Bentonites in Southern Spain. Characterization and Applications
Abstract
1. Introduction
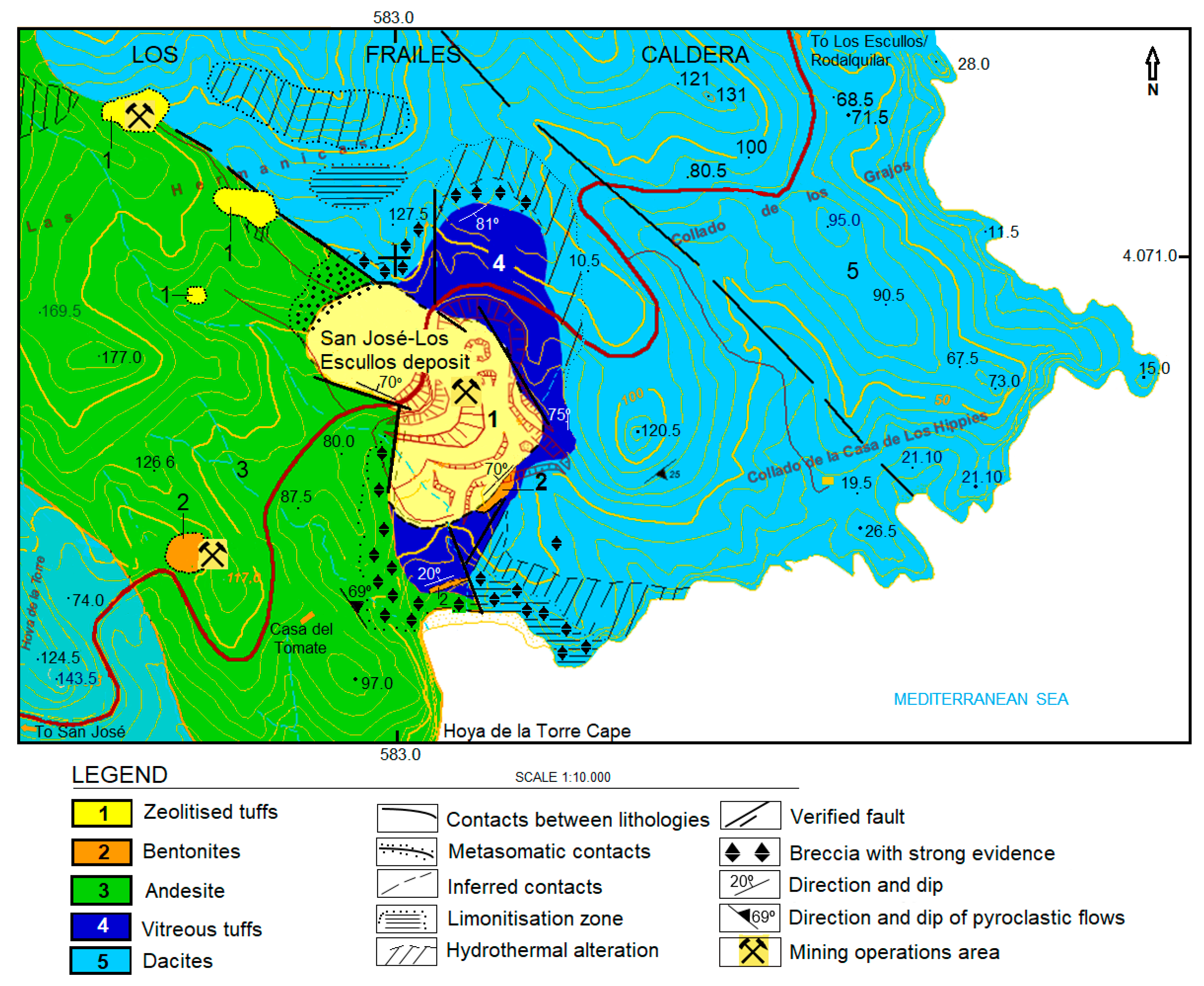
2. Geological Settings
3. Materials and Methods
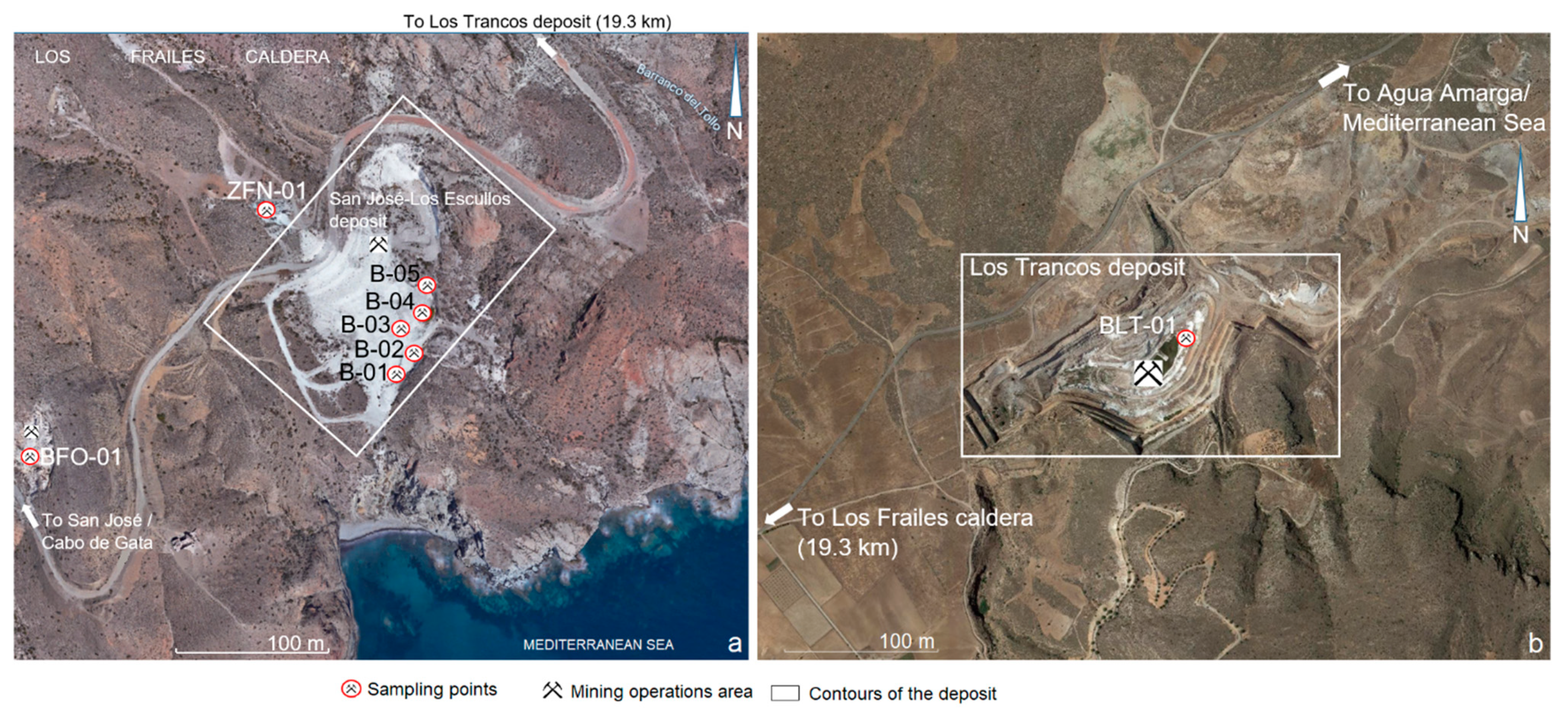
3.1. Materials
3.2. Methods
3.2.1. Petrography Characterisation by Thin Section (PTS)
3.2.2. X-ray Diffraction (XRD)
3.2.3. Oriented Aggregates (OA)
3.2.4. Thermogravimetric Analysis (TGA)
3.2.5. X-ray Fluorescence (XRF)
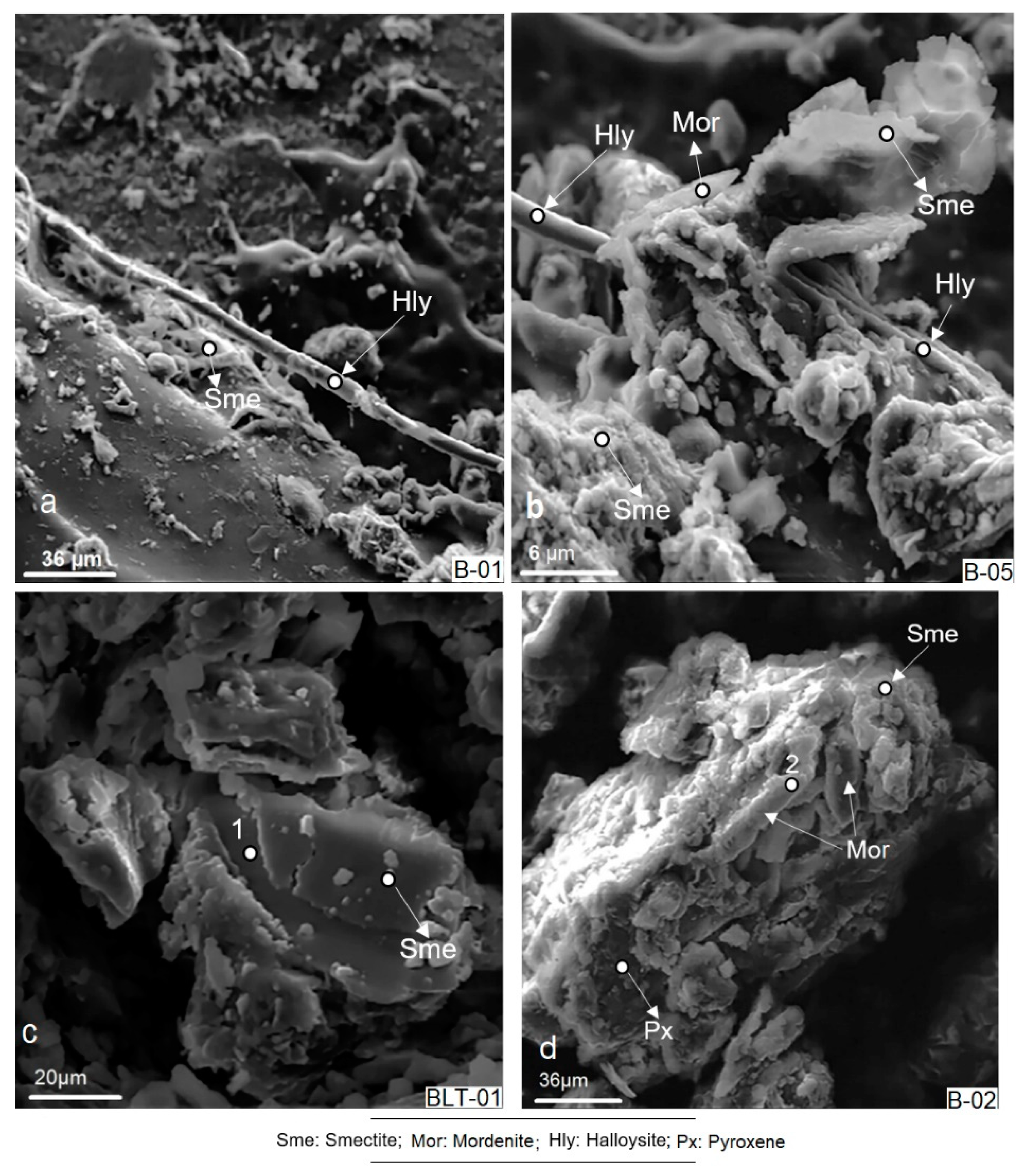
3.2.6. Scanning Electron Microscopy (SEM)
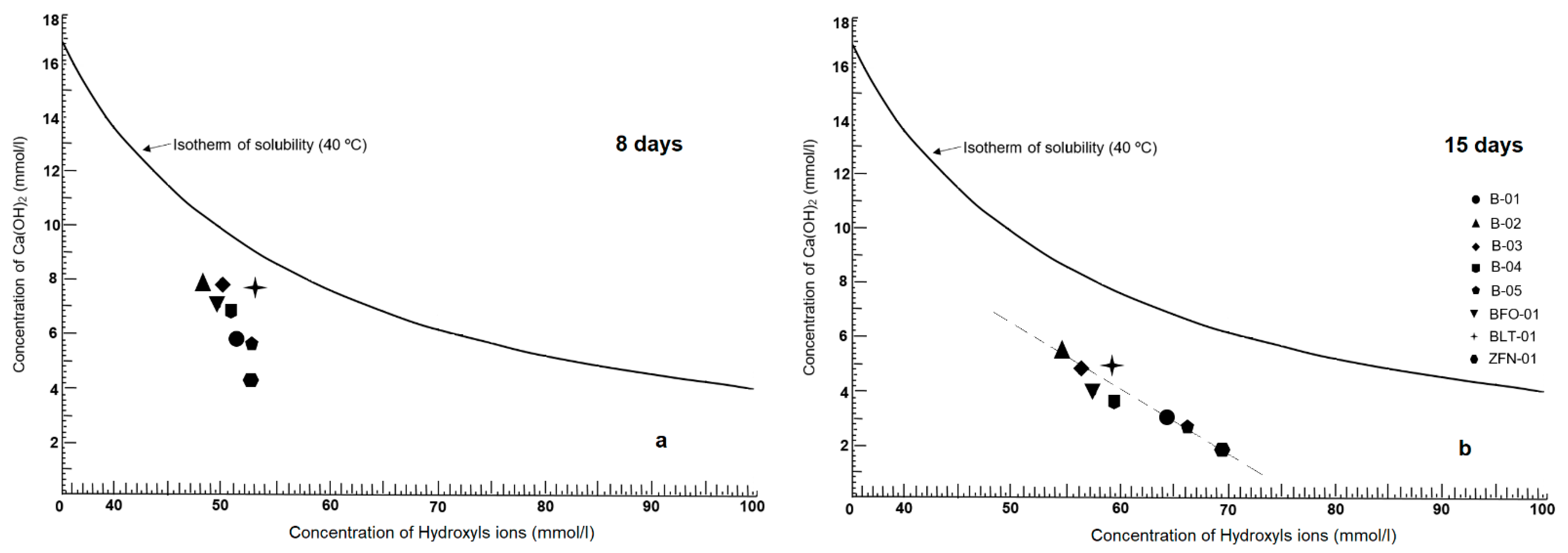
3.2.7. Chemical Pozzolanicity Analysis (CPA)
- [OH−]: is the concentration in hydroxyl ions (mmol/L).
- V3: is the volume of the hydrochloric acid solution (0.1 mol/L).
- f2: is the factor of the hydrochloric acid solution (0.1 mol/L).
- [CaO]: is the concentration in calcium oxide (mmol/L).
- V4: is the volume of EDTA solution used in the titration.
- f1: is the factor of the EDTA solution.
4. Results and Discussion
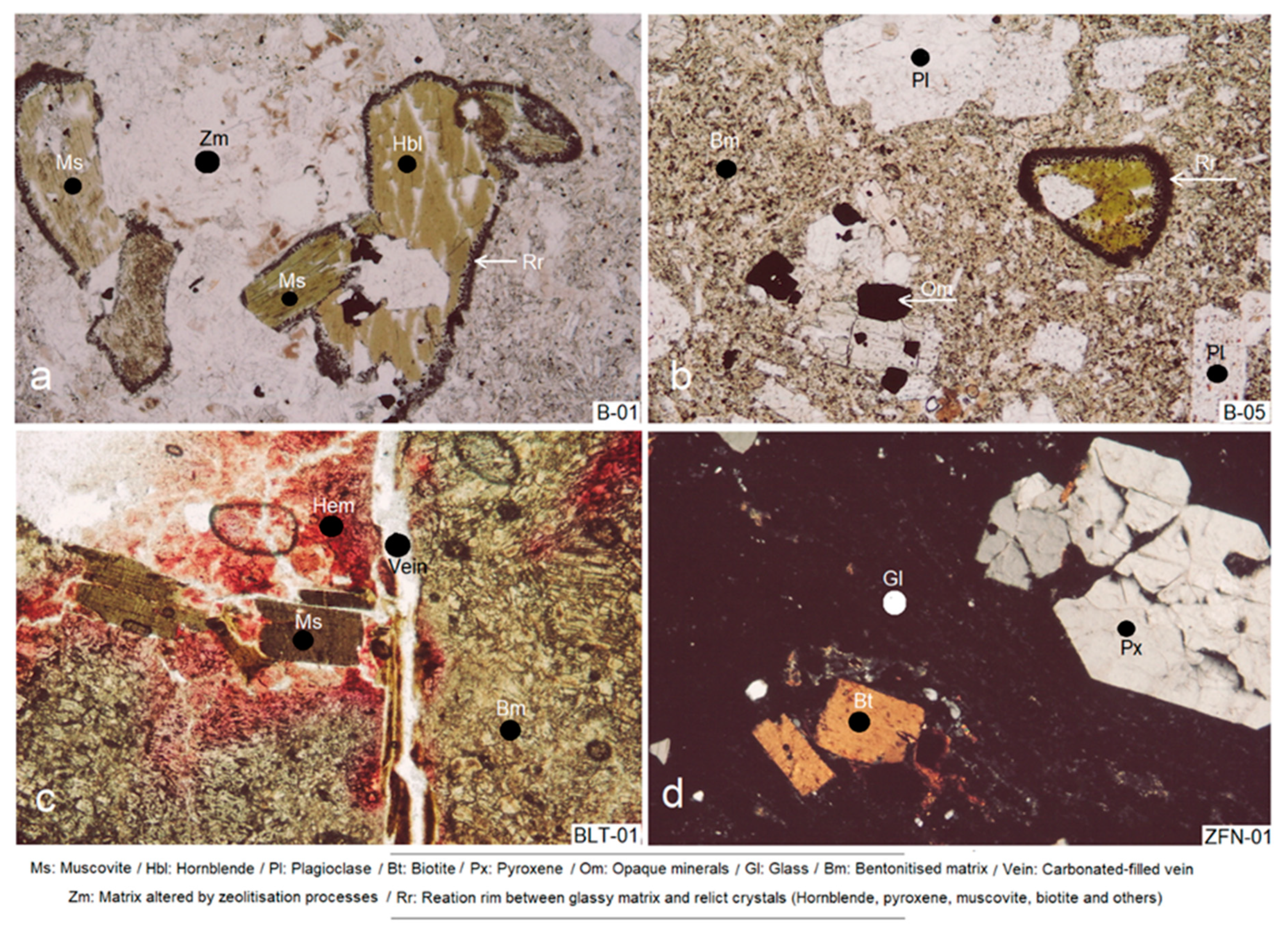
4.1. Petrographic Study by Thin Section
4.2. X-ray Diffraction
4.3. Oriented Aggregates
4.4. Thermogravimetric Analysis
4.5. X-ray Fluorescence
4.6. Scanning Electron Microscopy
4.7. Pozzolanicity Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cara, S.; Carcangiu, G.; Padalino, G.; Palomba, M.; Tamanini, M. The bentonites in pelotherapy: Thermal properties of clay pastes from Sardinia, Italy. Appl. Clay Sci. 2000, 16, 125–132. