Synthesis, Crystal Structure and Optical Properties of 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide for Anion Detection
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Apparatus
2.3. X-ray Crystallography
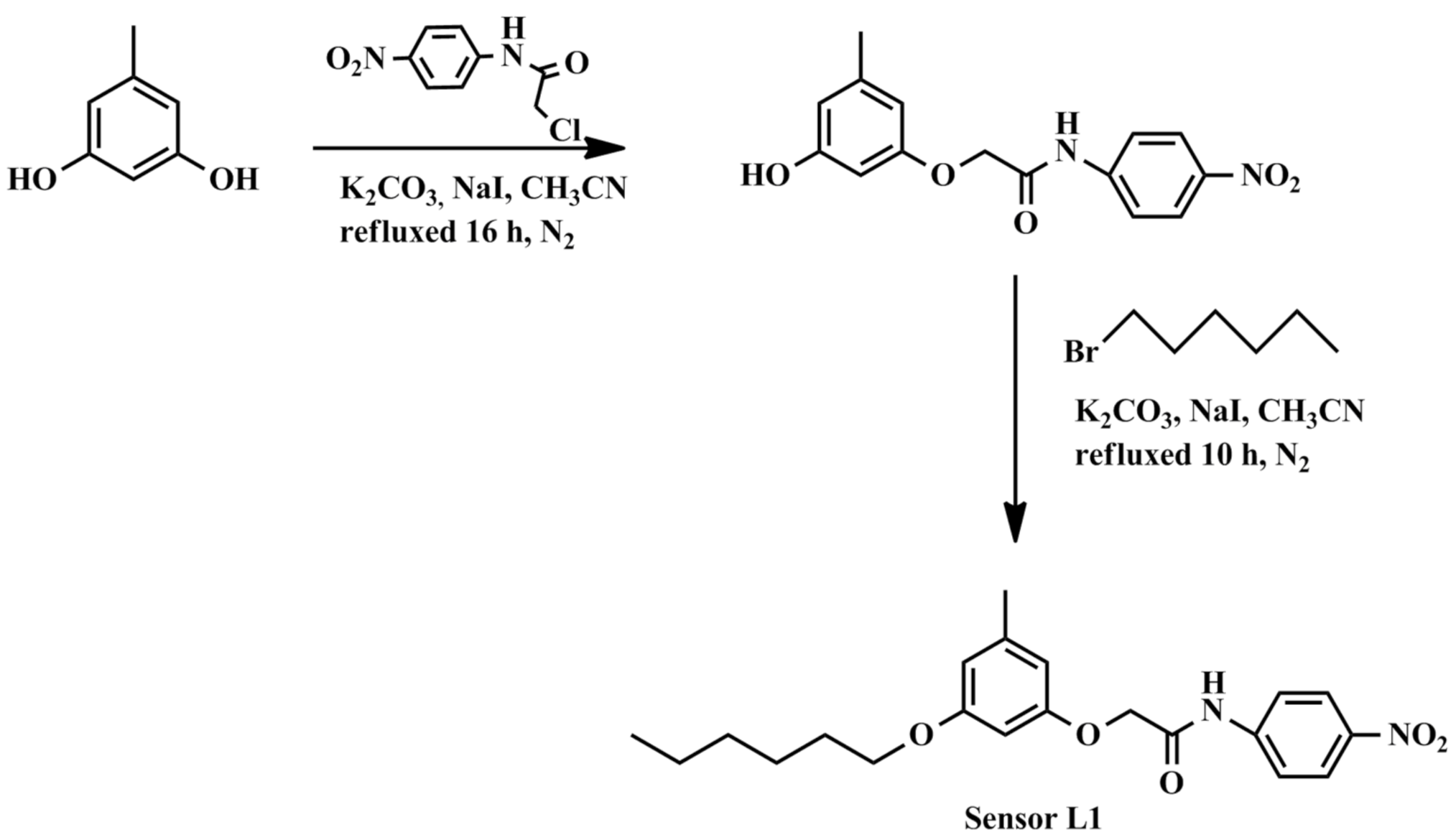
2.4. Synthesis of 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide, Sensor L1
2.4.1. 2-(3-Hydroxy-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide
2.4.2. 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide, (Sensor L1)
3. Results and Discussion
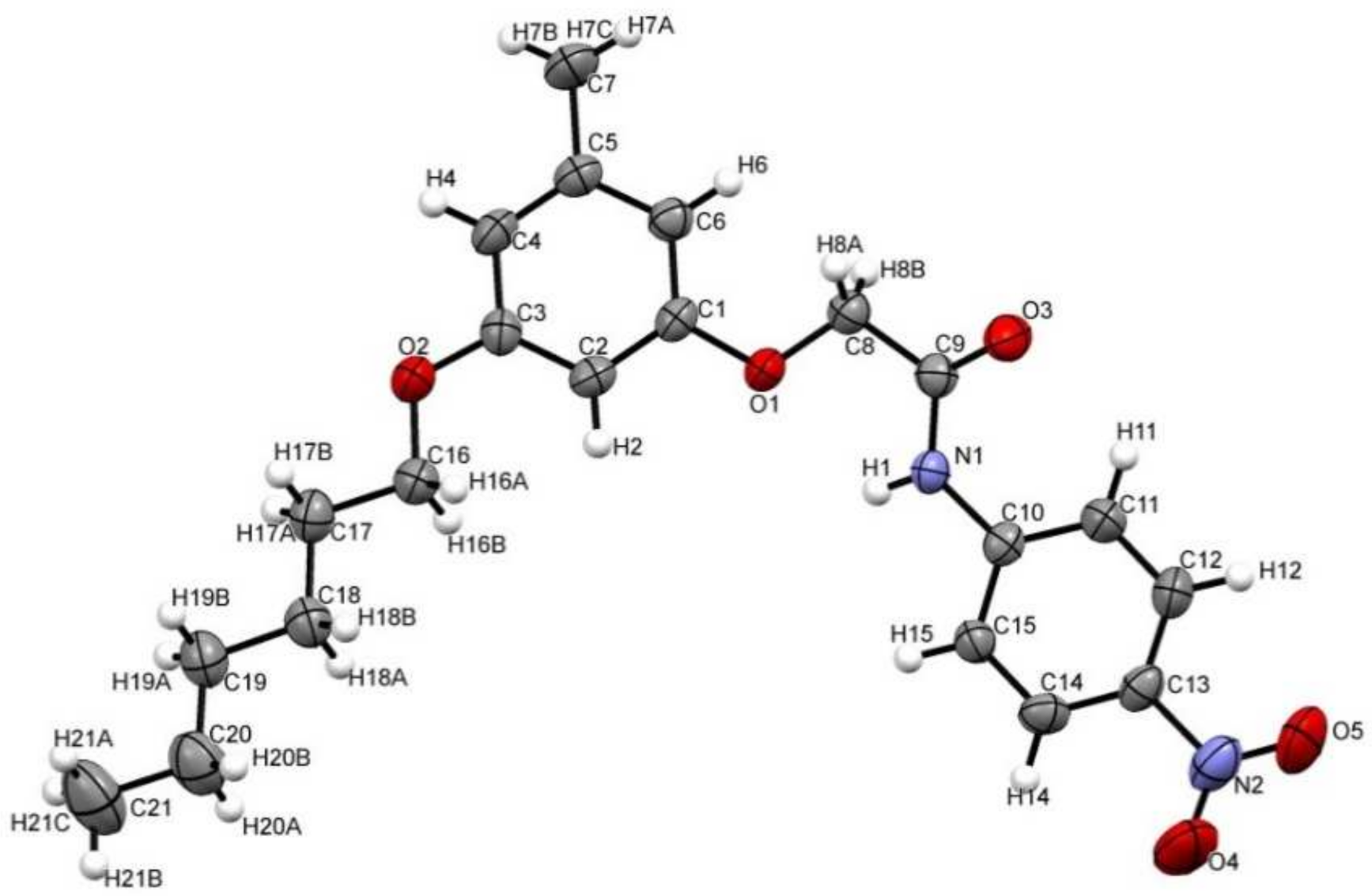
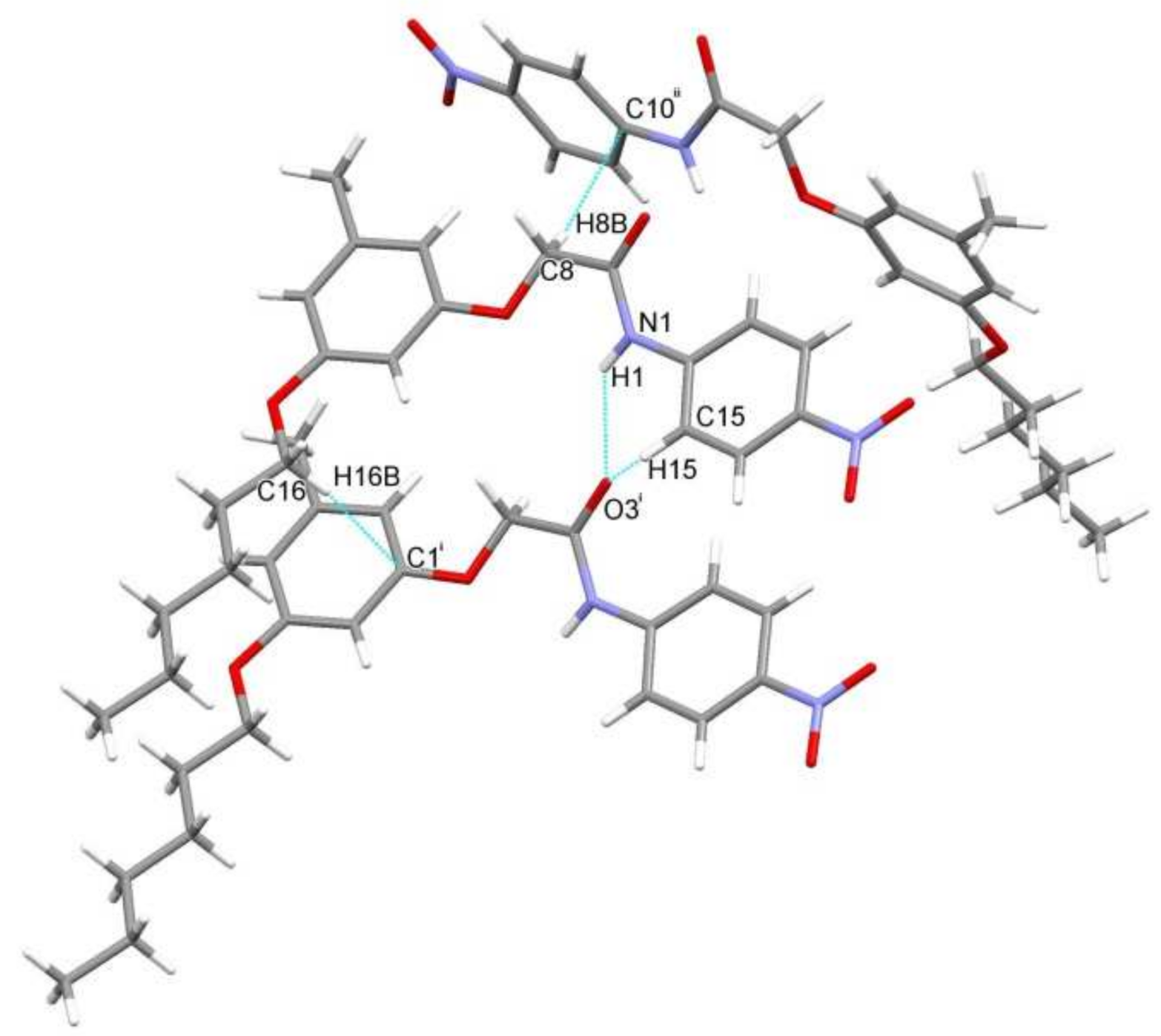
3.1. Crystal Structure and Structural Description
3.2. Interaction between Sensor L1 and Various Anions Using UV–Vis and 1H NMR Spectroscopy
3.2.1. Colorimetric Sensor L1
3.2.2. Interaction between Sensor L1 and Various Anions Using UV–Visible Spectroscopy
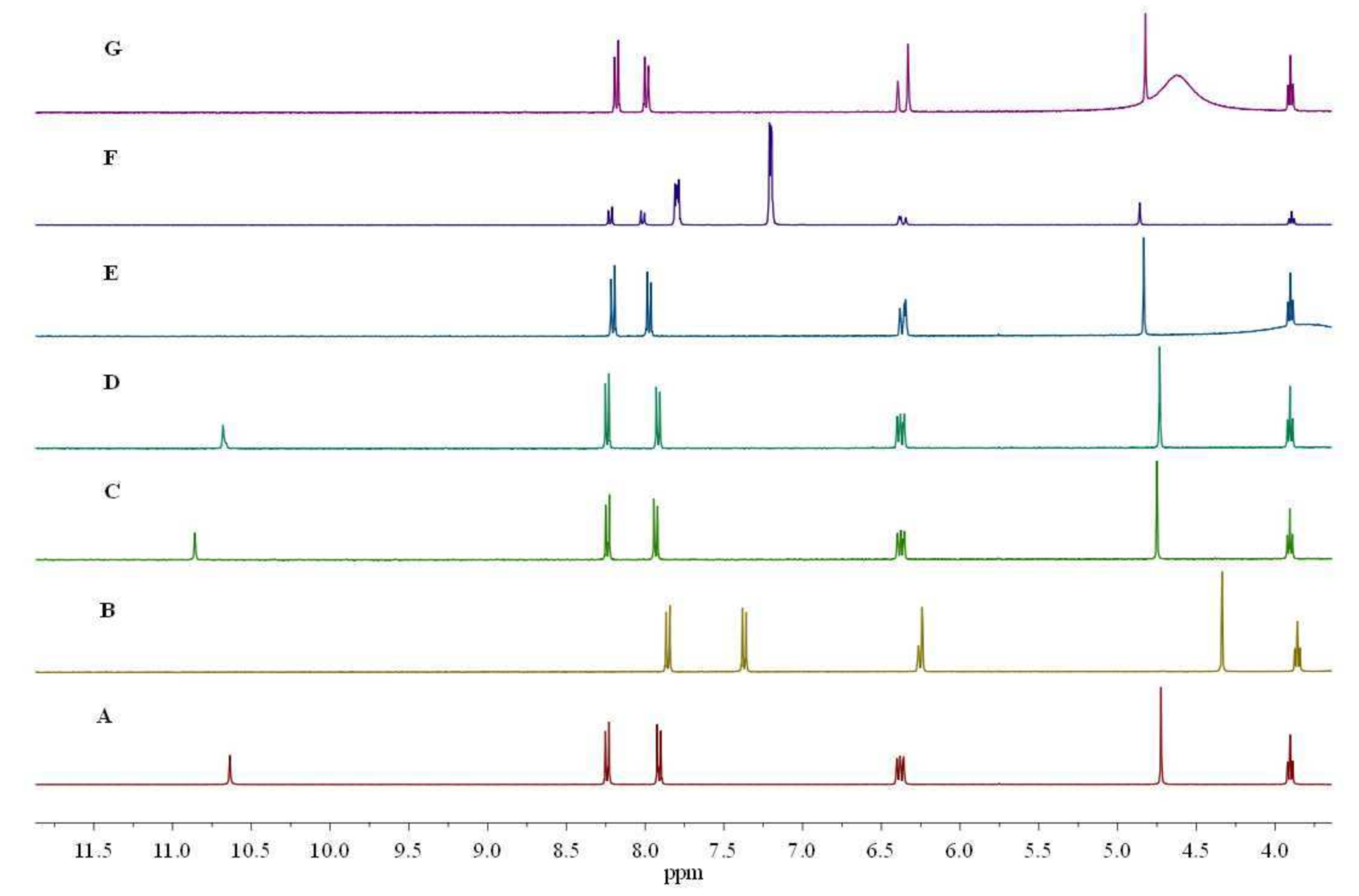
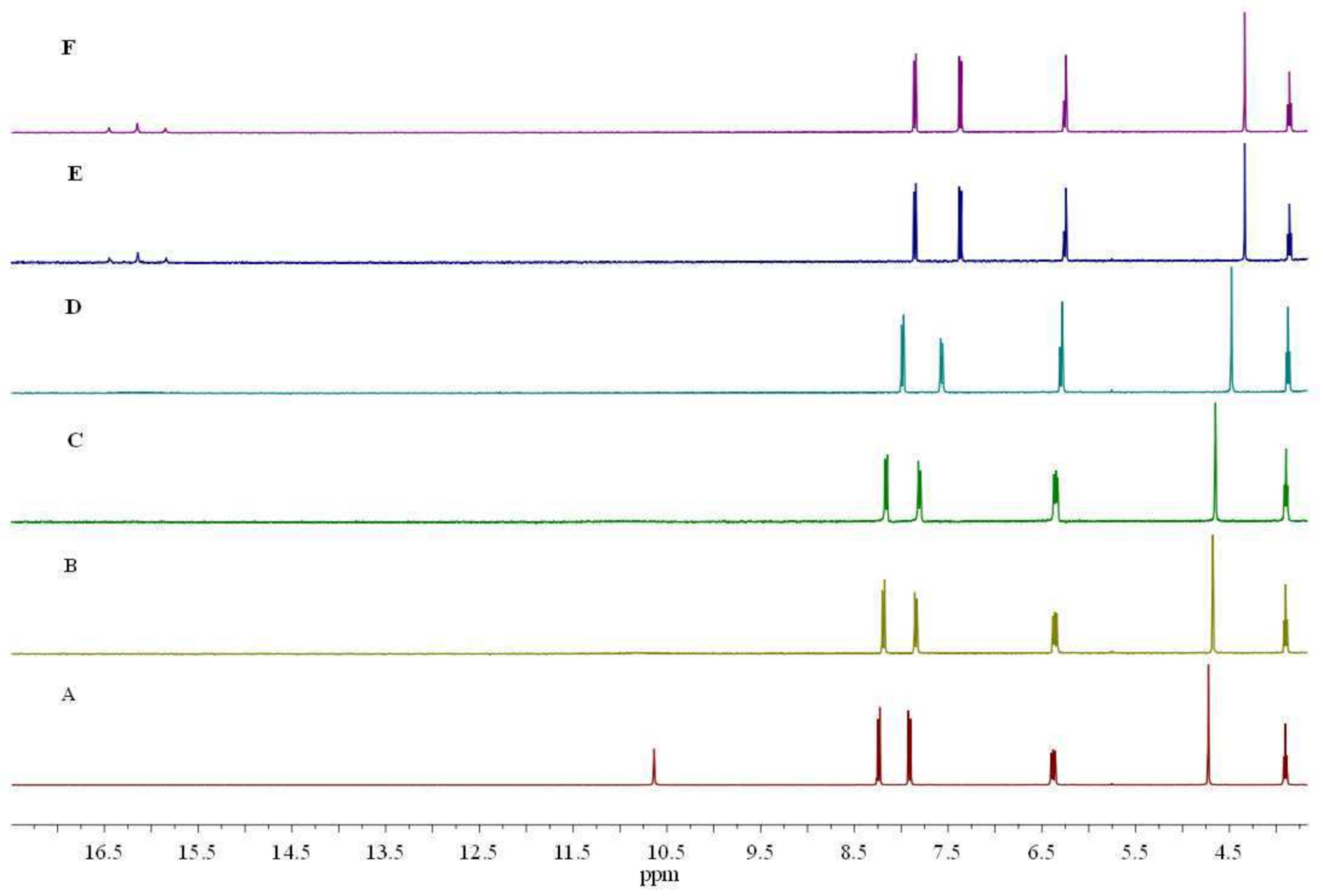
3.2.3. Interaction between Sensor L1 and Various Anions Using 1H NMR Spectroscopy
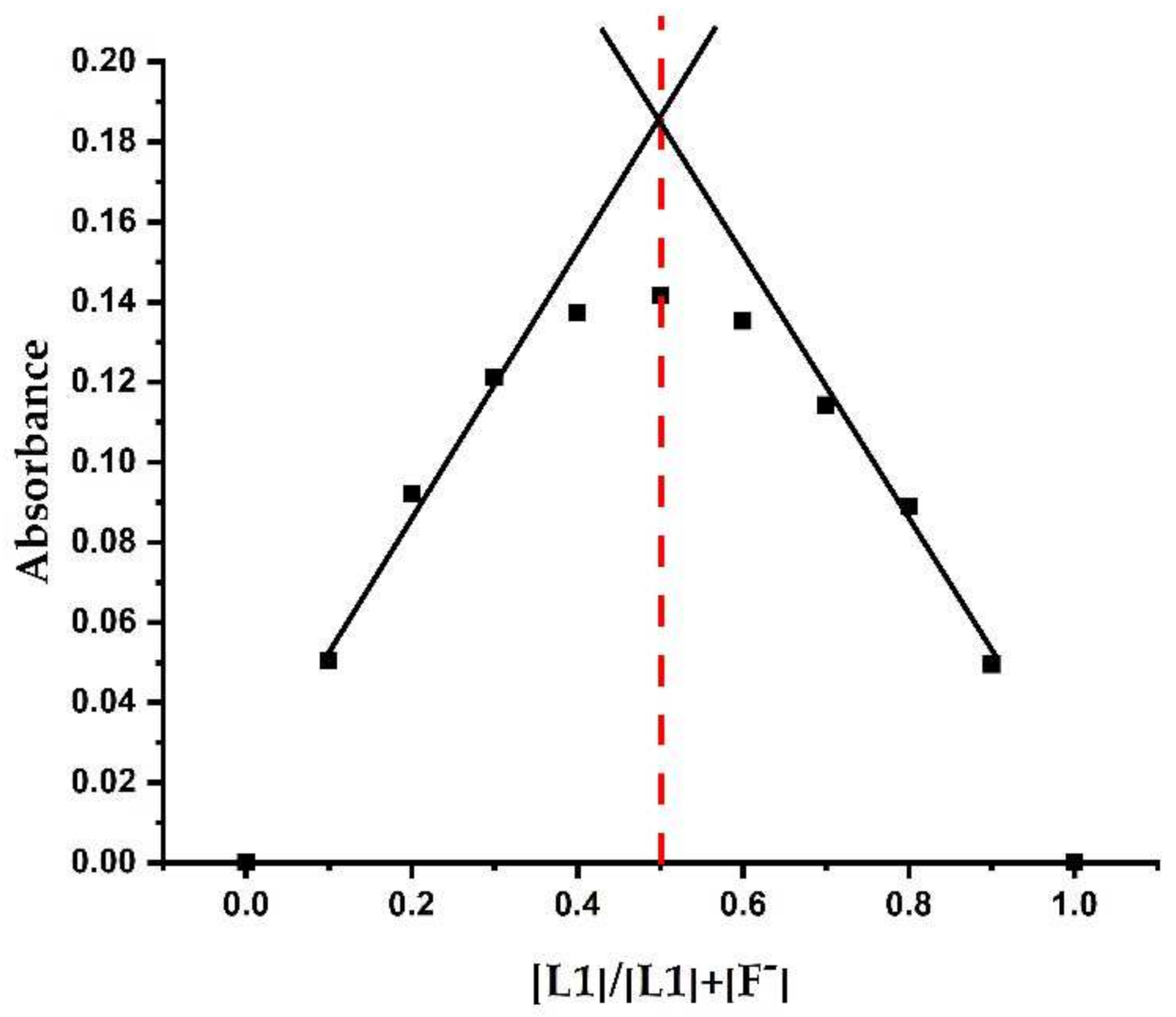
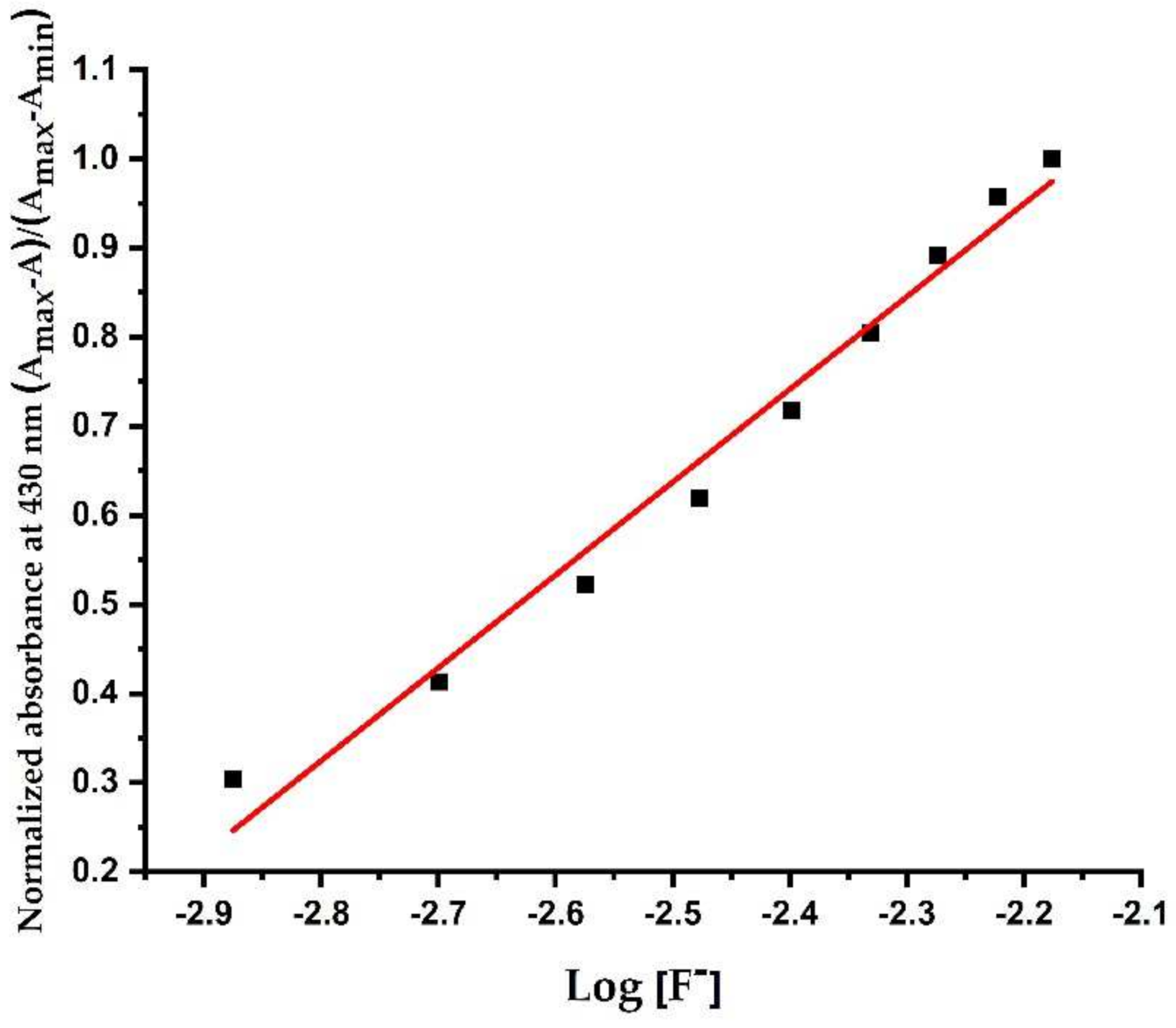
3.2.4. Determination of Association Constants (Ka) and Detection Limit of Sensor L1 with F− Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hein, R.; Beer, P.D.; Davis, J.J. Electrochemical Anion Sensing: Supramolecular Approaches. Chem. Rev. 2020, 120, 1888–1935. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rao, C.; Chen, L.; Fu, C.; Zhu, T.; Chen, X.; Liu, C. Addition of α-Cyanomethylpyridine to Naphthalimide via Trifluoromethyl-Directed CH Functionalization: Cyanide Sensing in Aqueous Media. J. Org. Chem. 2019, 84, 7518–7522. [Google Scholar] [CrossRef]
