% | (a) Retained 90% PCE from initial after 30 days (b) 16.10 as of final PCE after 320 s @ (0.87 V) | inert, unencapsulated, | control film morphology and crystallinity, improves optical and electrical properties reduces hysteresis | [98] |
| methylammonium acetate (MAAc) and thiosemicarbazide (TSC), combined | FTO/NiO/CH3NH3PbI3/PCBM/Ag | N/A | N/A | 19.19 | 10–15% MAAc (molar ratio) 3–5% TSC (molar ratio) | (a) Retained 90% PCE from initial after 1000 h (b) Retained 80% PCE from initial after 500 h | (a) light AM 1.5, 25 °C and RH < 25%, mppt (b) dark, 85 °C, RH < 25% | large-area (aperture area of 1.025 cm2) using one step solution process, high crystalline quality of film | [100] |
| ammonium acetate (NH4Ac) | FTO/TiO2/CH3NH3PbI3/spiro-OMeTAD/Au | 13.82 | N/A | 17.02 | 10 wt% | N/A | N/A | Improved film morphology and increased surface coverage | [101] |
| ammonium acetate CH3COONH4 (NH4Ac) | FTO/TiO2/CH3NH3PbI3/carbon devices | 11.11 | Remained 60% from initial PCE after 1900 h | 13.9 | molar ratio MAI/PbI2/NH4Ac 1:1:x = 0.08% | Remained 96% from initial PCE after 1900 h | Dark, RH = 40% | Carbon electrode-based device, improved crystallinity | [102] |
| zinc acetate Zn(CH3COO)2 (ZnAc2) | 12.30 | molar ratio MAI/ PbI2/ZnAc2 1:1−x:x = 7% | Remained 89% from initial PCE after 1900 h | ||||||
| lead acetate (PbAc2) | Glass/ ITO/PTAA/CH3NH3PbI3/PCBM/BCP/Ag | 17.25 | Retained 80% PCE from initial after 20 days | 19.07 | molar ratio PbAc2/PbI2=3% | Retained 95% PCE from initial after 20 days | unencapsulated, inert | PbAc2 additive aids cross-linking to form a strong hydrogen bond with MAI, leading to a more stable perovskite intermediate phase, retards crystallization process, resulting, perovskite thin films with better morphology and larger grains | [103] |
| barium acetate (BaAc2) | ITO/P3CTN/CH3NH3PbI3/PC61BM/C60/BCP/Ag | 18.99 | Retained only 20% from initial PCE after 400 h | 19.82 | 2 mg mL−1 | Retained 90% from initial PCE after 400 h | Non encapsulated, inert, 90 °C, dark, | suppression of ions migration, high quality perovskite film, grain boundary passivation | [104] |
| Additive in Active Layer | architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
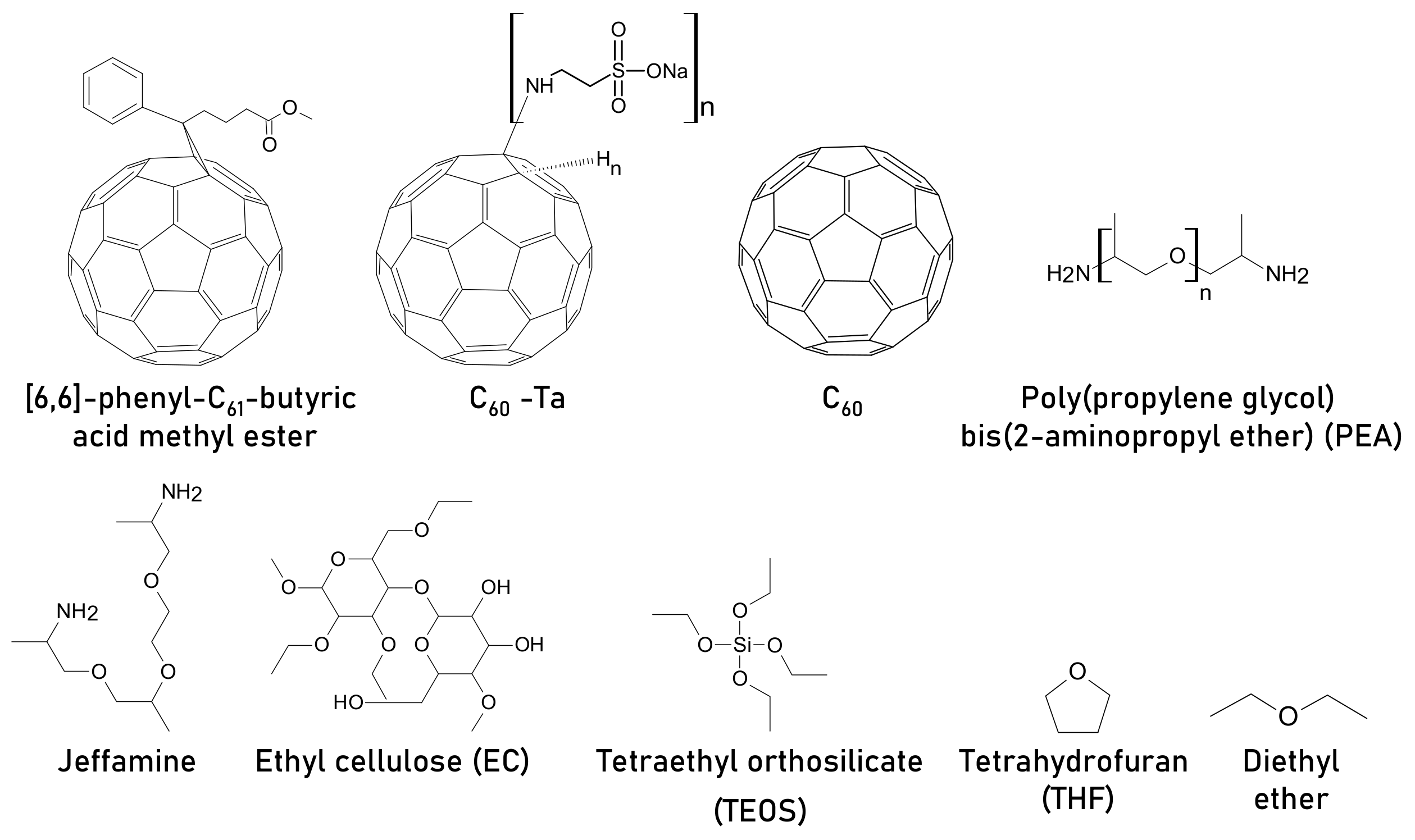
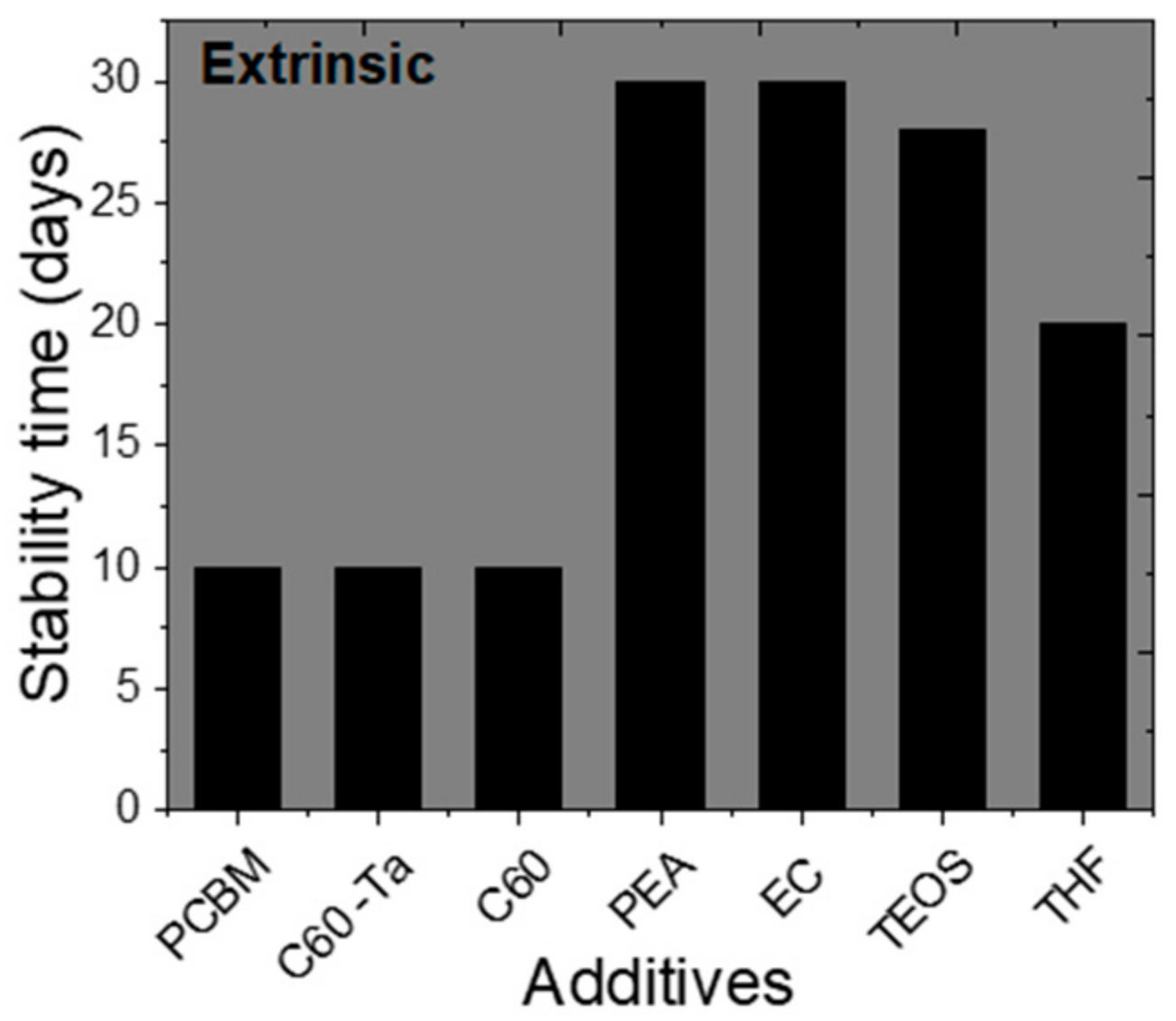
| [6,6]-phenyl-C61-butyric acid methyl ester | FTO/c-TiO2/m-TiO2/mZrO2/CH3NH3PbI3/m-carbon | 8.58 | N/A | 12.36 | 0.25 mg mL−1 | N/A | N/A | Improved morphology, intermediate formation between C=O of PCBM and PbI2 | [105] |
| PCBM | Glass/ITO/CH3NH3PbI3/PCBM/BPhen/Ag | 13.94 | Retained ~9% (absolute value) as of final PCE after 10 days | 15.94 | 3.5 mg mL−1 | Retained ~12% (absolute value) as of final PCE after 10 days | without encapsulation, ambient RH = 25–50%, | Improves crystallinity, passivates defects, suppress non radiative recombination | [106] |