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Handbook of Clay Science. Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Buchwald, A.; Hohmann, M.; Posern, K.; Brendler, E. The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl. Clay Sci. 2009, 46, 300–304. [Google Scholar] [CrossRef]
- Habert, G.; Choupay, N.; Escadeillas, G.; Guillaume, D.; Montel, J.M. Clay content of argillites: Influence on cement-based mortars. Appl. Clay Sci. 2009, 43, 322–330. [Google Scholar] [CrossRef]
- Kaci, A.; Chaouche, M.; Andréani, P.-A. Influence of bentonite clay on the rheological behaviour of fresh mortars. Cem. Concr. Res. 2011, 41, 373–379. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Kleinübing, S.J.; Carlos da Silva, G.M. Preparation and characterization of a Brazilian bentonite clay for removal of copper in porous beds. Appl. Clay Sci. 2011, 53, 73–79. [Google Scholar] [CrossRef]
- Slamova, R.; Trckova, M.; Vondruskova, H.; Zraly, Z.; Pavlik, I. Clay minerals in animal nutrition. Appl. Clay Sci. 2011, 51, 395–398. [Google Scholar] [CrossRef]
- Pelayo, M. Estudio del Yacimiento de Bentonita de Morrón de Mateo (Cabo de Gata, Almería) como Análogo Natural del Comportamiento de la Barrera de Arcilla de un Almacenamiento de Residuos Radiactivos. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2013; 311p. [Google Scholar]