- Xiao, Y.; Chu, W.-K.; Ng, C.-O.; Cheng, S.-C.; Tse, M.-K.; Yiu, S.-M.; Ko, C.-C. Design and Synthesis of Luminescent Bis(isocyanoborato) Rhenate(I) Complexes as a Selective Sensor for Cyanide Anion. Organometallics 2020, 39, 2135–2141. [Google Scholar] [CrossRef]
- Guo, C.; Sun, S.; He, Q.; Lynch, V.M.; Sessler, J.L. Pyrene-Linked Formylated Bis(dipyrromethane): A Fluorescent Probe for Dihydrogen Phosphate. Org. Lett. 2018, 20, 5414–5417. [Google Scholar] [CrossRef]
- Choi, S.-J.; Yoon, B.; Lin, S.; Swager, T.M. Functional Single-Walled Carbon Nanotubes for Anion Sensing. ACS Appl. Mater. Interfaces 2020, 12, 28375–28382. [Google Scholar] [CrossRef]
- Jo, Y.; Chidalla, N.; Cho, N.-G. Bis-ureidoquinoline as a Selective Fluoride Anion Sensor through Hydrogen-Bond Interactions. J. Org. Chem. 2014, 79, 9418–9422. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Chen, H.; You, Z.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Shear Exfoliated Metal–Organic Framework Nanosheet-Enabled Flexible Sensor for Real-Time Monitoring of Superoxide Anion. ACS Appl. Mater. Interfaces 2020, 12, 5429–5436. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Devi, M.; Jena, N.; Iqbal, M.M.; Nailwal, Y.; De Sarkar, A.; Pal, S.K. Proton-Triggered Fluorescence Switching in Self-Exfoliated Ionic Covalent Organic Nanosheets for Applications in Selective Detection of Anions. ACS Appl. Mater. Interfaces 2020, 12, 13248–13255. [Google Scholar] [CrossRef]
- Tretyakova, N.Y.; Lebedev, A.T.; Petrosyan, V.S. Degradetive Pathways for Aqueous Chlorination of Orcinol. Envion. Sci. Technol. 1994, 28, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhou, Y.; Song, B. Recognition and Sensing Propeties a Quinazonyl Aminothiourea-Based Anion Receptor in Non-Aqueous and Aqueous CH3CH-DMSO Medium. Sens. Actuators B Chem. 2012, 171–172, 550–555. [Google Scholar] [CrossRef]