| C60 -Ta | 16.46 | 1.5 mg mL−1 | Retained ~12% (absolute value) as of final PCE after 10 days | ||||||
| C60 | 16.59 | 0.1 mg mL−1 | Retained ~11% (absolute value) as of final PCE after 10 days | ||||||
| poly(propylene glycol) bis(2-aminopropyl ether) (PEA) | FTO/c-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 17.18 | Maintains ~55% of its original PCE after 30 days | 18.87 | 1 wt% | Maintains 95% of its original PCE after 30 days | dark, ambient, air, RH = 30 ± 5% | Grain boundary passivation, the oxygen atom from ether in PEA, acts as a crosslinking agent, reduces trap density and hysteresis | [107] |
| jeffamine | ITO/NiOx/CH3NH3PbI3/PC61BM/BCP/Ag | 14.5 | Showed major cracks | 16.8 | 0.05 wt% | Retained original morphology | 10 cycles of stretching at 30% strain | Defect passivation through Lewis acid–base reaction of N atom and O atom with Pb 2+/or interaction of MA+ and hydrogen bond of Jeffamine decrease trap density, enhances the ductility of the perovskite film to prevent cracking during stretching | [108] |
| ethyl cellulose (EC) | FTO/c-TiO2/CH3NH3PbI3/Spiro-OMeTAD/ MoO3/Ag | 17.11 | Completely died in 30 days | 19.27 | 0.1 mg mL−1 | Retained 80% PCE from initial after 30 days | non –encapsulated, dark, ambient, air, RH = 45% | Hydrogen bonding between EC and MAI passivates defects at the grain boundary, reduces hysteresis and improves stability | [109] |
| tetraethyl orthosilicate (TEOS) | TiO2/ CH3NH3PbI3/Spiro-OMeTAD/Ag | 15.96 | Retained 73% of its initial PCE after 28 days | 18.38 | 0.3 mol% | Retained 77% of its initial PCE after 28 days | ambient, 25 °C, RH = 30%, non-encapsulated, | Reduce trap density, improves carrier lifetime | [110] |
| THF | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 11.3 | Retained 35% PCE from initial after 20 days | 15.1 | THF: DMF (1:10) v/v | Retained 80% from initial PCE after 20 days | RH = 50%, unencapsulated, | Complex formation due to Lewis acid–base reaction | [113] |
| diethyl ether | Glass/ITO/NiOx/CH3NH3PbI3/PCBM/BCP/Ag | 13.28 | N/A | 15.09 | the molar ratio of 4% with matrix solvent | N/A | N/A | Grain size improvement | [114] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| dibutylhydroxytoluene (BHT) | ITO/PEDOT: PSS/CH3NH3PbI3/ PC61BM/ZnO/Al | 17.1 | Retained 85% from initial PCE after 180 min | 18.1 | 0.02 M | Retained 93% of initial PCE after 180 min | light 100 mW cm−2, RT, non-encapsulated, RH < 5% | intermolecular hydrogen bonds between the MA+ and −OH groups of the BHT additive improves film crystallinity, reducing the sub-Eg states and carrier traps, | [115] |
| methanol | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 16.53 | Retained 40% from initial PCE after 30 days | 19.51 | 5 vol% | Retained 90% from initial PCE after 30 days | Dark, ambient, nonencapsulated, | morphology control/enhancement | [116] |
