Investigations of LiNb1−xTaxO3 Nanopowders Obtained with Mechanochemical Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
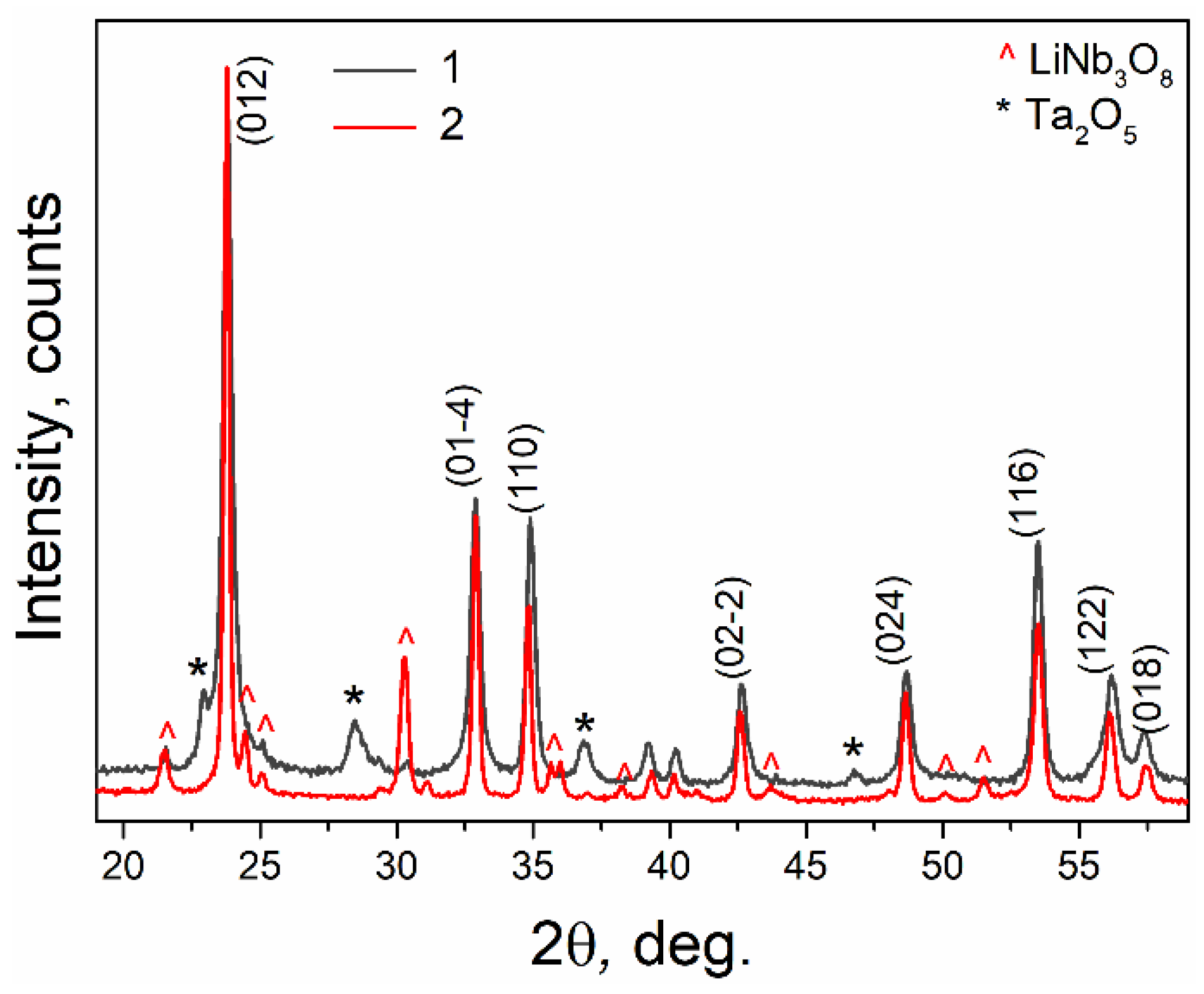
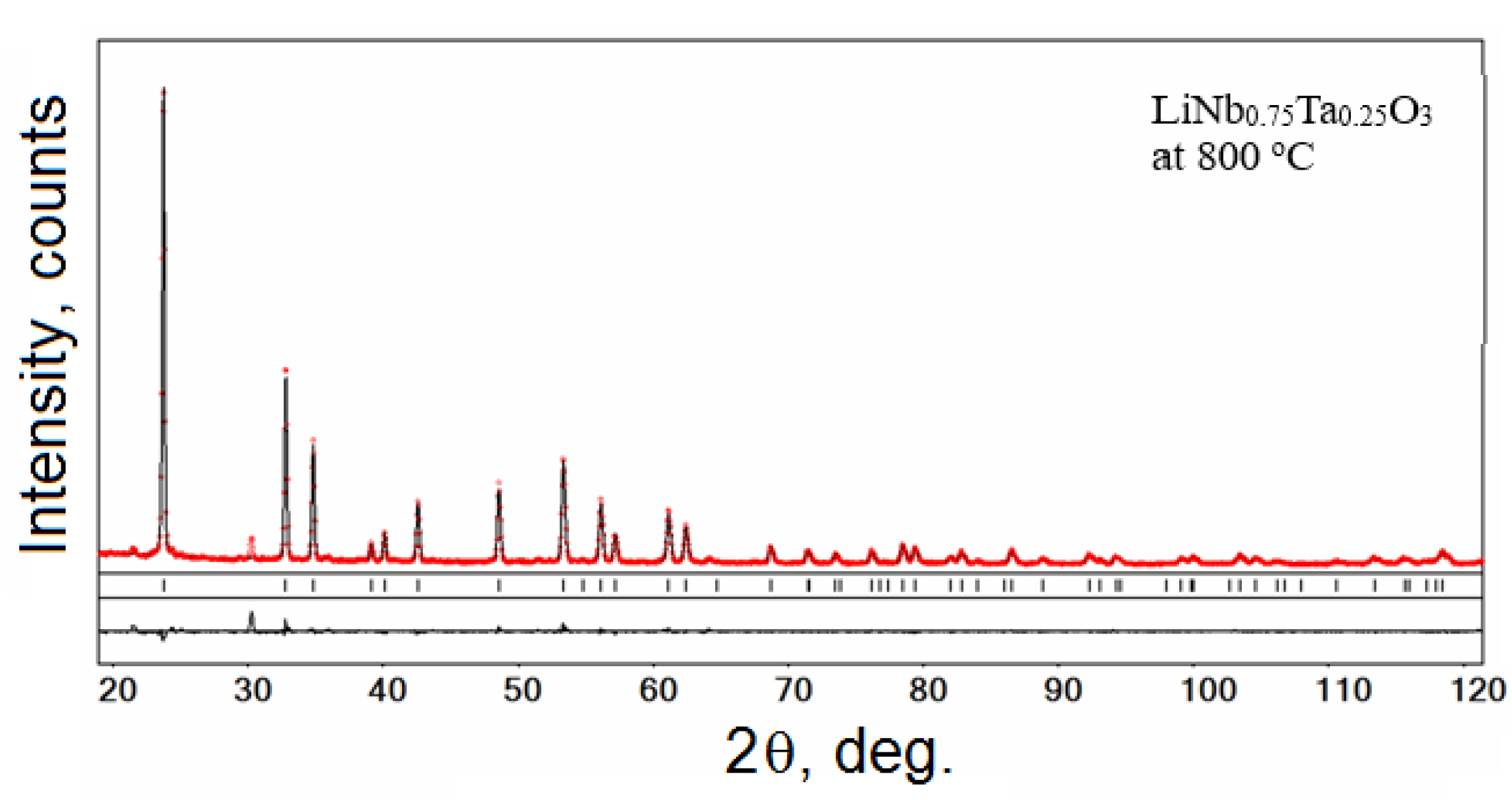
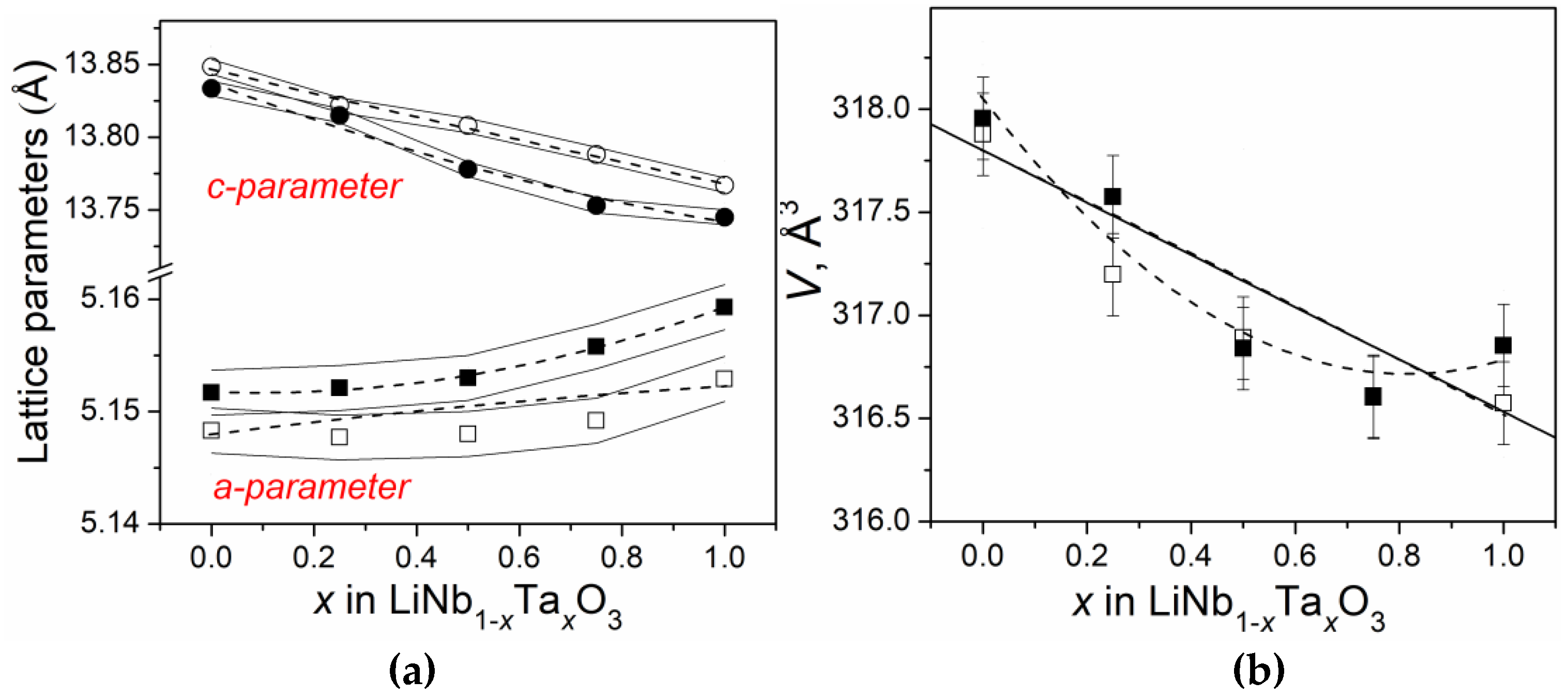
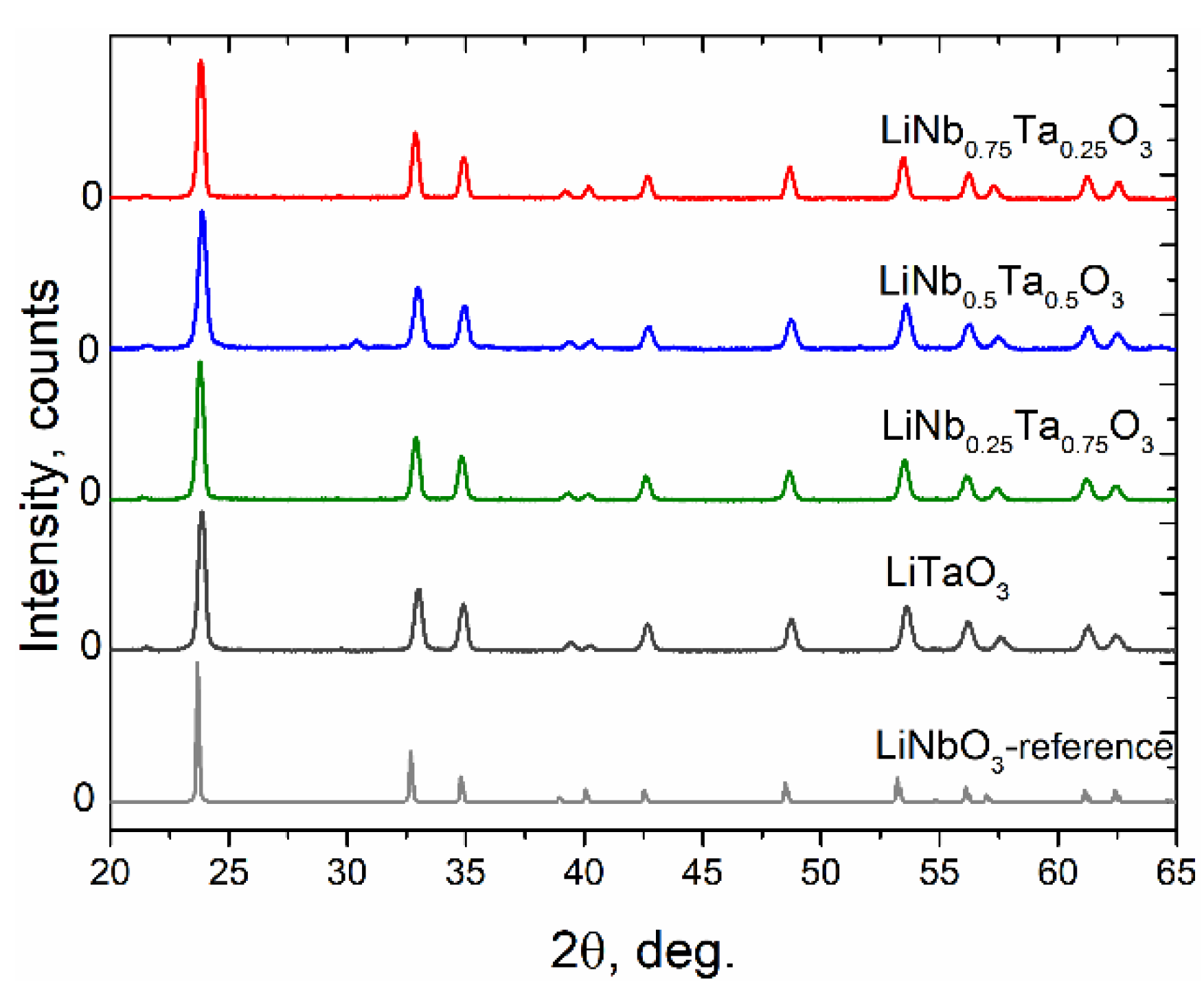
3.1. X-ray Diffraction
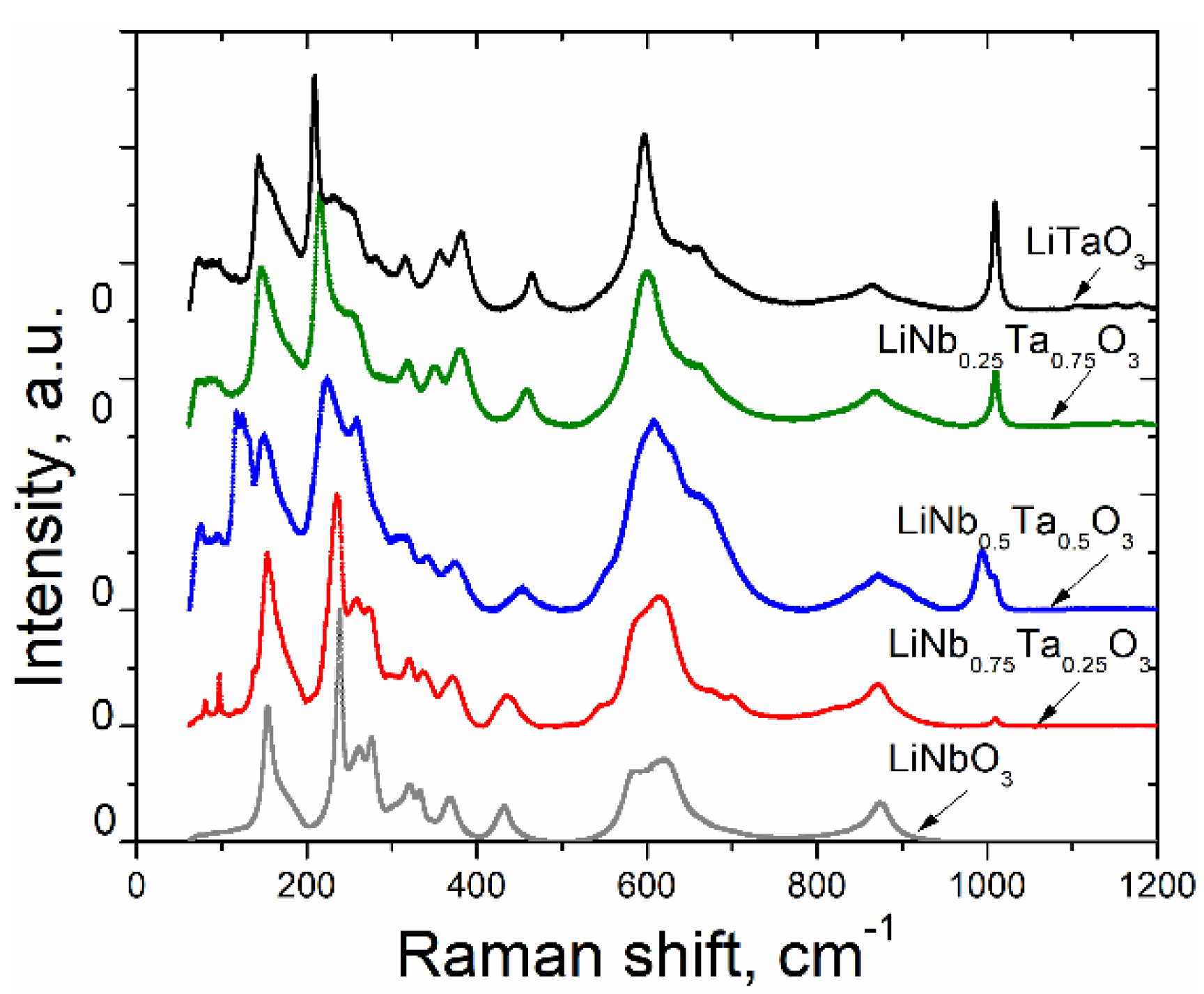
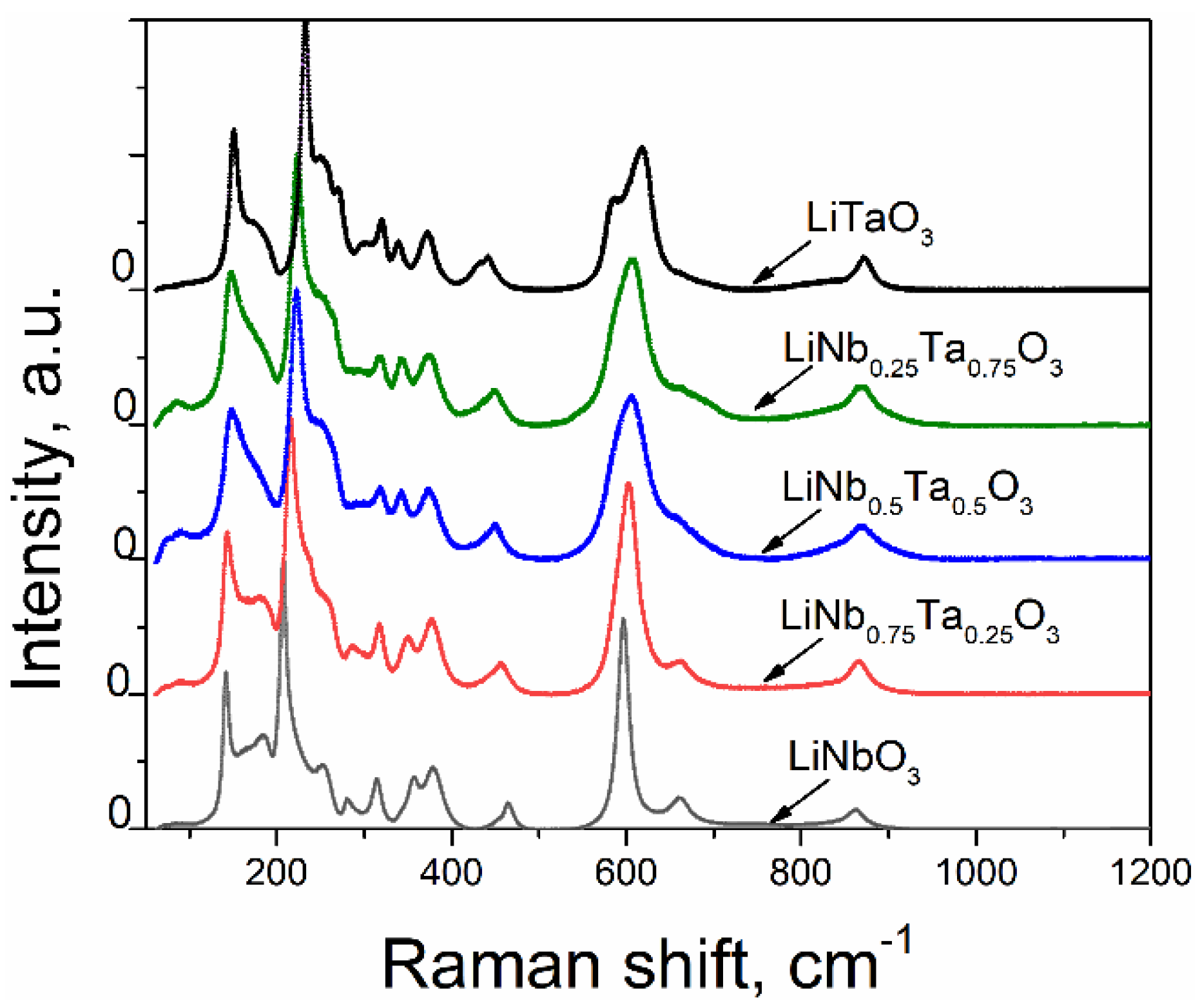
3.2. Raman Spectra
- Few low-frequency bands near 73–75 and 90–97 cm−1 are observed for the nanopowders with x ≠ 0. These bands cannot be linked with known Raman bands of LT [35,36,37]. In accordance with the results of [38], these bands could be induced by the presence of Li(Nb,Ta)3O8 phase, as identified by X-ray diffraction technique (see Table 2).
- A more intensive band near 117–125 cm−1 is observed only for the LN-LT sample with x = 0.5. The presence of this band could be attributed to the contribution of Li(Nb,Ta)3O8 and Ta2O5 additional phases in this sample. Note that the authors in [38] observed the close bands at 116 and 136 cm−1 and attributed them to LiNb3O8. The bands near 100 cm−1 were attributed to Ta2O5 by the authors of [39]. As it is followed from