- Joo, T.Y.; Singh, N.; Lee, G.W.; Jang, D.O. Benzimidazole-Based Ratiometric Fluorescent Receptor for Selective Recognition of Acetate. Tetrahedron Lett. 2007, 48, 8846–8850. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Jang, Y.J.; Kim, S.K.; Lee, K.-H.; Kim, J.S.; Yoon, J. Unique Hydrogen Bonds between 9-Anthracenyl Hydrogen and Anions. J. Org. Chem. 2004, 69, 5155–5157. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Liu, Y.; Shangguan, L.; Shi, B.; Zhu, H. Rationally Designed Self-Immolative Rotaxane Sensor Based on Pillar[5]arene for Fluoride Sensing. Org. Lett. 2020, 22, 6662–6666. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kwak, H.; Lee, S.J.; Lee, J.S.; Kwon, H.J.; Nam, S.H.; Lee, K.; Kim, C. Urea/Thiourea-Based Colorimetric Chemosensors for the Biologically Important Ions: Efficient and Simple Sensors. Tetrahedron 2006, 62, 9635–9640. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Lin, H.; Shao, J.; Cai, Z.-S.; Lin, H. A Novel Colorimetric Receptor Responding AcO− Anions based on an Azo Derivative in DMSO and DMSO/water Solution. J. Lumin 2010, 130, 466–472. [Google Scholar] [CrossRef]
- Ling, L.; Hu, J.; Zhang, H. Ferrocene Containing N-tosylhydrazones as Optical and Electrochemical Sensors for Hg2+, Cu2+ and F− Ions. Tetrahedron 2019, 75, 2472–2481. [Google Scholar] [CrossRef]
- Muwal, P.K.; Nayal, A.; Jaiswal, M.K.; Pandey, P.S. A Dipyrromethane based Receptor as a Dual Colorimetric Sensor for F− and Cu2+ Ions. Tetrahedron Lett. 2018, 59, 29–32. [Google Scholar] [CrossRef]