| 2-methoxyethanol ME | ITO/ZnO/CH3NH3PbI3/Spiro-OMeTAD/MoOx/Ag | 14.2 | N/A | 16.7 | 30 µL to 1 mL MAI solution | N/A | N/A | two-step interdiffusion protocol to prepare pin-hole free perovskite films, glycol ethers changes the lead iodide to perovskite conversion dynamics and enhances PCE resulting in more compact polycrystalline films, and it creates micrometer-sized perovskite crystals vertically-aligned across the photoactive layer | [118] |
| 2-ethoxyethanol (EE), | 13.2 | ||||||||
| 2-propoxyethanol (PE) | 15.1 | ||||||||
| isopropanol (IPA) | FTO/compact-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 16.02 | Retained 40% PCE from initial after 40 days | 19.70 | precursor: IPA volume ratio = 4:1 | Retained 85% PCE from initial after 40 days | unencapsulated, air, ambient, RT | morphology enhancement/control | [117] |
| n-butanol | FTO/CT-IO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 13.8 | N/A | 15.5 | 5.0 v% for 1 mL precursor | N/A | N/A | morphology enhancement | [119] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Reduced graphene oxide (rGO) | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 13.8 | Retained 20% of their initial PCE after 50 days | 16.5 | 9 μg mL−1 | Retained 40% of their initial PCE after 50 days | dark, RH = 10%, unencapsulated, | Defect-free perovskite films of enhanced crystallinity, larger and evenly distributed grains, enhanced light harvesting potential, increasing the photocurrent density and the resulting improved PCE | [120] |
| Poly(amic acid) (PAA) | ITO/NiOx/CH3NH3PbI3/PC61BM/BCP/Ag | 14.16 | (a) Remained 13% as of final PCE after 500 h (b) Retained 50% from initial PCE after 70h | 17.85 | 0.0497 mg mL−1 | (a) Remained 16.57% as of final PCE after 500 h (b) Retained 88% from initial PCE after 70 h | Inert, dark, non-encapsulated, (b) 85 °C, RH = 45% without encapsulation, dark, in the glovebox | The O atoms in PAA and PI form hydrogen bonds with H atoms in CH3NH3 + and lone pair of electrons of N atoms interact with Pb ions, which stabilized the PVSK framework. | [121] |
| polyimide (PI) | 16.49 | 0.0990 mg mL−1 | N/A | N/A |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| DS | MgF2/PET/ITO/Nb2O5/CH3NH3PbI3/Spiro-OMeTAD/Au | 17.03 | (a) Retained 50% PCE from initial after 60 days (b) N/A | 18.40 | 10 vol% in 1 mL precursor | (a) Retained 86% PCE from initial after 60 days (b) 15.24 (83% of initial) as of final PCE after 5000 cycles | (a) unencapsulated, RH = 35%, dark, RT (b) bending stability- bending radius of 4 mm | Intermediate between Pb2+ and S atom | [122] |
| thiourea | ITO/PEDOT:PSS/CH3NH3PbI3 /PCBM/Ag | 13.4 | N/A | 16.2 | 0.5 vol% | N/A | N/A | Thiourea promotes grain growth, reduce trap states, passivate grain boundaries | [123] |