the XRD data (see Table 1), the simultaneous presence of Li(Nb,Ta)3O8 and Ta2O5 phases occurs only in the sample with x = 0.5, so the overlay of corresponding bands can result in a peculiar form of its spectrum. Furthermore, the sample with x = 0.5, i.e., with the composition intermediate between pure LN and pure LT, ought to essentially reveal the bands of both crystals, so it is no wonder that the spectrum of this sample has the most complex character.Furthermore, the sample with x = 0.5 reveals a significant increase of the bands’ intensities near 260 and 630–670 cm−1 that visually looks like a widening of intensive neighboring peaks. This result is in good agreement with [40], where two intensive neighboring bands in the region of 600 cm−1 were also observed for the LN-LT sample with x = 0.553.Finally, it should be noted that the bands near 600 cm−1 are considerably broad for all investigated samples in comparison with the other observed bands. This is consistent with the results in [41] where it is concluded that the band at 600 cm−1 is broader for non-poled LN samples (particularly, nanopowders) than for polarized.
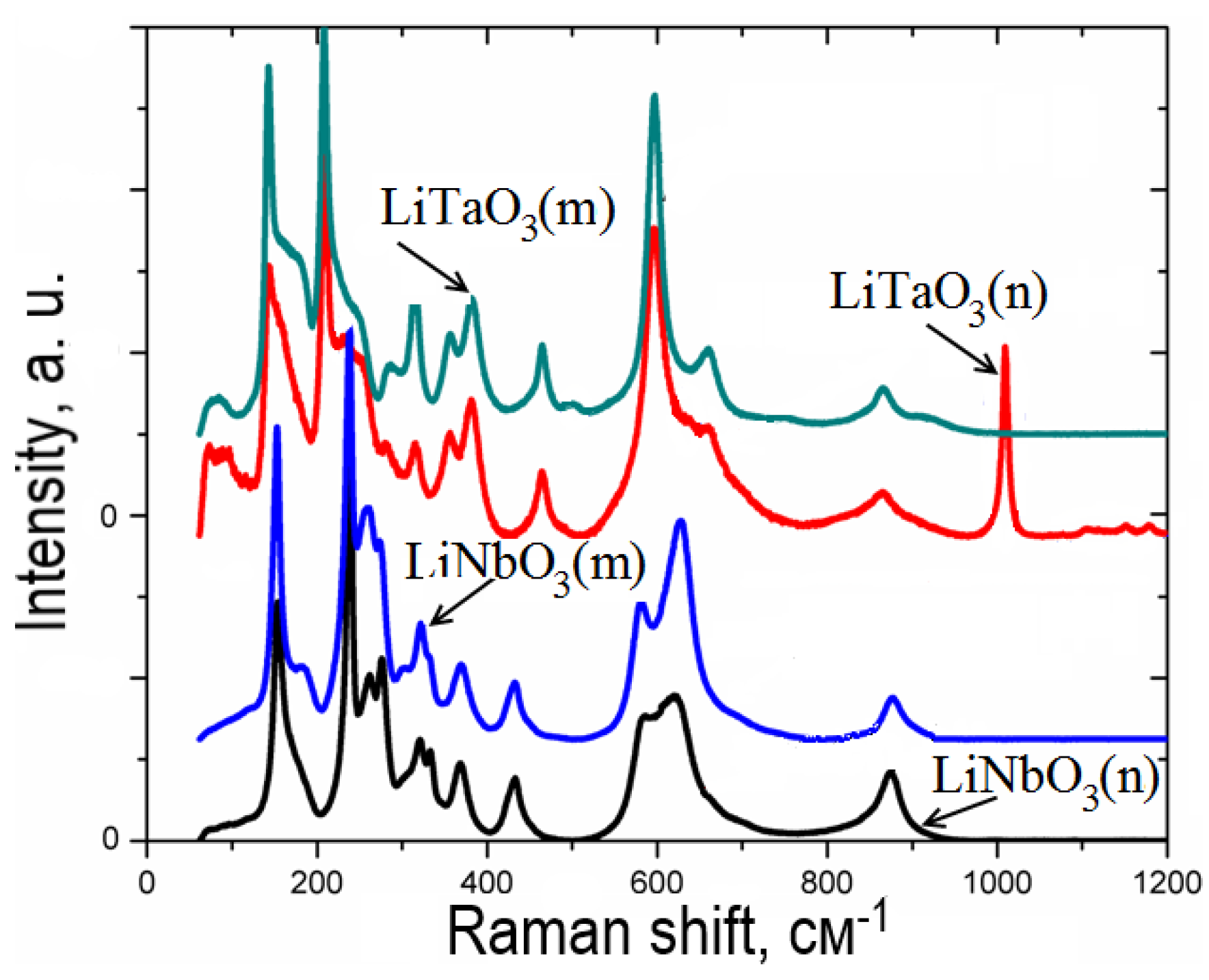
- The Raman spectra of LN and LT nano- and micropowders are shown in Figure 10 for comparison purposes. The latter were obtained by the crushing of LN and LT single crystals grown at SRC ‘Electron-Carat’. As seen from Figure 10, the band observed at about 1008–1009 cm−1 for LT nanopowder is not pronounced for LT micropowder as well as for LN compounds. The similar band can be observed in Figure 9 for nanopowders with x≠ 0. As it is seen from Figure 9, the intensity of this band increases with increasing of x. Moreover, for x = 0.5, this band splits into two with the frequencies of 994 and 1008 cm−1. As it is shown in [39], this band is absent in Ta2O5 Raman spectrum. Since the data about Raman scattering in LiNb3O8 are not available in this spectral range, we cannot exclude that this band is linked with the LiNb3O8 (or LiTa3O8) phase. However, as it is seen from Table 2, the LiTa3O8 phase is absent in the pure LT sample annealed at 550 °C as well as in the sample with x = 0.75 (within the limits of accuracy). Thus, we have to conclude that the nature of this band cannot be clearly determined from current experiments and requires additional studies.