- Hassan, A.Z.A.; Abdel, W.M.M. The combined effect of bentonite and natural zeolite on sandy soil properties and productivity of some crops. Topclass J. Agric. Res. 2013, 1, 22–28. [Google Scholar]
- Zhou, C.H.; Zhao, L.Z.; Wang, A.Q.; Chen, T.H.; He, H.P. Current fundamental and applied research into clay minerals in China. Appl. Clay Sci. 2016, 119, 3–7. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ramontja, J. Natural bentonite clay and its composites for Dye removal: Current state and future potential. Am. J. Chem. Appl. 2016, 3, 8–19. [Google Scholar]
- Luqman, A.; Enobong, R. Bioactivity of quaternary glass prepared from bentonite clay. J. Adv. Ceram. 2016, 5, 47–53. [Google Scholar] [CrossRef][Green Version]
- Aravindhraj, M.; Sapna, B.T. Influence of Bentonite in Strength and Durability of High Performance Concrete. Int. Res. J. Eng. Technol. 2016, 3, 5. [Google Scholar]
- Masindi, V. Application of cryptocrystalline magnesite-bentonite Clay hybrid for defluoridation of underground water resources: Implication for point of use treatment. J. Water Reuse Desalination 2017, 7, 3. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.R.; Yoon, S.; Jeon, J.S.; Kim, M.S. Optimal initial condition of a bentonite buffer with regard to thermal behavior in a high-level radioactive waste repository. Comput. Geotech. 2018, 104, 109–117. [Google Scholar] [CrossRef]