| thioacetamide (TAA) | ITO/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 17 | Retains ~75.1% of its initial performance after aging 816 h | 18.9 | 1.0% (molar ratio to PbI2) | Retains 88.9% of its initial performance after aging 816 h | RH = 25–35%, unsealed, air, | Interaction of TAA with Pb2+ improved grain size | [124] |
| 2-pyridylthiourea | FTO/C-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.5 | (a) Retained only ~10% PCE from initial after 30 days (b) Retained~55% PCE from initial after 30 days | 18.2 | 0.5 mg mL−1 | (a) Retained~95% PCE from initial after 30 days (b) Retained~92% PCE from initial after 30 days | ambient, air, dark (a) RH = 55 ± 5%, dark, RT (b) 65 °C, RH = 30%,dark | N-donor and S-donor coordinate with PbI2 and slow down the formation of PbI2. improved morphology, larger crystalline size, smooth compact, homogeneous film | [125] |
| ITU for I− and thiourea | ITO/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 17.75 | Retained 30% of the initial PCE after 100 h | 20.3 | 0.003 mM | Retained 80% of the initial PCE after 100 h | AM 1.5 light, ambient atmosphere | Reduction of iodine ions and reduces defect state concentration, and defect passivation of grain boundaries crystalline quality, improve charge transport | [126] |
| lead thiocyanate Pb(SCN)2 | FTO/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.57 | N/A | 17.80 | 5% Pb(SCN)2 (molar ratio with respect to PbI2 | N/A | N/A | Forms volatile products HSCN and CH3NH2, resulting in formation of excess PbI2 that passivates GB | [127] |
| potassium thiocyanate (KSCN) | FTO/c-TiO2/ m-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 19.38 | N/A | 19.6 | 1 mol% | N/A | N/A | Grain size and crystallinity improvement reduced recombination density | [129] |
| methylammonium thiocyanate (MASCN) additive | Glass/FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.81 | Died in 150 h. | 18.71 | 40 mol% | Retained 16.34% as of final PCE after 1000 h ~89.7% from initial | air, RH = 20–25%, without encapsulation, | Rapid vacuum-based drying approach, high crystallinity, large carrier lifetimes | [131] |
| guanidinium thiocyanate (GuSCN) | FTO/TiO2/CH3NH3PbI3/PCBM/Ag | 15.57 | Retained 60% PCE from initial PCE after 15 days | 16.70 | 10% mmol | Retained 90% PCE from initial PCE after 15 days | unencapsulated, dark, RH = 30–40%, | Enhance the crystallinity, enlarge the crystal size, and reduce the trap density of the perovskite film | [132] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| poly(vinylidenefluoride-co-hexafluoropropylene) (PVDF-HFP) | FTO/cTiO2/CH3NH3PbI3/Spiro-MeOTAD/Au | 7.2 | N/A | 10.6 | N/A | N/A | N/A | Interact with uncoordinated MAI molecule through hydrogen bonds between fluorine atoms. This can improve the carrier lifetimes and reduce the charge transfer resistance, which contributes to enhancing PCE | [135] |