- Increasing the spectral range up to 4000 cm−1 allows revealing the weak vibrations at 1600 and 3400 cm−1 (looking as low-intensive wide bands) that can be caused by the traces of OH– groups, which are always present in LN and LT as well as the traces of HCO3− groups (near 1750 and 2900 cm−1) present in synthesized compounds, which is probably due to the use of lithium carbonate as a component of the initial mixture.
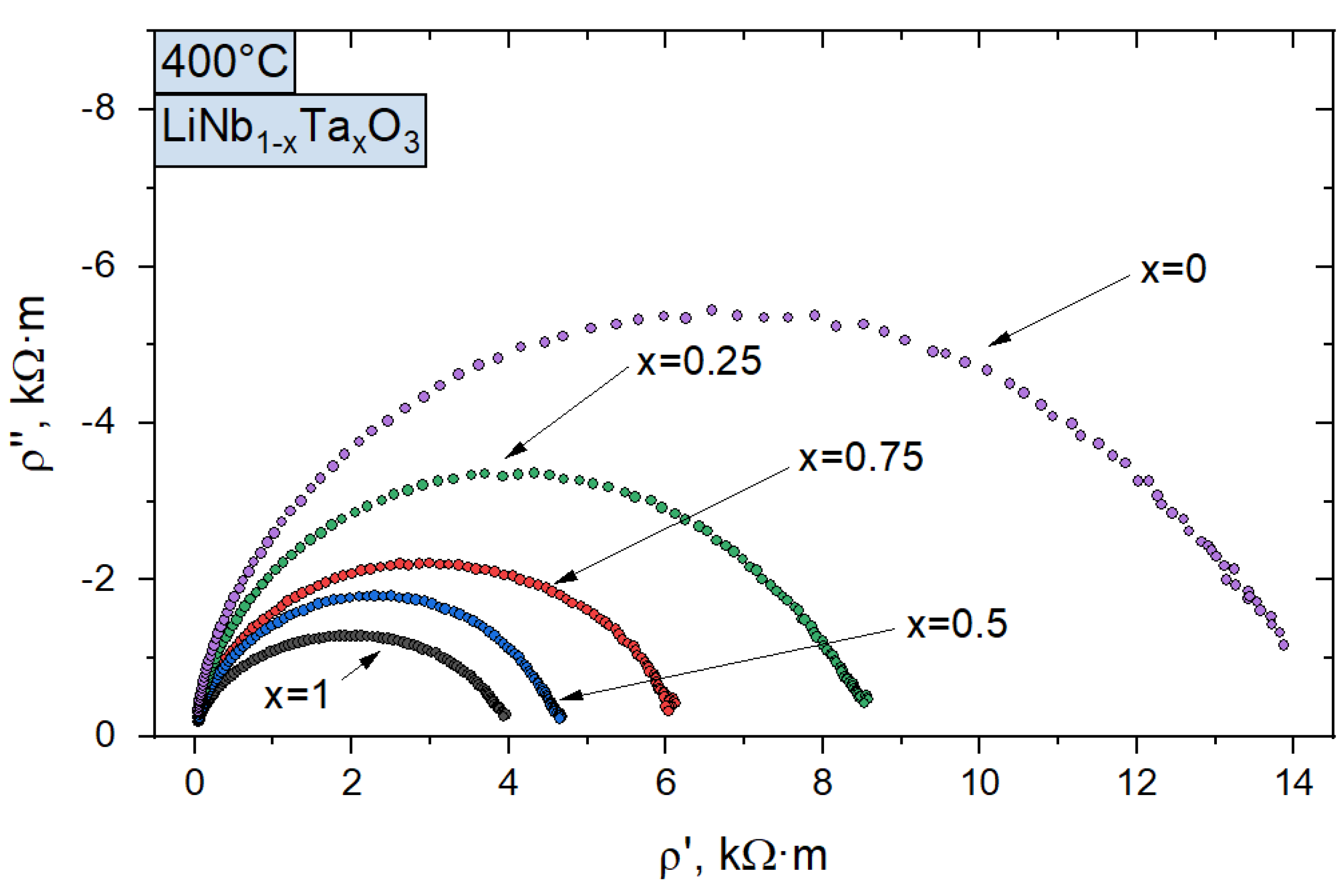
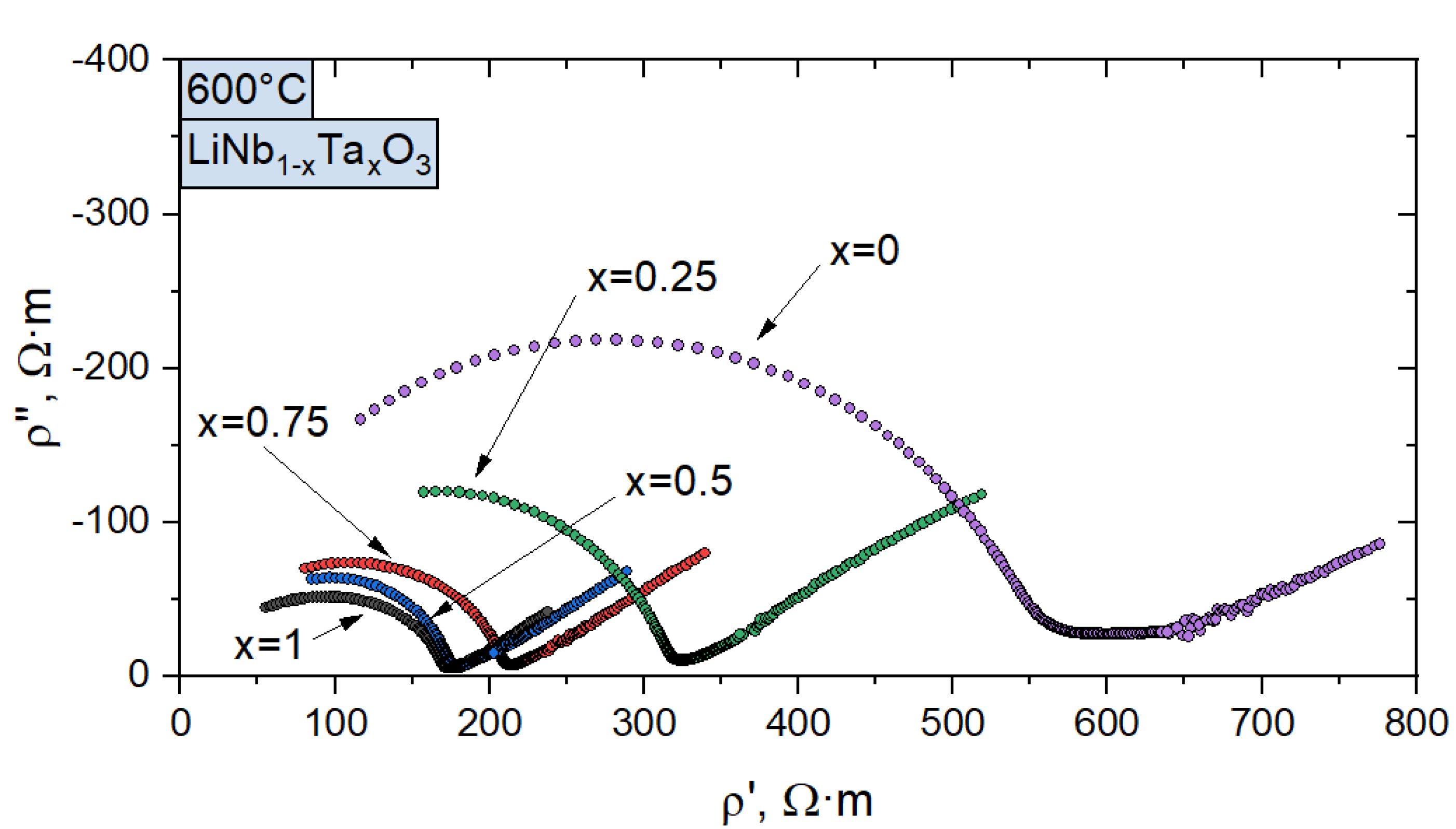
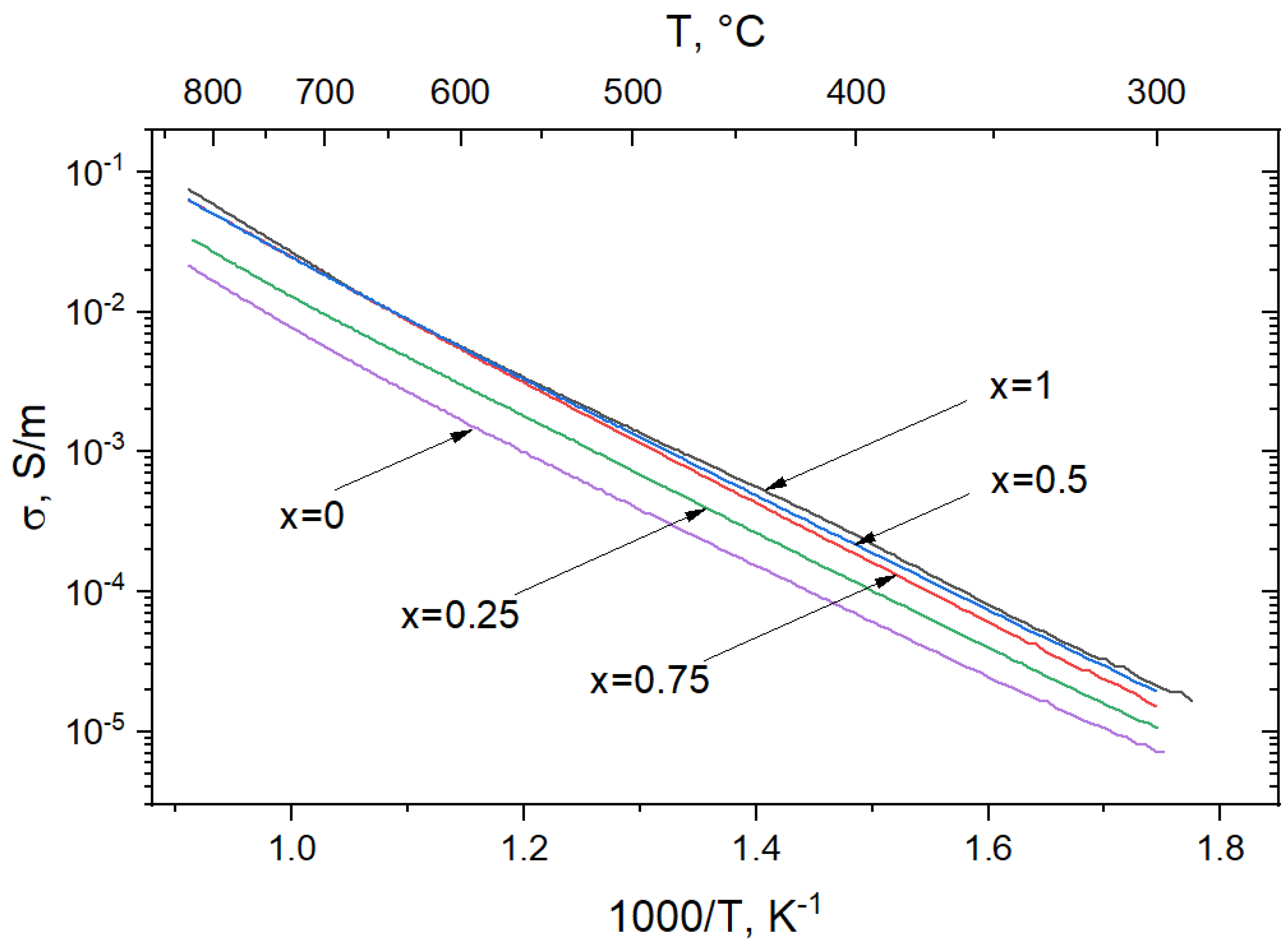
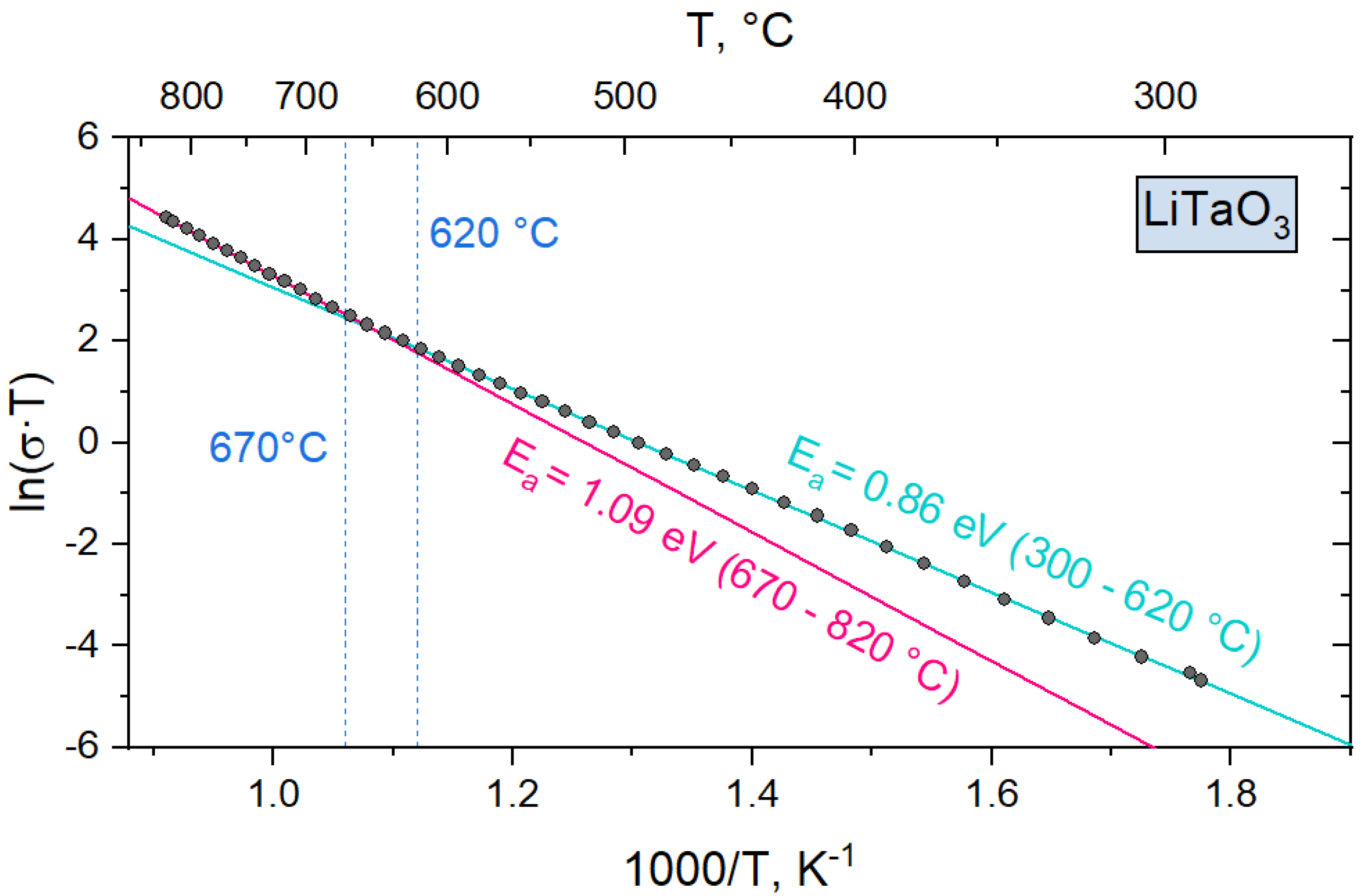
3.3. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LiNbO3 Crystal Market by Type (Acoustic Grade, Optical Grade, Others), by Application (Electro-Optical, Surface Acoustic Wave, Piezoelectric Sensors, Non-Linear Optical, Other) and Region—Global Forecasts to 2027. Available online: https://www.reportsanddata.com/report-detail/global-linbo3-crystal-market-by-manufacturers-countries-type-and-application-forecast-to-2022 (accessed on 21 May 2021).
- Lithium Niobate Modulator Market—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast. 2019–2027. Available online: https://www.transparencymarketresearch.com/lithium-niobate-modulator-market.html (accessed on 21 May 2021).
- Global Lithium Tantalate Crystal Market 2019–2027—Industry Analysis, Size, Share, Growth, Trends and Forecast. Available online: https://www.prnewswire.com/news-releases/global-lithium-tantalate-crystal-market-2019–2027--industry-analysis-size-share-growth-trends-and-forecast-300980802.html (accessed on 21 May 2021).
- Lithium Tantalate Crystal Market (Grade: Optical Grade, SAW Grade, and Others; and Application: Electro-Optical Modulators, Pyroelectric Detectors, Piezoelectric Transducers, Acoustic Wave Devices, and Others)—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast. 2019–2027. Available online: https://www.transparencymarketresearch.com/lithium-tantalate-crystal-market.html (accessed on 21 May 2021).