- Gautham, B.U.; Narayana, B.; Sarojini, B.K.; Manjunatha, J.G.; Suresh, N.K. Colorimetric ‘Naked Eye’ Sensor for Fluoride Ion based on Isatinhydrazones via Hydrogen Bond Formation: Design, Synthesis and Characterization ascertained by Nuclear Magnetic Resonance, Ultraviolet–Visible, Computational and Electrochemical studies. Inorg. Chem. Commun. 2020, 121, 108216. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; He, Q.; Chi, X.; Lynch, V.M.; Zhang, Z.; Su, J.; Tian, H.; Sessler, J.L. Molecular Cursor Caliper: A Fluorescent Sensor for Dicarboxylate Dianions. J. Am. Chem. Soc. 2019, 141, 14798–14806. [Google Scholar] [CrossRef] [PubMed]
- Reena, V.; Suganya, S.; Velmathi, S. Synthesis and anion binding studies of azo-Schiff bases: Selective colorimetric fluoride and acetate ion sensors. J. Fluor. Chem. 2013, 153, 89–95. [Google Scholar] [CrossRef]
- Yu, X.-F.; Xiao, B.; Cheng, J.; Liu, Z.-B.; Yang, X.; Li, Q. Theoretical Design of Near-Infrared Fluorescent Sensor for F Anion Detection Based on 10-Hydroxybenzo[h]quinoline Backbone. ACS Omega 2019, 4, 10516–10523. [Google Scholar] [CrossRef]
- Lv, T.-T.; Ju, C.-G.; Liu, B.-N.; Ren, X.-H.; Shan, G.-S.; Jia, T.-Z. Determination of Orcinol Glucoside by LC-MS in Curculigo Orchiioides and Its Application to a Pharmacokinetic Study. Rev. Bras. Farmacogn. 2019, 29, 744–748. [Google Scholar] [CrossRef]
- Rautenberg, M.; Bhattacharya, B.; Akhmetova, I.; Emmerling, F. Mechanochemical and Solution Syntheses of Two Novel Cocrystals of Orcinol with Two N,N′-Dipyridines: Structural Diversity with Varying Ligand Flexibility. J. Mol. Struct. 2020, 1217, 128303. [Google Scholar] [CrossRef]