| polyvinylidene fluoride-trifluoroethylene polymer P(VDF-TrFE) | FTO/c-TiO2/mesoporous-TiO2/CH3NH3PbI3/Spiro-MeOTAD/Ag | 9.57 ± 0.25% | N/A | 12.54 ± 0.40 | N/A | N/A | N/A | Two-step deposition, improved crystallinity and morphology, reduced carrier recombination of charge carrier, increased carrier lifetime | [136] |
| diiodomethane CH2I2 | Glass/FTO/TiO2/MAPbI3/Spiro-OMeTAD/Au | 10.0 | N/A | 16.5 | 0.25 mL in 1 mL precursor | N/A | N/A | Iodine liberation, morphology enhancement | [138] |
| Diiodooctane DIO | Glass/ITO/PEDOT:PSS/MAPbI3/PCBM/Ag | 10.61 | N/A | 17.74 | 1 vol% in 1 mL precursor | N/A | N/A | Intermediate formation and morphology enhancement | [137] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| carbon quantum dot (CQD) | ITO/NiOx/CH3NH3PbI3/PCBM/Ag | 15.25 | Remained ~10% PCE from initial after 48 h | 18.24 | 0.15 mg mL−1 | Remained~73.4% PCE from initial after 48 h | unencapsulated, dark, RT, RH = 80%, | Reduce non-radiative recombination loss, improve the crystallinity of film; thus, passivate grain boundaries and improve stability | [139] |
| carbon nanodots (CNDs) | ITO/NiOx/CH3NH3PbI3/PC61BM/BCP/Ag | 14.48% ± 0.39% | N/A | 16.47% ± 0.26% | 10 mg mL−1 | Remained ~18% as of an absolute value of PCE after 500 h | dark, 25 °C, RH = 40% air, unencapsulated, | Increase in the crystal size, lower content of grain boundary defects, longer carrier lifetimes. | [142] |
| carbon quantum dots (CQDs) | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 17.59 | (a) Retained 74% from initial PCE after 1500 h (b) Retained 17% from initial PCE after 216 h | 18.81 | 0.05 mg mL−1 | (a) Retained 90% from initial PCE after 1500 h (b) Retained 70% from initial PCE after 216 h | (a) unencapsulated, inert. (b) unencapsulated, RH = 50–60%, | Passivate the grain boundaries and decrease the trap-state density. The bonding between CQDs and MAPbI3 leads to a stable absorption of CQDs on the MAPbI3 surface, forming a protective layer to prevent the perovskite from coming in contact with water, thereby enhancing the stability of PSCs | [141] |
| nitrogen doped CQDs (N-CQDs), | ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/Al | 8.34 | N/A | 13.936 | 3 vol% | N/A | N/A | N-CQDs act like an intermediate and help to form dense and smooth perovskite; passivate the trap states and decrease the non-radiative charge recombination | [140] |
| Nitrogen-doped carbon dots (NCDs) | FTO/blTiO2/mL-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 12.12 ± 0.28% | N/A | 15.93 ± 0.15% | 0.05 mg mL−1 | N/A | N/A | Coordinate with the iodide ions and lead cations on the surface of perovskite, which effectively passivates the surface traps and help reduce non-radiative charge carrier recombination; the nitrogen dopants with lone electron pairs in NCDs optimize the interfacial energy level to enhance the charge carrier extraction efficiency at photoactive layer/TiO2 interface | [144] |