- Masood, B.; Elahi, A.; Barbhuiya, S.; Ali, B. Mechanical and durability performance of recycled aggregate concrete incorporating low calcium bentonite. Constr. Build. Mater. 2020, 237, 117760. [Google Scholar] [CrossRef]
- Wu, H.L.; Jin, F.; Zhou, A.; Du, Y.J. The engineering properties and reaction mechanism of MgO-activated slag cement-clayey sand-bentonite (MSB) cut-off wall backfills. Constr. Build. Mater. 2021, 271, 121890. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Tan, Y.; Zhu, J. Sealing performance of compacted block joints backfilled with bentonite paste or a particle-powder mixture. Soils Found. 2021, 61, 496–505. [Google Scholar] [CrossRef]
- Cunningham, C.; Arribas, A., Jr.; Rytuba, J.; Arribas, A. Mineralized and unmineralized calderas in Spain. Part I: Evolution of the Los Frailes Caldera. Mineral. Depos. 1990, 25, 21–28. [Google Scholar] [CrossRef]
- Arribas, A. Las Mineralizaciones de Metales Preciosos de la Zona Central del Cabo de Gata (Almería) en el Contexto Metalogénico del Sureste de España. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 1992; pp. 109–148, 186–237. [Google Scholar]
- Rytuba, J.; Arribas, A., Jr.; Cunningham, C.; McKee, E.; Podwysocki, M.; Smith, J.; Kelly, W.; Arribas, A. Mineralized and unmineralized calderas in Spain; Part II. Evolution of the Rodalquilar caldera complex and associates gold-alunite deposits. Mineral. Deposita 1990, 25, 529–535. [Google Scholar] [CrossRef]
- Fernández-Soler, J.M. El Volcanismo Calco-Alcalino de Cabo de Gata (Almería). Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1992; 243p. [Google Scholar]