- Xue, D.; Betzler, K.; Hesse, H. Dielectric properties of lithium niobate-tantalate crystals. Solid State Commun. 2000, 115, 581–585. [Google Scholar] [CrossRef]
- Wood, I.G.; Daniels, P.; Brown, R.H.; Glazer, A.M. Optical birefringence study of the ferroelectric phase transition in lithium niobate tantalate mixed crystals: LiNb1-xTaxO3. J. Phys. Condens. Matter 2008, 20, 235237. [Google Scholar] [CrossRef] [PubMed]
- Bartasyte, A.; Glazer, A.M.; Wondre, F.; Prabhakaran, D.; Thomas, P.A.; Huband, S.; Keebleand, D.S.; Margueron, S. Growth of LiNb1−xTaxO3 solid solution crystals. Mater. Chem. Phys. 2012, 134, 728–735. [Google Scholar] [CrossRef]
- Roshchupkin, D.; Emelin, E.; Plotitcyna, O.; Fahrtdinov, R.; Irzhak, D.; Karandashev, V.; Orlova, T.; Targonskaya, N.; Sakharov, S.; Mololkin, A.; et al. Single crystals of ferroelectric lithium niobate–tantalate LiNb1–xTaxO3 solid solutions for high-temperature sensor and actuator applications. Acta Crystallogr. B 2020, 76, 1071–1076. [Google Scholar] [CrossRef]
- Nashimoto, K.; Cima, M.J. Epitaxial LiNbO3 thin films prepared by a sol-gel process. Mat. Lett. 1991, 10, 348–354. [Google Scholar] [CrossRef]
- Hirano, S.; Yogo, T.; Kikuta, K.; Morishita, T.; Ito, Y.; Hirano, S.; Yogo, T.; Kikuta, K.; Morishita, T. Preparation of potassium tantalate niobate by sol-gel method. J. Amer. Cer. Soc. 1992, 75, 1701–1704. [Google Scholar] [CrossRef]
- Ono, S.; Hirano, S. Processing of highly oriented lithium tantalate films by chemical solution deposition. J. Mater. Res. 2002, 17, 2532–2539. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamauchi, K.; Yagi, T.; Nishiwaki, A.; Wakita, K.; Ohnishi, N.; Hotta, K.; Sahashi, I. Preparation and characterization of high-quality stoichiometric LiNbO3 thick films prepared by the sol–gel method. Thin Solid Film. 2004, 458, 108–113. [Google Scholar] [CrossRef]
- Satapathy, S.; Gupta, P.K.; Srivastava, H.; Srivastava, A.K.; Wadhawan, V.K.; Varma, K.B.R.; Sathe, V.G. Effect of capping ligands on the synthesis and on the physical properties of the nanoparticles of LiTaO3. J. Cryst. Growth 2007, 307, 185–191. [Google Scholar] [CrossRef]
- Gou, J.; Wang, J.; Huang, Z.H.; Jiang, Y.D. Preparation of LiTaO3 nano-crystalline films by sol-gel process. Key Engin. Mat. 2013, 531–532, 446–449. [Google Scholar] [CrossRef]
- Johann, F.; Jungk, T.; Lisinski, S.; Hoffmann, Á.; Ratke, L.; Soergel, E. Sol-gel derived ferroelectric nanoparticles investigated by piezoresponse force microscopy. Appl. Phys. Lett. 2009, 95, 202901. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.G.; Hu, J.H.; Huang, Z.H.; Fang, M.H. Preparation of LiTaO3 nanoparticles by a sol–gel route. J. Sol. Gel. Sci. Technol. 2011, 58, 664–668. [Google Scholar] [CrossRef]
- Mathew, T.V.; Kuriakose, S. Synthesis and characterization of sodium–lithium niobate ceramic structures and their composites with biopolymers. J. Adv. Cer. 2013, 2, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-Y.; Chen, H.-L.; Chen, G.-J.; Kuo, C.-L.; Hsieh, P.-H.; Hwang, W.-S. Investigation of the defect structure of congruent and Fe-doped LiNbO3 powders synthesized by the combustion method. Materials 2017, 10, 380. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Chen, H.; Wang, Z.; Zou, X. Solvothermal synthesis and characterization of lithium tantalate nanoparticles. Key Eng. Mater. 2014, 602–603, 19–22. [Google Scholar] [CrossRef]
- de Figueiredo, R.S.; Messai, A.; Hernandes, A.C.; Sombra, A.S.B. Piezoelectric lithium niobate obtained by mechanical alloying. J. Mater. Sci. Lett. 1998, 17, 449–451. [Google Scholar] [CrossRef]
- Khalameida, S.; Sydorchuk, V.; Leboda, R.; Skubiszewska-Zięba, J.; Zazhigalov, V. Preparation of nano-dispersed lithium niobate by mechanochemical route. J. Therm. Anal. Calorim. 2014, 115, 579–586. [Google Scholar] [CrossRef]
- Fierro-Ruiz, C.D.; Sánchez-Dena, O.; Cabral-Larquier, E.M.; Elizalde-Galindo, J.T.; Farías, R. Structural and magnetic behavior of oxidized and reduced Fe doped LiNbO3 powders. Crystals 2018, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Logad, S.; Choudhary, O.P.; Debnath, C.; Verma, S.; Bartwal, K.S. Preparation of lithium niobate nanoparticles by high energy ball milling and their characterization. Univers. J. Mater. Sci. 2013, 1, 18–24. [Google Scholar] [CrossRef]
- Kocsor, L.; Péter, L.; Corradi, G.; Kis, Z.; Gubicza, J.; Kovács, L. Mechanochemical reactions of lithium niobate induced by high-energy ball-milling. Crystals 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Timoshevskii, A.N.; Ktalkherman, M.G.; Emel’kin, V.A.; Pozdnyakov, B.A.; Zamyatin, A.P. High-temperature decomposition of lithium carbonate at atmospheric pressure. High. Temperature 2008, 46, 414–421. [Google Scholar] [CrossRef]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Marsh, P. Defect structure dependence on composition in lithium niobate. Acta Crystallogr. Sect. B Struct. Sci. 1986, 42, 61–68. [Google Scholar] [CrossRef]