| potassium cation (K+) functionalized carbon nanodots (CNDs@K) | ITO/PTAA:F4TCNQ/CH3NH3PbI3/PCBM/BPhen/Ag | 18.25 | N/A | 21.04 | N/A | N/A | Defects passivation and crystallization control of the perovskite film; K+ in the grain boundary, and prevents excessive cations from occupying interstitial sites, thereby reducing microstrain of polycrystalline film, the synergistic effect of tailored crystal size, and suppressed grain boundary defects could reduce charge trap density, facilitate charge generation, and lengthen carrier lifetime | [143] | |
| graphene quantum dots (GQDs) | FTO/c-TiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 16.83 | Remained ~15.6% as of final PCE after 30 days | 18.34 | 1.5 mg mL−1 | Remained ~17.4% as of final PCE after 30 days | encapsulation, RH = 36%, atm | Promote charge transfer from the perovskite layer to TiO2 film. Faster electron extraction and a slower recombination rate passivate hanging bonds at the perovskite GBs | [145] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| hydroiodic acid (HI) | ITO/PTAA/CH3NH3PbI3/PCBM/Ti/Au | 16.1 | N/A | 18.21 | 0.004 vol% | N/A | N/A | Grain size improvement | [149] |
| hydrochloric acid (HCl) | Glass/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 9.96 | N/A | 14.49 | volume ratio 8%, | N/A | N/A | Grain size improvement and morphology enhancement | [156] |
| hydrobromic acid (HBr) | 13.53 | volume ratio 6% | N/A | N/A | |||||
| hydroiodic acid (HI) | 15.21 | volume ratio 8% | N/A | N/A | |||||
| lithium iodide (LiI) | FTO/TiO2/CH3NH3PbI3/electrode | 11.3 | Remained ~1% PCE as of final value after 25 days | 17.01 | 12mg/mL | Retained ~6% PCE as of final value after 70 days | unencapsulated, ambient, air, RH = 40%, | Larger grain size and higher crystallinity and reduced PbI2 residue | [155] |
| sodium iodide (NaI) | FTO/compact TiO2/mesoporous TiO2/CH3 NH3PbI3/SpiroMeOTAD/Au | 14.01 | N/A | 15.14 | 0.02 mol L−1 | N/A | N/A | Improved crystallinity and grain boundary passivation | [150] |
| lithium chloride (LiCl) | Glass/ITO/PEDOT:PSS/CH3NH3PbI3/PC61BM/C60/BCP/Al | 11.40 | Died in 50 days | 9.98 | 0.25%, wt% | N/A | N/A | Improved crystallinity and homogenous nucleation, and crystallization | [151] |
| sodium chloride (NaCl) | 12.77 | 1.0%, wt% | N/A | N/A | |||||
| potassium chloride (KCl) | 15.08 | 0.75%, wt% | Remained ~85% after 50 days | unencapsulated, inert, dark |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| n-type goethite (FeOOH) | FTO/cTiO2/mp-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.4 | (a) Remained only 5.7% as of final PCE after 360 h. (b) Retained 61% from initial PCE after 60 days. (c) Retained only 42% from initial PCE after 480 h | 19.7 | 0.1 mg mL−1 | (a) Retained 94% after 360 h, i.e., 17.9%. (b) Retained 97% from initial PCE after 60 days. (c) Retained 92% from initial PCE after 480 h | (a) inert, 85 °C, dark, non-encapsulated, (b) 10 ± 5% RH, (c) light 100 mW cm−2, inert, RT, mpp tracking, | Improved quality of perovskite and inhibition of iodine and methylamine ion migration | [157] |