- Costafreda, J.L. Geología, Caracterización y Aplicaciones de las Rocas Zeolíticas del Complejo Volcánico de Cabo de Gata (Almería). Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2008; 515p. [Google Scholar]
- Reyes, E. Mineralogía y Geoquímica de las Bentonitas de la Zona Norte de Cabo de Gata (Almería). Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1977; 650p. [Google Scholar]
- Leone, G.; Reyes, E.; Cortecci, G.; Pochini, A.; Linares, J. Genesis of bentonitas from Cabo de Gata, Almería, Spain: A stable isotope study. Clay Miner. 1983, 18, 227–238. [Google Scholar] [CrossRef]
- Pelayo, M.; García-Romero, E.; Labajo, M.A.; Pérez del Villar, L. Occurrence of Fe-Mg-rich smectites and corrensite in the Morrón de Mateo bentonite deposit (Cabo de Gata region, Spain): A natural analogue of the bentonite barrier in a radwaste repository. Appl. Geochem. 2011, 26, 1153–1168. [Google Scholar] [CrossRef][Green Version]
- Google Earth. Available online: https://earth.google.com/web/@36.78396874,-2.07282152,311.26637664a,8201.3672037d,35y,0h,0t,0r (accessed on 17 May 2021).
- British Standard Institution. UNE-EN 196-5:2011. Methods of Testing Cement–Part 5: Pozzolanicity Test for Pozzolanic Cement; British Standard Institution: London, UK, 2011. [Google Scholar]