- Ohgaki, M.; Tanaka, K.; Marumo, F. Structure refinement of lithium (I) niobium (V) trioxide, LiNbO3, withanharmonic thermal vibration model. Mineral. J. 1992, 16, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Zotov, N.; Boysen, H.; Schneider, J.; Frey, F. Application of combined neutron and X-ray powder diffraction refinements to the structure of congruent lithium niobate. Mater. Sci. Forum 1994, 166, 631–636. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Reddy, J.M.; Bernstein, J.L. Ferroelectric lithium niobate. 3. Single crystal X-ray diffraction study at 24 °C. J. Phys. Chem. Solids 1966, 27, 997–1012. [Google Scholar] [CrossRef]
- Iyi, N.; Kitamura, K.; Izumi, F.; Yamamoto, J.K.; Hayashi, T.; Asano, H.; Kimura, S. Comparative study of defect structures in lithium niobate with different compositions. J. Solid State Chem. 1992, 101, 340–352. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Ferroelectric lithium tantalate. 1. single crystal X-ray diffraction study at 24 °C. J. Phys. Chem. Solids 1967, 28, 1685–1692. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Hamilton, W.C.; Sequeira, A. Ferroelectric lithium tantalate. 2. Single crystal neutron diffraction study at 24 °C. J. Phys. Chem. Solids 1967, 28, 1693–1698. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiang, H. Synthesis and Characterization of LiNbO3 Powders by Thermal Decomposition Method at Low Temperature. Ferroelectrics 2011, 413, 212–219. [Google Scholar] [CrossRef]
- Gorelik, V.S.; Abdurakhmonov, S.D.; Sidorov, N.V.; Palatnikov, M.N. Raman scattering in lithium niobate and lithium tantalate single crystals and ceramics. Inorg. Mater. 2019, 55, 524–532. [Google Scholar] [CrossRef]
- Ridah, A.; Bourson, P.; Fontana, M.D.; Malovichko, G. The composition dependence of the Raman spectrum and new assignment of the phonons in LiNbO3. J. Phys. Cond. Matter 1997, 9, 9687–9693. [Google Scholar] [CrossRef]
- Repelin, Y.; Husson, E.; Bennani, F.; Proust, C. Raman spectroscopy of lithium niobate and lithium tantalate. Force field calculations. J. Phys. Chem. Solids 1999, 60, 819–825. [Google Scholar] [CrossRef]
- Bartasyte, A.; Plausinaitiene, V.; Abrutis, A.; Stanionyte, S.; Margueron, S.; Boulet, P.; Kobata, T.; Uesu, Y.; Gleize, J. Identification of LiNbO3, LiNb3O8 and Li3NbO4 phases in thin films synthesized with different deposition techniques by means of XRD and Raman spectroscopy. J. Phys.: Condens. Matter. 2013, 25, 205901. [Google Scholar] [CrossRef]
- Verma, N.; Mari, B.; Singh, K.C.; Jindal, J.; Mollar, M.; Rana, R.; Pereira, A.L.J.; Manjón, F.J. Structural and optical properties of Ta2O5:Eu3+: Mg2+ or Ca2+ phosphor prepared by molten salt method. AIP Conf. Proc. 2016, 1724, 020082. [Google Scholar] [CrossRef]
- Rüsing, M.; Sanna, S.; Neufeld, S.; Berth, G.; Schmidt, W.G.; Zrenner, A.; Yu, H.; Wang, Y.; Zhang, H. Vibrational properties of LiNb1-xTaxO3 mixed crystals. Phys. Rev. B. 2016, 93, 284305. [Google Scholar] [CrossRef]
- Golubović, A.; Gajić, R.; Hinić, I.; Šćepanović, M. IR and Raman spectra of poled and unpoled LiNbO3 single crystals. J. Alloy. Comp. 2008, 460, 74–78. [Google Scholar] [CrossRef]
- Heitjans, P.; Masoud, M.; Feldhoff, M.; Wilkening, M. NMR and impedance studies of nanocrystalline and amorphous ion conductors: Lithium niobate as a model system. Faraday Discuss. 2007, 134, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.H.; Chen, L.F.; Chia, C.T. Impedance spectroscopic studies on congruent LiNbO3 single crystal. J. Phys. Condens. Matter 2007, 19, 086225. [Google Scholar] [CrossRef]
- Huanosta, A.; West, A.R. The electrical properties of ferroelectric LiTaO3 and its solid solutions. J. Appl. Phys. 1987, 61, 5386–5391. [Google Scholar] [CrossRef]
- Lanfredi, S.M.; Rodrigues, A.C. Impedance spectroscopy study of the electrical conductivity and dielectric constant of polycrystalline. J. Appl. Phys. 1999, 86, 2215–2219. [Google Scholar] [CrossRef]
- Suhak, Y.; Roshchupkin, D.; Redkin, B.; Kabir, A.; Jerliu, B.; Ganschow, S.; Fritze, H. Correlation of Electrical Properties and Acoustic Loss in Single Crystalline Lithium Niobate-Tantalate Solid Solutions at Elevated Temperatures. Crystals 2021, 11, 398. [Google Scholar] [CrossRef]
- Rahn, J.; Huger, E.; Dorrer, L.; Ruprecht, B.; Heitjans, P.; Schmidt, H. Li self-diffusion in lithium niobate single crystals at low temperatures. Phys. Chem. Chem. Phys. 2012, 14, 2427–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidenfelder, A.; Schulz, M.; Fielitz, P.; Shi, J.; Borchardt, G.; Becker, K.-D.; Fritze, H. Electronic and Ionic Transport Mechanisms of Stoichiometric Lithium Niobate at High-Temperatures. MRS Online Proc. Libr. 2013, 1519, 330. [Google Scholar] [CrossRef]
- Barik, R.; Satpathy, S.K.; Behera, B.; Biswal, S.K.; Mohapatra, R.K. Synthesis and Spectral Characterizations of Nano-Sized Lithium Niobate (LiNbO3) Ceramic. Micro Nanosyst. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Efremov, V.V.; Shcherbina, O.B.; Palatnikov, M.N.; Masloboeva, S.M. Comparative investigation of electrophysical characteristics of ceramic and single crystal LiNbO3. J. Phys. Conf. Ser. 2020, 1658, 012010. [Google Scholar] [CrossRef]