- Kanekar, Y.; Basha, K.; Duche, S.; Gupte, R.; Kapat, A. Regioselective Synthesis of Phenanthrenes and Evaluation of their Anti-Oxidant based Anti-Inflammatory Potential. Eur. J. Med. Chem. 2013, 67, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.R.; Thummuri, D.; Naidu, V.G.M.; Ramakrishna, S.; Mallavadhani, U.V. Synthesis of Some Novel Orcinol based Coumarin Triazole Hybrids with Capabilities to Inhibit RANKL-Induced Osteoclastogenesis through NF-κB Signaling Pathway. Bioorg. Chem. 2018, 78, 94–102. [Google Scholar] [CrossRef]
- Pham, P.J.; Hernandez, R.; French, W.T.; Estill, B.G.; Mondala, A.H. A Spectrophotometric Method for Quantitative Determination of Xylose in Fermentation Medium. Biomass Bioenergy 2011, 35, 2814–2821. [Google Scholar] [CrossRef]
- Monde, K.; Satoh, H.; Nakamura, M.; Tamura, M.; Takasygi, M. Organochloride Compounds from a Terrestrial Higher Plant: Structure and Origin of Chlorinated Orcinol Derivatives from Diseased Bulbs of Lilium Maximowiczii. J. Nat. Prod. 1998, 61, 913–921. [Google Scholar] [CrossRef]
- Mukherjee, A.; Grobelny, P.; Thakur, T.S.; Desiraju, G.R. Polymorphs, Pseudopolymorphs, and Co-Crystals of Orcinol: Exploring the Structural Landscape with High Throughput Crystallography. Cryst. Growth Des. 2011, 11, 2637–2653. [Google Scholar] [CrossRef]
- Singh, A.; Nishith, U.; Trivedi, D.R. Spectroscopic Studies of Colorimetric Receptors for Detection of Biologically Important Inorganic F−, AcO− and H2PO4− Anions in Organo-Aqueous Medium: Real-Life Application. Inorg. Chem. Commun. 2020, 115, 107874. [Google Scholar] [CrossRef]
- Erdemir, S.; Kocyigit, O.; Alici, O.; Malkondu, S. ‘Naked-Eye’ Detection of F− Ions by Two Novel Colorimetric Receptors. Tetrahedron Lett. 2013, 54, 613–617. [Google Scholar] [CrossRef]
- Bruker, S. APEX3, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.J.; van de Streek, J.; Wood, P. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Li, X.-M.; Xu, L.-L.; Tang, X.-F.; Zhang, S.-S. 2-Chloro-N-(4-nitrophenyl)acetamide. Acta Cryst. 2006, E62, o4427–o4428. [Google Scholar] [CrossRef]