- Martín-Vivaldi, J.L. The Bentonites of Cabo de Gata (Southeast Spain) and of Guelaya Volcanic Province (North Morocco). Clays Clay Miner. 1962, 11, 327–357. [Google Scholar] [CrossRef]
- Linares, J. Chemical evolutions related to the genesis of hydrothermal smectites, Almería, SE Spain. In Geochemistry and Mineral Formation in the Earth Surface; Rodríguez-Clemente, R., Tardy, Y., Eds.; CSIC-CNRS: Madrid, Spain, 1987; pp. 567–584. [Google Scholar]
- Delgado, A.; Reyes, E. Isotopic study of the diagenetic and hydrothermal origins of the bentonite deposits at Los Escullos (Almería, Spain). In Current Research in Geology Applied to Ore Deposits; Fenoll, P., Torres-Ruiz, J., Gervilla, F., Eds.; Universidad de Granada: Granada, Spain, 1993; pp. 657–678. [Google Scholar]
- García-Romero, E.; Manchado, E.M.; Suárez, M.; García-Rivas, J. Spanish Bentonites: A review and new data on their geology, mineralogy, and crystal chemistry. Minerals 2019, 9, 696. [Google Scholar] [CrossRef]
- Martínez, J.A.; Caballero, E.; Jiménez, C.; Linares, J. The effect of a volcanic dome over the Cala del Tomate bentonite (Almería). Cadernos Lab. Xeolóxico Laxe 2000, 25, 67–69. [Google Scholar]
- Caballero, E. Quimismo del Proceso de Bentonitización en la Región Volcánica de Cabo de Gata (Almería). Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1985; 328p. [Google Scholar]
- Benito, R.; García-Guinea, J.; Valle-Fuentes, F.J.; Recio, P. Mineralogy, geochemistry and uses of mordenite-bentonite ash-tuff beds of Los Escullos, Almería, Spain. J. Geochem. Explor. 1998, 62, 229–240. [Google Scholar] [CrossRef]