| silver iodide (AgI) | FTO/compact TiO2/mesoporous TiO2/ CH3NH3PbI3/SpiroMeOTAD/Au | 14.01 | N/A | 14.18 | 0.02 mol L−1 | N/A | N/A | Improved crystallinity and grain boundary passivation | [150] |
| copper iodide (CuI) | 15.25 | N/A | N/A | ||||||
| copper bromide (CuBr) | 15.61 | N/A | N/A | ||||||
| nickel chloride (NiCl2) | ITO/SnO2/CH3NH3PbI3/SpiroMeOTAD/Au | 17.25 | Retained ~70% PCE after 100 h | 20.61 | 0.03 mM | Retained ~70% PCE after 100 h | en-capsulated, air, light 65 mW cm−2, ∼25 °C, RH = 45%, without ultraviolet filter | High crystallinity, GB passivation | [158] |
| copper chloride (CuCl2) | FTO/NiOX/ CH3NH3PbI3/PCBM/BCP/Ag | 9.73 | N/A | 15.22 | 2.5 mol% | N/A | N/A | Improves morphology | [159] |
| cadmium chloride (CdCl2) | FTO/SnO2 /CH3NH3PbI3 /Spiro-OMeTAD/Au | 11.7 | N/A | 13.2 | 1% molar ratio | N/A | N/A | Enhance grain size and crystallinity | [160] |
| zinc chloride (ZnCl2) | 13.76 | 0.1% molar ratio | N/A | N/A | |||||
| rhodium iodide (RhI3) | ITO/SnO2/CH3NH3PbI3/Spiro OMeTAD/Ag | 19.09 | Retained 75% of efficiency after 500 h | 20.71 | 1 mol% | Retained 92% of efficiency after 500 h | unencapsulated, dry air | Improved crystallinity and grain boundary passivation | [162] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| lead iodide (PbI2) | FTO/c-TiO2/m-TiO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.95 | N/A | 18.42 | 10% excess PbI2 | N/A | N/A | Improves the crystallinity | [163] |
| lead iodide (PbI2) | Glass/FTO/CH3NH3PbI3/Spiro-OMeTAD/Electrode/Encapsulation(UV-epoxy) | 16.2 | Retained 80% PCE till 12 h | 16.5 | 10 mg mL−1 | Retained 80% PCE till 10 days | ambient air, 85 °C, 60% RH, light 1 sun | N/A | [171] |
| lead iodide (PbI2) | Glass/ITO/SnO2/CH3NH3PbI3/PTAA/MoO3/Al | ~8.9% | Remained ~ 40% PCE after 1500 h | ~15.6% | 15% excess | Remained ~ 95% PCE after 1500 h | light 30 mW cm−2, 40 °C, inert | Excess PbI2 forms adduct with the cosolvent (NMP), improving PCE and stability. | [172] |
| lead iodide (PbI2) | FTO/TiO2/CH3NH3PbI3/Spiro-OMeTAD/Ag | 7.23 ± 0.10 | N/A | 14.32 ± 0.28% | concentration –N/A, by annealing @130 °C | N/A | N/A | Grain boundary passivation | [176] |
| lead sulfide (PbS QDs) | FTO/SnO2/CH3NH3PbI3/Spiro-OMeTAD/Au | 15.2 | N/A | 15.7 | 12.5 mg mL−1 | N/A | N/A | Capping ligand for surface functionalization to improve crystallinity | [175] |
| Additive in Active Layer | Architecture | PCE, % (Pristine) | Stability (Pristine) | PCE, % (with Additive) | Amount of Additive | Stability with Additive | Stability Conditions | Role of Additive | Ref |
|---|---|---|---|---|---|---|---|---|---|