- El Bachiri, A.; Bennani, F.; Bousselamti, M. Dielectric and electrical properties of LiNbO3 ceramics. J. Asian Ceram. Soc. 2016, 4, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Masoud, M.; Heitjans, P. Impedance Spectroscopy Study of Li Ion Dynamics in Single Crystal, Microcrystalline, Nanocrystalline and Amorphous LiNbO3. Defect Diffus. Forum 2005, 237–240, 1016–1021. [Google Scholar] [CrossRef]
- El Bachiri, A.; Bennani, F.; Bousselamti, M. Dielectric and electrical properties of LiNbO3 ceramics. J. Asian Ceram. Soc. 2016, 4, 46–54. [Google Scholar] [CrossRef] [Green Version]
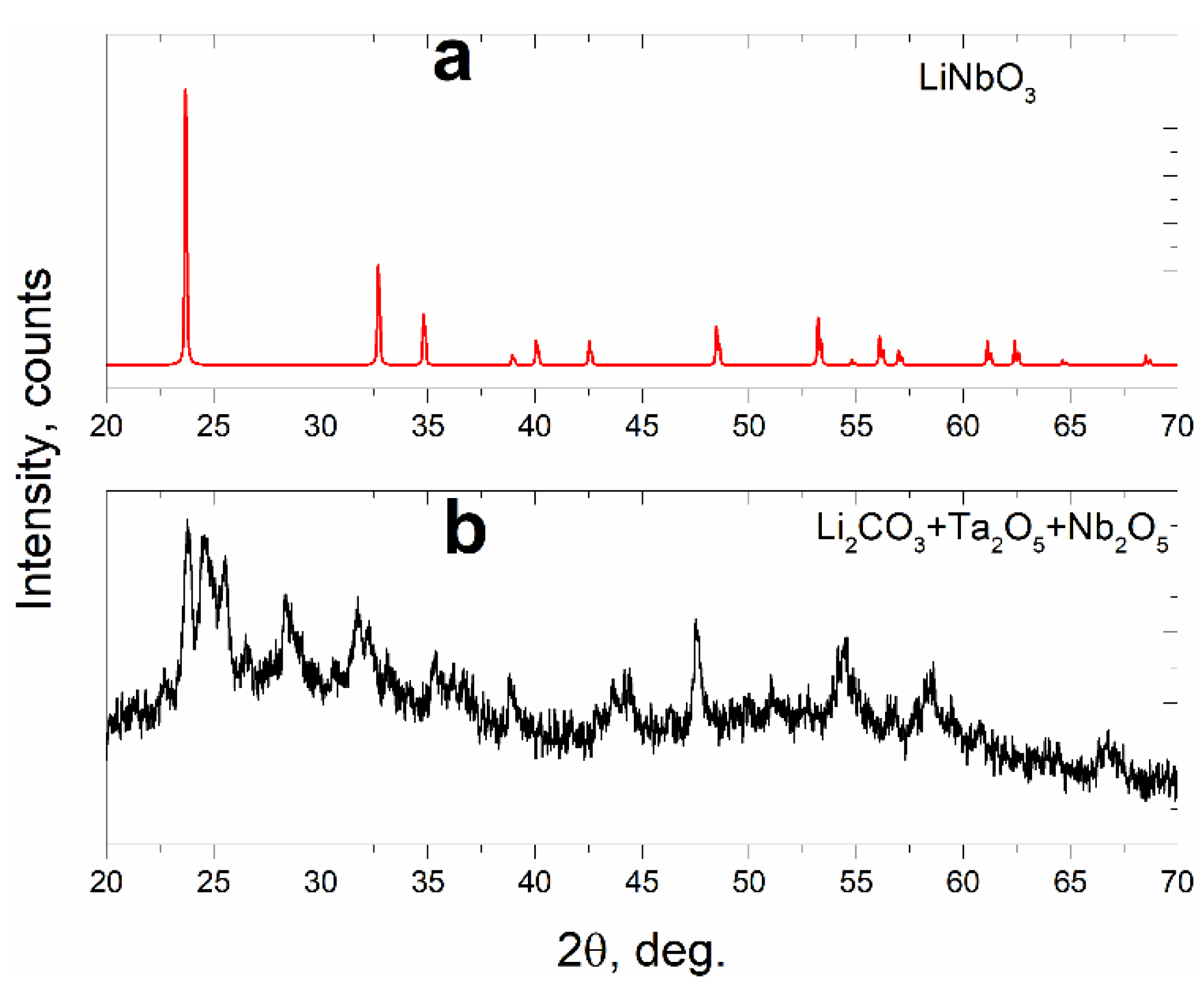
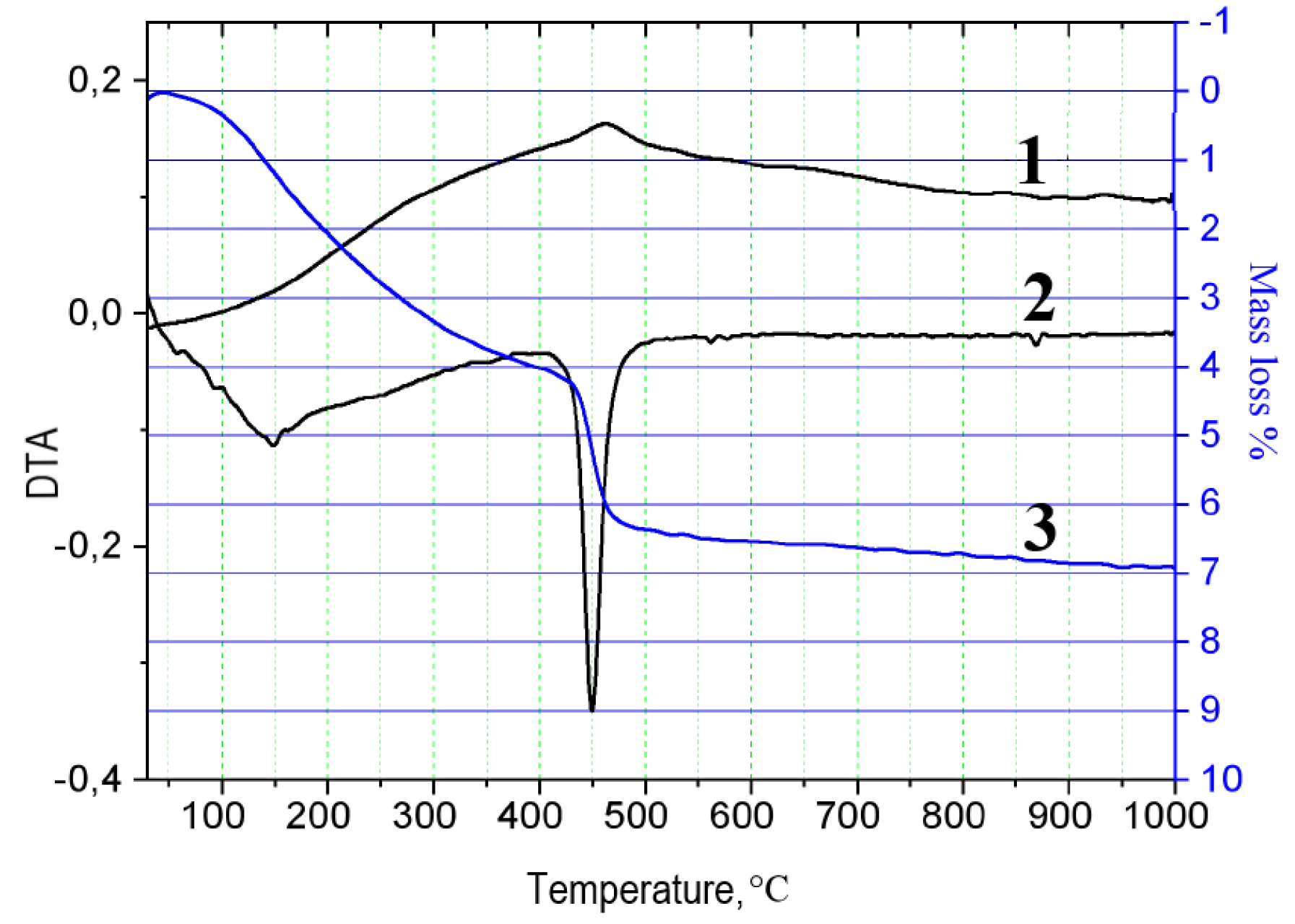
| Sample No. | Composition | Weight, g | ||
|---|---|---|---|---|
| Li2CO3 | Nb2O5 | Ta2O5 | ||
| S01 (x = 0) | LiNbO3 | 2499 | 8989 | – |
| S02 (x = 0.25) | LiNb0.75Ta0.25O3 | 2175 | 5868 | 3252 |
| S03 (x = 0.5) | LiNb0.5Ta0.5O3 | 1926 | 3463 | 5758 |
| S04 (x = 0.75) | LiNb0.25Ta0.75O3 | 1727 | 1554 | 7748 |
| S05 (x = 1) | LiTaO3 | 1566 | – | 9367 |
| Sample | x | T, °C | Parasitic Phases | a, Å | c, Å | Dave, nm | <ε>, % |
|---|---|---|---|---|---|---|---|
| S01 | 0 | 550 | – | 5.1483(3) | 13.8484(9) | 41 | 0.088 |
| 800 | – | 5.1517(2) | 13.8335(7) | 206 | 0.108 | ||
| S02 | 0.25 | 550 | Li(Nb,Ta)3O8 | 5.1477(3) | 13.822(1) | 50 | 0.143 |
| 800 | Li(Nb,Ta)3O8 | 5.1521(3) | 13.8148(8) | 171 | 0.105 | ||
| S03 | 0.5 | 550 | Ta2O5 + LiNb3O8 | 5.1534(4) | 13.808(1) | 63 | 0.107 |
| 800 | Li(Nb,Ta)3O8 | 5.153(1) | 13.778(3) | 97 | 0.128 | ||
| S04 | 0.75 | 550 | Ta2O5 | 5.149(2) | 13.788(5) | 31 | 0.093 |
| 800 | Li(Nb,Ta)3O8 | 5.1558(7) | 13.753(2) | 92 | 0.139 | ||
| S05 | 1 | 550 | Ta2O5 | 5.1529(6) | 13.767(2) | 66 | 0.114 |
| 800 | Li(Nb,Ta)3O8 | 5.1593(4) | 13.745(2) | 80 | 0.135 |
| x = 0 | x = 0.25 | x = 0.5 | x = 0.75 | x = 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Raman Shift, cm−1 | Intensity, a. u. | Raman Shift, cm−1 | Intensity, a. u. | Raman Shift, cm−1 | Intensity, a. u. | Raman Shift, cm−1 | Intensity, a. u. | Raman Shift, cm−1 | Intensity, a. u. |
| 117.3 | 556 | 63.5 | 151 | 75.5 | 3779 | 73.8 | 2037 | 73.1 | 2275 |
| 153.8 | 5873 | 73.2 | 391 | 95.8 | 3327 | 90.2 | 2046 | 97 | 2194 |
| 180 | 1089 | 80.8 | 1117 | 117.1 | 8657 | 146.9 | 6845 | 143.2 | 6681 |
| 238.8 | 10,002 | 97.5 | 2255 | 124.2 | 8534 | 168.4 | 4115 | 162 | 4985 |
| 262.6 | 4068 | 117.7 | 715 | 131 | 7601 | 215.3 | 10,093 | 209.1 | 10,133 |
| 276.7 | 4481 | 138 | 2445 | 150.2 | 7676 | 240.2 | 5079 | 232.5 | 4917 |
| 303 | 1582 | 153.8 | 7500 | 224.3 | 10,117 | 252.7 | 4891 | 250.5 | 4384 |
| 321 | 2491 | 168.8 | 4176 | 258.3 | 8375 | 319 | 2778 | 280.8 | 2294 |
| 333 | 2220 | 208.6 | 1390 | 312.4 | 3266 | 351.1 | 2550 | 316 | 2327 |
| 369 | 1904 | 235.9 | 9978 | 342.3 | 2388 | 380.5 | 3293 | 356.2 | 2579 |
| 432.8 | 1550 | 259 | 5508 | 375.7 | 2129 | 458.3 | 1570 | 381.5 | 3387 |
| 584.6 | 3074 | 272.7 | 5113 | 453.1 | 997 | 600.1 | 6663 | 465.1 | 1618 |
| 621.3 | 3557 | 298.6 | 2242 | 608.4 | 8264 | 660.7 | 2598 | 597 | 7575 |
| 700.8 | 464 | 320.8 | 2914 | 629.7 | 7113 | 868.2 | 1535 | 637 | 2886 |
| 875 | 1681 | 336.4 | 2391 | 664.1 | 4978 | 1009.7 | 2344 | 660.2 | 2741 |
| 371.7 | 2150 | 849.6 | 1035 | 865.8 | 1122 | ||||
| 436 | 1339 | 872.9 | 1571 | 1009.2 | 4684 | ||||
| 547.3 | 945 | 906 | 954 | ||||||
| 597.7 | 5018 | 994.1 | 2675 | ||||||
| 615.5 | 5608 | 1008.1 | 1536 | ||||||
| 676 | 1572 | ||||||||
| 700 | 1300 | ||||||||
| 821.8 | 808 | ||||||||
| 872 | 1841 | ||||||||
| 1009.7 | 341 | ||||||||
| Composition | 300 ÷ 620 °C | 670 ÷ 820 °C | ||
|---|---|---|---|---|
| EA, eV | σ0, ·106 S/m | EA, eV | σ0, ·106 S/m | |
| LiNbO3 | 0.88 ± 0.031 | 0.17 | 1.05 ± 0.071 | 1.54 |
| LiNb0.75Ta0.25O3 | 0.88 ± 0.011 | 0.32 | 1.01 ± 0.073 | 1.59 |
| LiNb0.5Ta0.5O3 | 0.88 ± 0.015 | 0.58 | 1 ± 0.07 | 2.61 |
| LiNb0.25Ta0.75O3 | 0.91 ± 0.015 | 0.83 | 0.99 ± 0.069 | 2.58 |
| LiTaO3 | 0.86 ± 0.015 | 0.48 | 1.09 ± 0.072 | 8.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasylechko, L.; Sydorchuk, V.; Lakhnik, A.; Suhak, Y.; Wlodarczyk, D.; Hurskyy, S.; Yakhnevych, U.; Zhydachevskyy, Y.; Sugak, D.; Syvorotka, I.I.; et al. Investigations of LiNb1−xTaxO3 Nanopowders Obtained with Mechanochemical Method. Crystals 2021, 11, 755. https://doi.org/10.3390/cryst11070755
Vasylechko L, Sydorchuk V, Lakhnik A, Suhak Y, Wlodarczyk D, Hurskyy S, Yakhnevych U, Zhydachevskyy Y, Sugak D, Syvorotka II, et al. Investigations of LiNb1−xTaxO3 Nanopowders Obtained with Mechanochemical Method. Crystals. 2021; 11(7):755. https://doi.org/10.3390/cryst11070755
Chicago/Turabian StyleVasylechko, Leonid, Volodymyr Sydorchuk, Andrey Lakhnik, Yuriy Suhak, Damian Wlodarczyk, Stepan Hurskyy, Uliana Yakhnevych, Yaroslav Zhydachevskyy, Dmytro Sugak, Ihor I. Syvorotka, and et al. 2021. "Investigations of LiNb1−xTaxO3 Nanopowders Obtained with Mechanochemical Method" Crystals 11, no. 7: 755. https://doi.org/10.3390/cryst11070755
APA StyleVasylechko, L., Sydorchuk, V., Lakhnik, A., Suhak, Y., Wlodarczyk, D., Hurskyy, S., Yakhnevych, U., Zhydachevskyy, Y., Sugak, D., Syvorotka, I. I., Solskii, I., Buryy, O., Suchocki, A., & Fritze, H. (2021). Investigations of LiNb1−xTaxO3 Nanopowders Obtained with Mechanochemical Method. Crystals, 11(7), 755. https://doi.org/10.3390/cryst11070755