- Huertas, F.J.; Carretero, P.; Delgado, J.; Linares, J.; Samper, J. An experimental study on the ion-exchange behaviour of the smectite of Cabo de Gata (Almería, Spain): FEBEX Bentonite. J. Colloid Interface Sci. 2001, 239, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Pérez del Villar, L.; Delgado, A.; Pelayo, M.; Fernández Soler, J.M.; Tsige, A.M.; Cózar, J.S.; Reyes, A. Natural Thermal Effects Induced on the Bentonite from de Cala del Tomate Deposit (Cabo de Gata, Almería); BARRA II Project, Termal Effect; CIEMAT/DIAE/54450/1/04 Report No. 82; Internal Report of CIEMAT: Madrid, Spain, 2004. [Google Scholar]

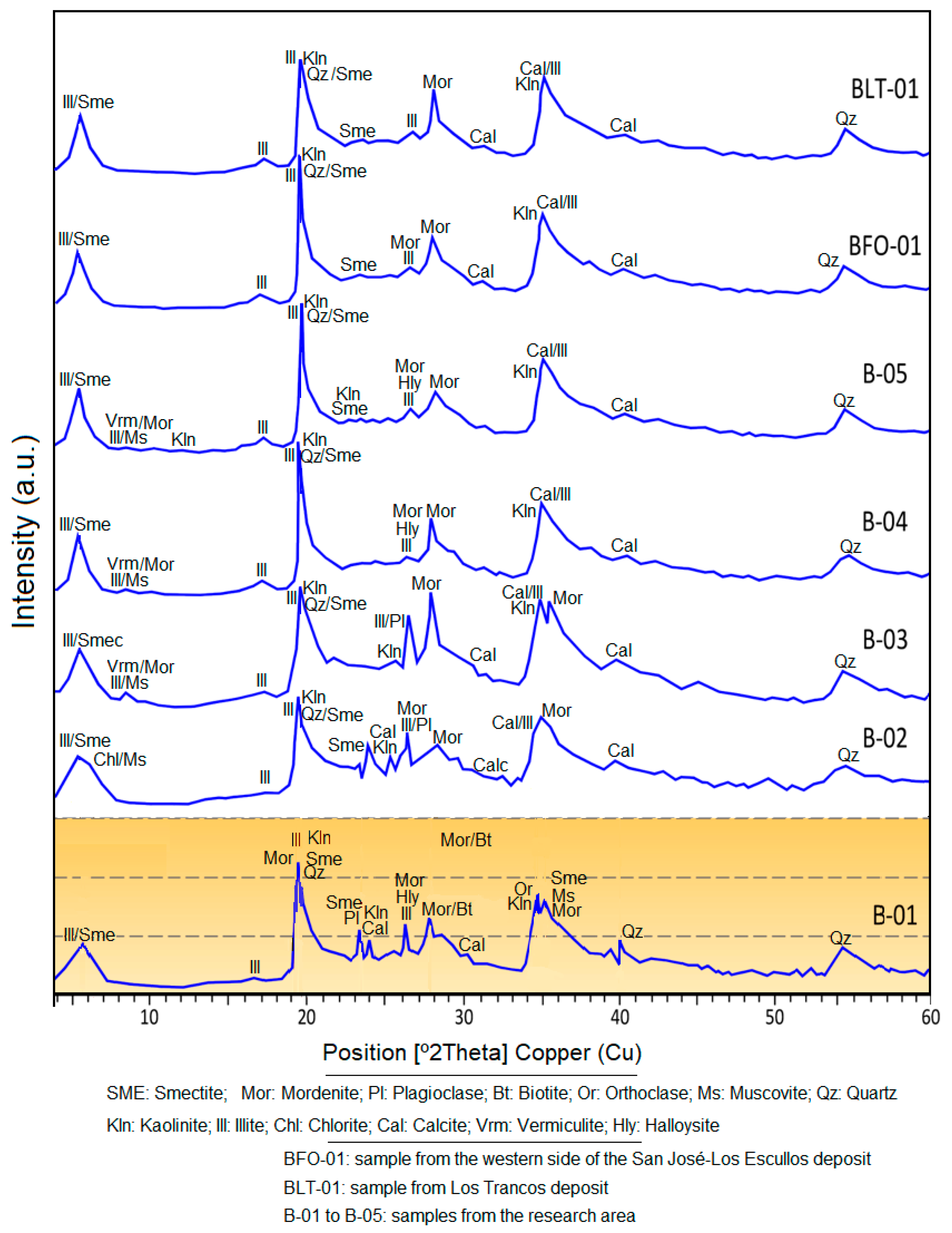
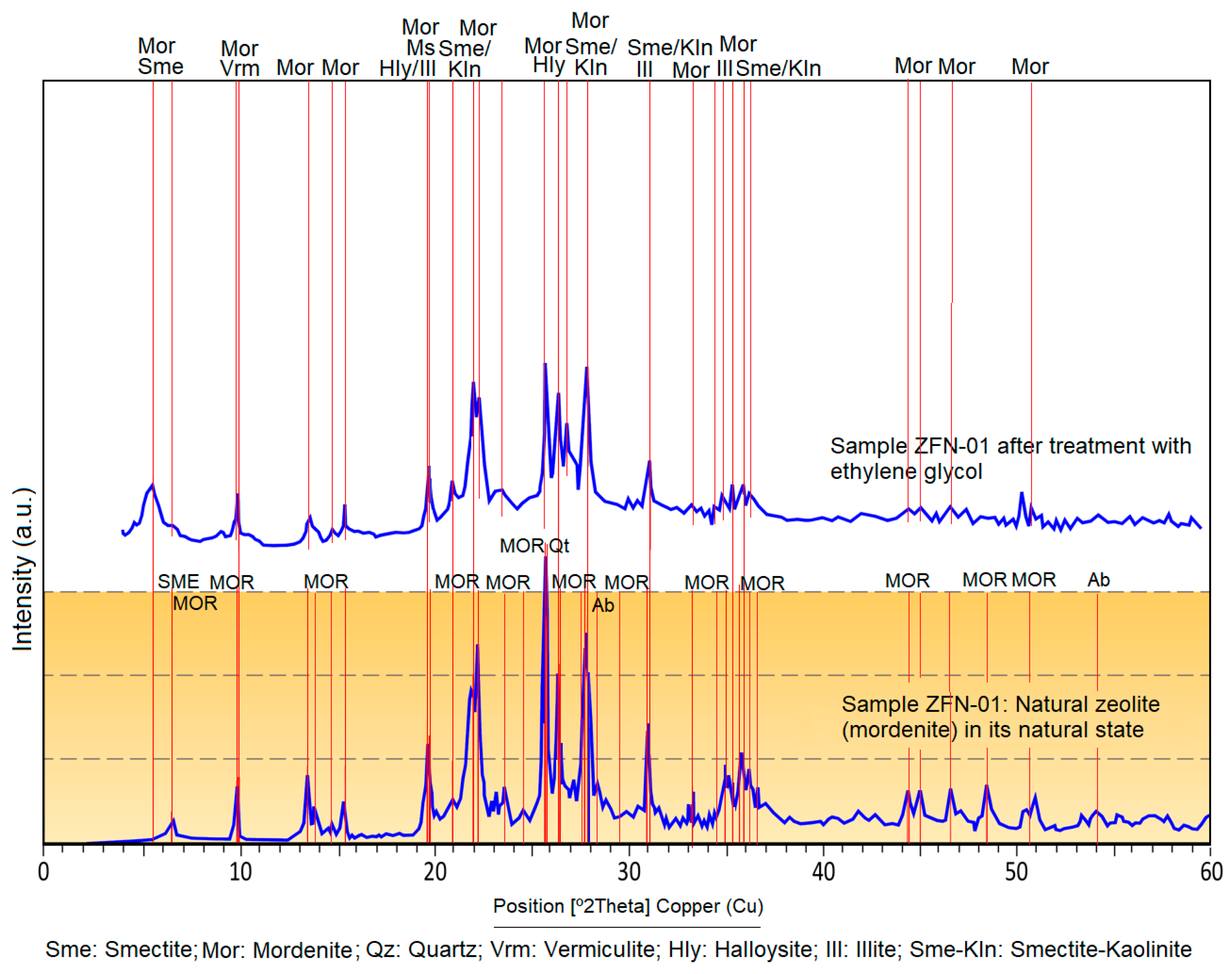
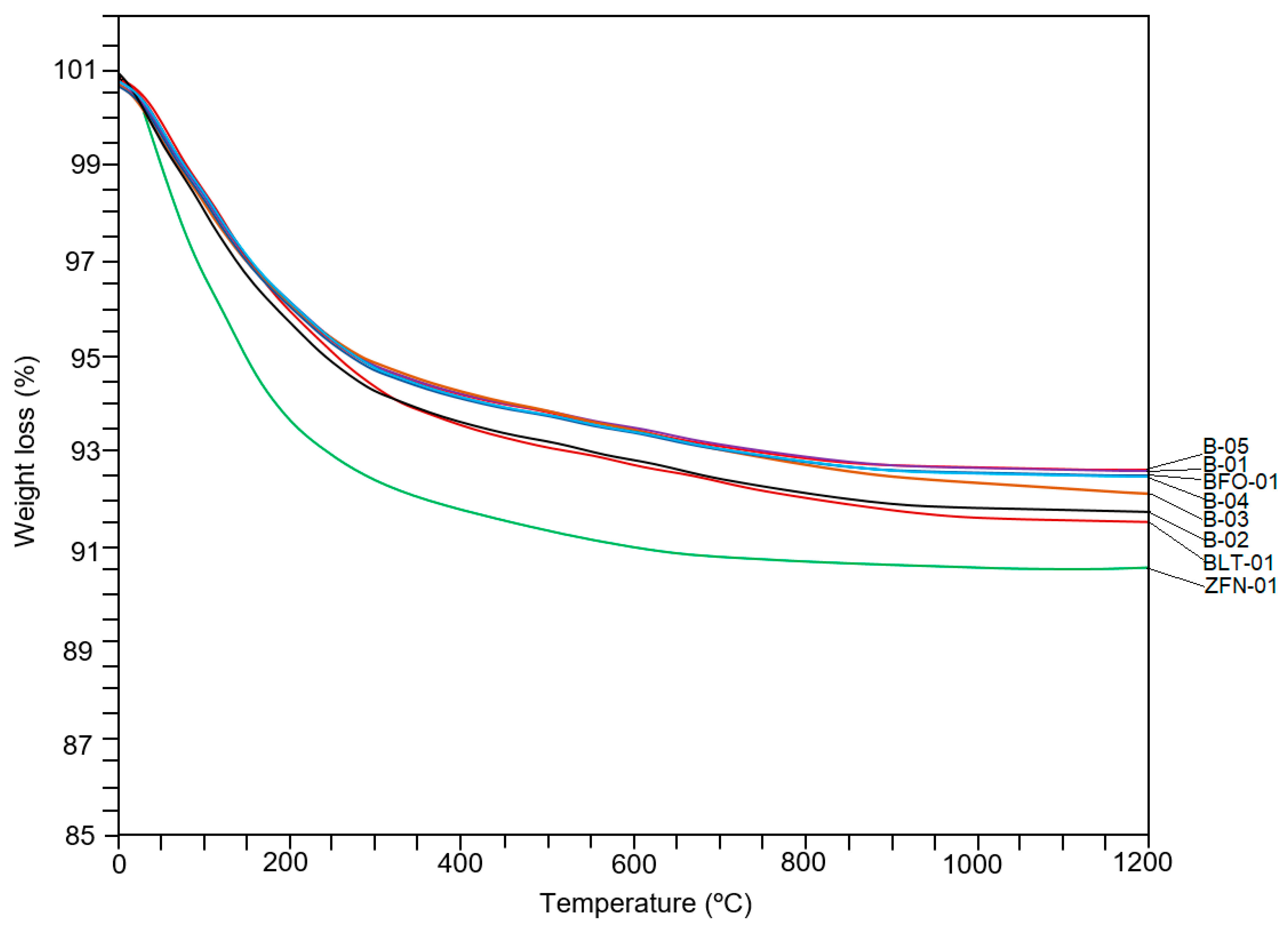
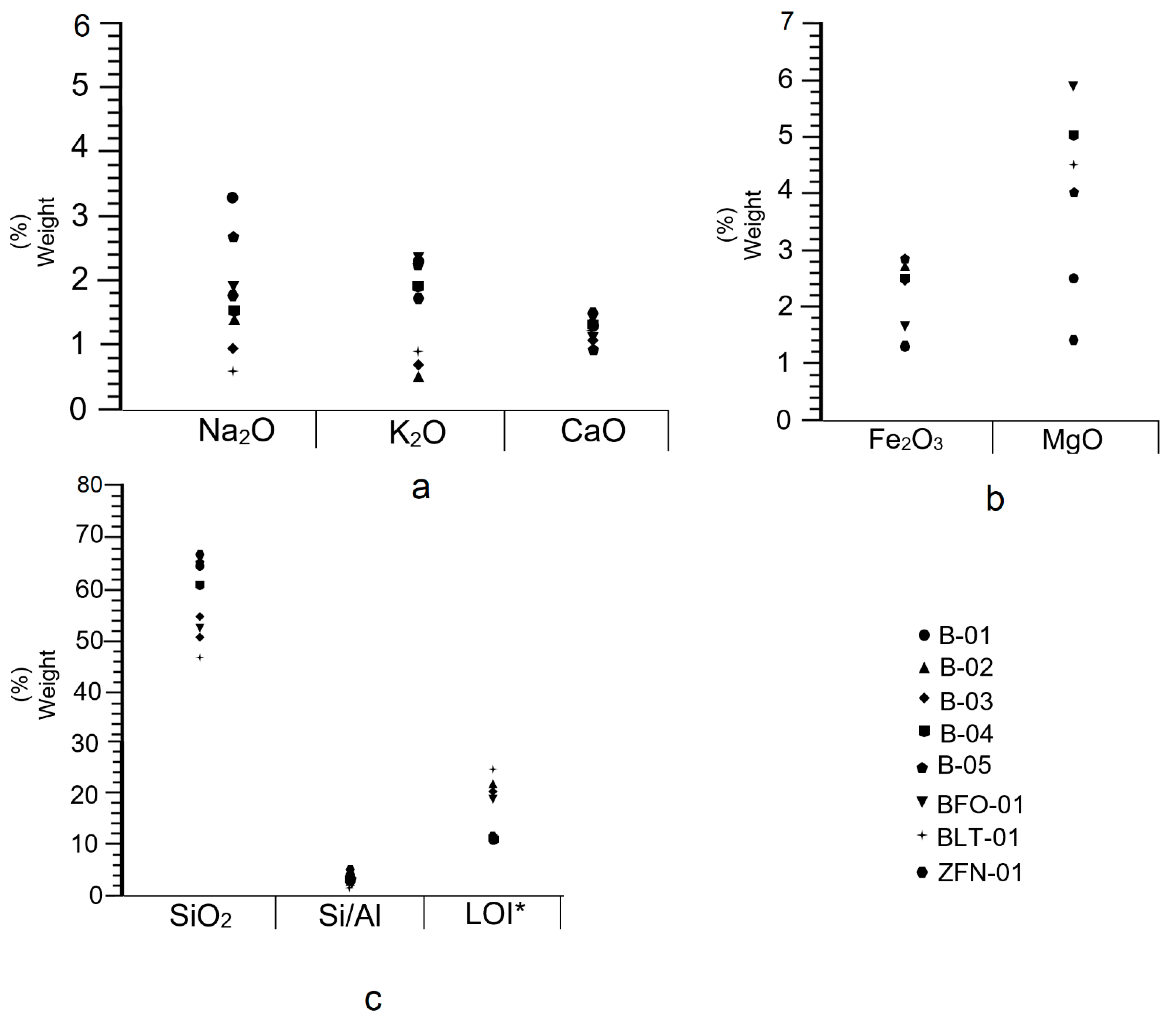
| Sample | SiO2 | Al2O3 | CaO | Na2O | K2O | MgO | Fe2O3 | TiO2 | LOI * | Na2O/CaO | MgO/Al2O3 | Si/Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-01 | 64.30 | 13.71 | 1.03 | 3.3 | 2.3 | 2.5 | 1.3 | 0.123 | 11.5 | 0.58 | 0.18 | 4.1 |
| B-02 | 51.29 | 13.13 | 1.20 | 1.40 | 0.53 | 7.61 | 2.75 | 0.122 | 22.0 | 1.17 | 0.58 | 3.4 |
| B-03 | 52.53 | 13.69 | 1.11 | 0.99 | 0.70 | 7.67 | 2.43 | 0.133 | 20.5 | 0.89 | 0.56 | 3.3 |
| B-04 | 61.3 | 14.76 | 1.24 | 1.56 | 1.92 | 5.0 | 2.46 | 0.116 | 11.5 | 1.26 | 0.34 | 3.6 |