| ammonium iodide (NH4I) | Glass/ITO/PTAA/CH3NH3PbI3/PCBM/Ag | 15.2 | Retained ~70% PCE after 20 days | 17.4 | 5 wt% | Retained ~80% PCE after 20 days | RH = 50 ± 5%, unencapsulated, ambient, air | Improved morphology, increased crystalline size and grain size, lower trap density | [177] |
| ammonium chloride (NH4Cl) | ITO/PEDOT:PSS/CH3NH3PbI3/PC61BM/Al | 7.97 | N/A | 9.93 | 17.5 mg mL−1 | N/A | N/A | Improved morphology, increased crystalline size and grain size, lower trap density | [178] |
| ammonium fluoride (NH4F) | ITO/PEDOT:PSS/CH3NH3PbI3 /PC61BM/LiF/Al | 13.74 | Retained 70% PCE after 5 weeks | 15.15 | 10 mol% | Retained 82% PCE after 5 weeks | encapsulated, air | Improved morphology, increased crystalline size and grain size, lower trap density | [179] |
| ammonium chloride (NH4Cl) | 16.88 | Retained 87% PCE after 5 weeks | |||||||
| ammonium bromide (NH4Br) | 16.85 | Retained 89% PCE after 5 weeks | |||||||
| ammonium iodide (NH4I) | 17.44 | Retained 91% PCE after 5 weeks | |||||||
| ammonium hypophosphite NH4H2PO2 | ITO/PTAA:F4-TCNQ/CH3NH3PbI3/PC61BM/TrNBr/Ag | 9.4 ± 1.0% | N/A | 16.5 ± 0.7% | 2.5 mg mL−1 | N/A | N/A | Intermediate with Pb and improved crystallinity grain size | [182] |
| hydrazinium iodide (N2H5I) | Glass/ITO/SnO2/CH3NH3PbI3/PTAA/MoOx/Al | 14.98 | Retained ~60% PCE after 4400 h | 17.03 | 0.5%, wt% | Retained ~80% PCE after 4400 h | inert, light 60-70 mW cm−2, 50 °C. | Intermediate between PbI2 and N2H5I, improved crystallinity, grain size | [183] |
| hydrazinium chloride (N2H5Cl) | ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/C60/BCP/Ag | 7.14 | N/A | 12.66 | PbI2/MAI/N2H5Cl molar ratio = 1:1:0.2 | N/A | N/A | Intermediate between PbI2 and N2H5Cl, improved crystallinity, grain size | [184] |
| hypophosphorous acid (HPA) | Glass/FTO/TiO2/CH3NH3PbI3/ETL/electrode | 5.1 | N/A | 8 | 0.75 uL mg−1 solution in 1 mL of MAI | N/A | N/A | Larger grains with a smoother surface, additive acts as a reducing agent for iodine | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangrulkar, M.; Stevenson, K.J. The Progress of Additive Engineering for CH3NH3PbI3 Photo-Active Layer in the Context of Perovskite Solar Cells. Crystals 2021, 11, 814. https://doi.org/10.3390/cryst11070814
Mangrulkar M, Stevenson KJ. The Progress of Additive Engineering for CH3NH3PbI3 Photo-Active Layer in the Context of Perovskite Solar Cells. Crystals. 2021; 11(7):814. https://doi.org/10.3390/cryst11070814
Chicago/Turabian StyleMangrulkar, Mayuribala, and Keith J. Stevenson. 2021. "The Progress of Additive Engineering for CH3NH3PbI3 Photo-Active Layer in the Context of Perovskite Solar Cells" Crystals 11, no. 7: 814. https://doi.org/10.3390/cryst11070814
APA StyleMangrulkar, M., & Stevenson, K. J. (2021). The Progress of Additive Engineering for CH3NH3PbI3 Photo-Active Layer in the Context of Perovskite Solar Cells. Crystals, 11(7), 814. https://doi.org/10.3390/cryst11070814