- Deka, B.; Sarma, R.J. Concentration Dependent Colour Switching of Tryptophan-Derived 3,5-dinitrobenzamide Ligands in the Presence of Fluoride Anions. Sens. Actuators B Chem. 2014, 197, 321–325. [Google Scholar] [CrossRef]
- Okudan, A.; Erdemir, S.; Kocyigit, O. ‘Naked-Eye’ Detection of Fluoride and Acetate Anions by Using Simple and Efficient Urea and Thiourea based Colorimetric Sensors. J. Mol. Struct. 2013, 1048, 392–398. [Google Scholar] [CrossRef]
- Peng, X.; Wu, Y.; Fan, J.; Tian, M.; Han, K. Colorimetric and Ratiometric Fluorescence Sensing of Fluoride: Tuning Selectivity in Proton Transfer. J. Org. Chem. 2005, 70, 10524–10531. [Google Scholar] [CrossRef]
- Zou, L.; Yan, B.; Pan, D.; Tan, Z.; Bao, X. A Colorimetric and Absorption Ratiometric Anion Sensor Based on Indole & Hydrazide Binding Units. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 78–84. [Google Scholar]
- Wang, C.; Li, G.; Zhang, Q. A Novel Heteroacene, 2-(2,3,4,5-tetrafluorophenyl)-1H-imidazo[4,5-b]phenazine as a Multi-Response Sensor for F− Detection. Tetrahedron Lett. 2013, 54, 2633–2636. [Google Scholar] [CrossRef]





| Compound | Sensor L1 |
|---|---|
| CCDC Number | 1420420 |
| Empirical Formula | C21H26N2O5 |
| Formula Weight | 386.44 |
| Temperature | 296 |
| Wavelength (Å) | 0.71073 |
| Crystal System | monoclinic |
| Space Group | P21 |
| a (Å) | 9.7080(6) |
| b (Å) | 5.3052(3) |
| c (Å) | 19.8563(13) |
| α (°) | 90 |
| β (°) | 98.554(2) |
| γ (°) | 90 |
| Volume /Å3 | 1011.28(11) |
| Z | 2 |
| Density (calculated) (g/cm3) | 1.269 |
| Absorption Coefficient (mm−1) | 0.091 |
| F(000) | 412.0 |