| B-05 | 62.46 | 14.57 | 0.94 | 2.64 | 2.21 | 4.0 | 1.83 | 0.133 | 11.5 | 2.8 | 0.27 | 3.7 |
| BFO-01 | 52.13 | 17.05 | 1.12 | 1.96 | 0.35 | 5.91 | 1.61 | 0.123 | 19.2 | 1.75 | 0.35 | 2.7 |
| BLT-01 | 47.26 | 19.02 | 1.22 | 0.30 | 0.87 | 4.5 | 2.45 | 0.173 | 24.4 | 0.25 | 0.24 | 2.1 |
| ZFN-01 | 67.04 | 12.55 | 1.54 | 2.64 | 1.79 | 1.40 | 1.17 | 0.085 | 11.7 | 1.75 | 0.11 | 4.7 |
| Sample | SiO2 | Al2O3 | CaO | Na2O | K2O | MgO | Fe2O3 | TiO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| LTBB | 52.12 | 17.75 | 1.29 | 0.51 | 0.14 | 4.56 | 2.41 | 0.20 | 20.77 |
| LTBV | 51.25 | 17.01 | 1.32 | 0.51 | 0.43 | 4.78 | 2.31 | 0.17 | 20.61 |
| LTBN | 53.47 | 17.21 | 1.05 | 1.07 | 0.27 | 4.87 | 2.21 | 0.16 | 19.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costafreda, J.L.; Martín, D.A. Bentonites in Southern Spain. Characterization and Applications. Crystals 2021, 11, 706. https://doi.org/10.3390/cryst11060706
Costafreda JL, Martín DA. Bentonites in Southern Spain. Characterization and Applications. Crystals. 2021; 11(6):706. https://doi.org/10.3390/cryst11060706
Chicago/Turabian StyleCostafreda, Jorge Luis, and Domingo Alfonso Martín. 2021. "Bentonites in Southern Spain. Characterization and Applications" Crystals 11, no. 6: 706. https://doi.org/10.3390/cryst11060706
APA StyleCostafreda, J. L., & Martín, D. A. (2021). Bentonites in Southern Spain. Characterization and Applications. Crystals, 11(6), 706. https://doi.org/10.3390/cryst11060706