| Crystal Size (mm3) | 0.26 × 0.14 × 0.14 |
| Theta Range for Data Collection (°) | 6.224 to 52.792 |
| Index Ranges | −10 ≤ h ≤ 12, −6 ≤ k ≤ 6, −24 ≤ l ≤ 24 |
| Reflections Collected | 12,231 |
| Independent Reflections | 4117 [Rint = 0.0402] |
| Max. and Min. Transmission | 0.7454 and 0.6568 |
| Data/Restraints/Parameters | 4117/1/255 |
| Goodness-of-fit on F2 | 1.066 |
| Final R Indices [I > 2sigma(I)] | R1 = 0.0437, wR2 = 0.0873 |
| R Indices (all data) | R1 = 0.0733, wR2 = 0.0976 |
| Largest Diff. Peak and Hole | 0.15/−0.14 e Å−3 |
| D–H∙∙∙A | D–H | H∙∙∙A | D∙∙∙A | D–H∙∙∙A |
|---|---|---|---|---|
| N1–H1···O3i | 0.86 | 2.37 | 3.096(3) | 143 |
| C15–H15···O3i | 0.930 | 2.636 | 3.178 | 117.88 |
| C16–H16B···C1i | 0.970 | 2.882 | 3.793 | 156.87 |
| C8–H8B···C10ii | 0.969 | 2.888 | 3.764 | 150.69 |
| Parameters | Bond Length (Å) | Parameters | Bond Length (Å) |
| O1–C1 | 1.384(3) | C5–C6 | 1.393(4) |
| O1–C8 | 1.404(3) | C5–C7 | 1.513(4) |
| O2–C3 | 1.363(3) | C8–C9 | 1.505(4) |
| O2–C16 | 1.435(4) | C10–C11 | 1.385(4) |
| O3–C9 | 1.214(3) | C10–C15 | 1.391(4) |
| O4–N2 | 1.218(4) | C11–C12 | 1.378(4) |
| O5–N2 | 1.208(4) | C12–C13 | 1.374(4) |
| Atom–atom–atom | Angle (/°) | Atom–atom–atom | Angle (/°) |
| C1–O1–C8 | 116.6(2) | O3–C9–N1 | 124.3(2) |
| C3–O2–C16 | 117.6(2) | O3–C9–C8 | 119.4(2) |
| C9–N1–C10 | 126.0(2) | N1–C9–C8 | 116.3(2) |
| O4–N2–C13 | 117.3(3) | C11–C10–N1 | 122.1(2) |
| O5–N2–O4 | 123.9(3) | C11–C10–C15 | 119.3(2) |
| O5–N2–C13 | 118.8(3) | C15–C10–N1 | 118.6(2) |
| O1–C1–C2–C3 | −179.6(3) | C4–C5–C6–C1 | −1.2(4) |
| O1–C1–C6–C5 | 179.5(2) | C6–C1–C2–C3 | 0.0(4) |
| O1–C8–C9–O3 | −163.6(3) | C7–C5–C6–C1 | 179.5(3) |
| O1–C8–C9–N1 | 17.7(3) | C8–O1–C1–C2 | −160.3(2) |
| O2–C3–C4–C5 | 177.0(3) | C8–O1–C1–C6 | 20.1(4) |
| O2–C16–C17–C18 | −176.9(3) | C9–N1–C10–C11 | −29.2(4) |
| O4–N2–C13–C12 | 179.1(3) | C9–N1–C10–C15 | 151.6(3) |
| O4–N2–C13–C14 | 0.3(5) | C10–N1–C9–C3 | 0.8(4) |
| O5–N2–C13–C12 | −2.4(4) | C10– N1–C9–C8 | 179.4(2) |
| Chemical Shifts (ppm) | Assignments |
|---|---|
| 10.64 | NH Proton at Amide Group |
| 8.24 and 7.91 | CH Protons (aromatic protons at nitroaniline) |
| 6.40 | CH Protons (aromatic protons at 3,5-dihydroxy toluene) |
| 4.73 | CH2 Protons at Methyl Group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansukra, P.; Duangthongyou, T.; Suramitr, S.; Chainok, K.; Wannalerse, B. Synthesis, Crystal Structure and Optical Properties of 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide for Anion Detection. Crystals 2021, 11, 671. https://doi.org/10.3390/cryst11060671
Jansukra P, Duangthongyou T, Suramitr S, Chainok K, Wannalerse B. Synthesis, Crystal Structure and Optical Properties of 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide for Anion Detection. Crystals. 2021; 11(6):671. https://doi.org/10.3390/cryst11060671
Chicago/Turabian StyleJansukra, Piangkwan, Tanwawan Duangthongyou, Songwut Suramitr, Kittipong Chainok, and Boontana Wannalerse. 2021. "Synthesis, Crystal Structure and Optical Properties of 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide for Anion Detection" Crystals 11, no. 6: 671. https://doi.org/10.3390/cryst11060671
APA StyleJansukra, P., Duangthongyou, T., Suramitr, S., Chainok, K., & Wannalerse, B. (2021). Synthesis, Crystal Structure and Optical Properties of 2-(3-(Hexyloxy)-5-Methylphenoxy)-N-(4-Nitrophenyl)acetamide for Anion Detection. Crystals, 11(6), 671. https://doi.org/10.3390/cryst11060671




_CHAINOK.jpg)


