Effect of Unburned Pulverized Coal on the Melting Characteristics and Fluidity of Blast Furnace Slag
Abstract
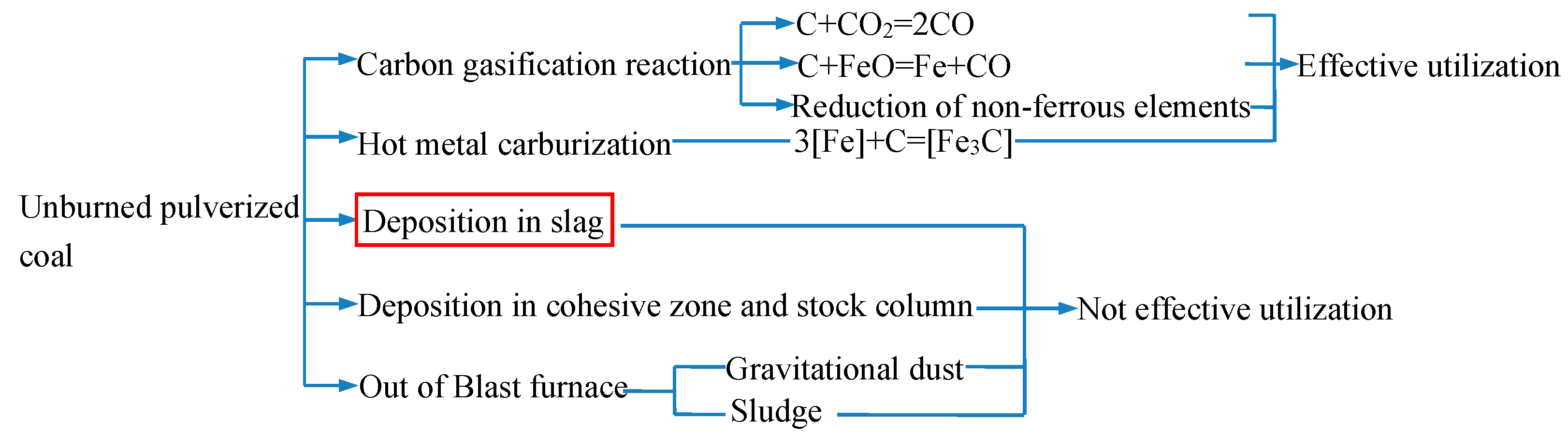
1. Introduction
2. Materials and Methods
2.1. Materials
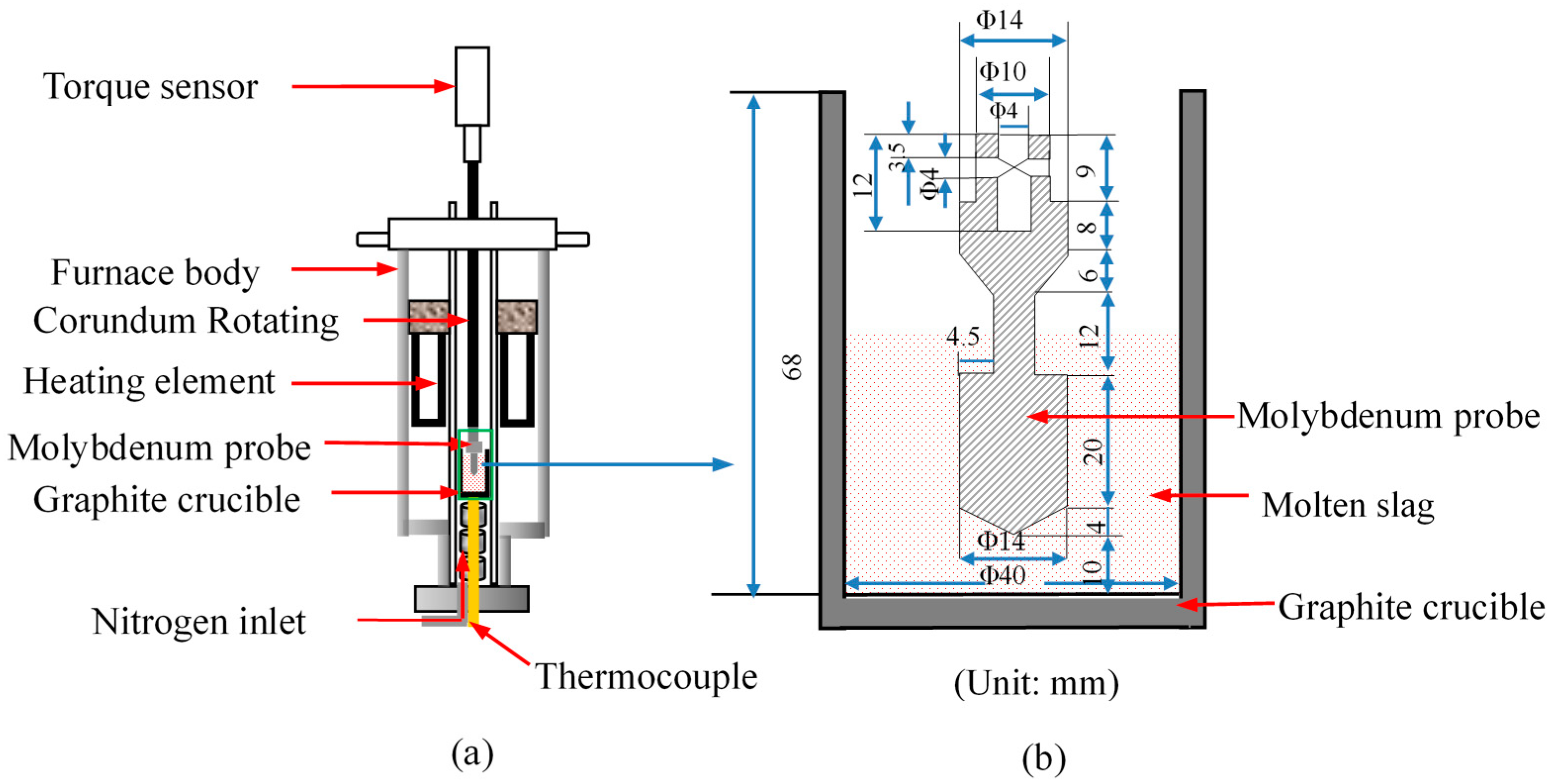
2.2. Experimental Equipment and Methods
2.2.1. Proportion of UPC
2.2.2. Experiment on Effect of UPC on BF Slag Viscosity
2.2.3. Microscopic Characterization
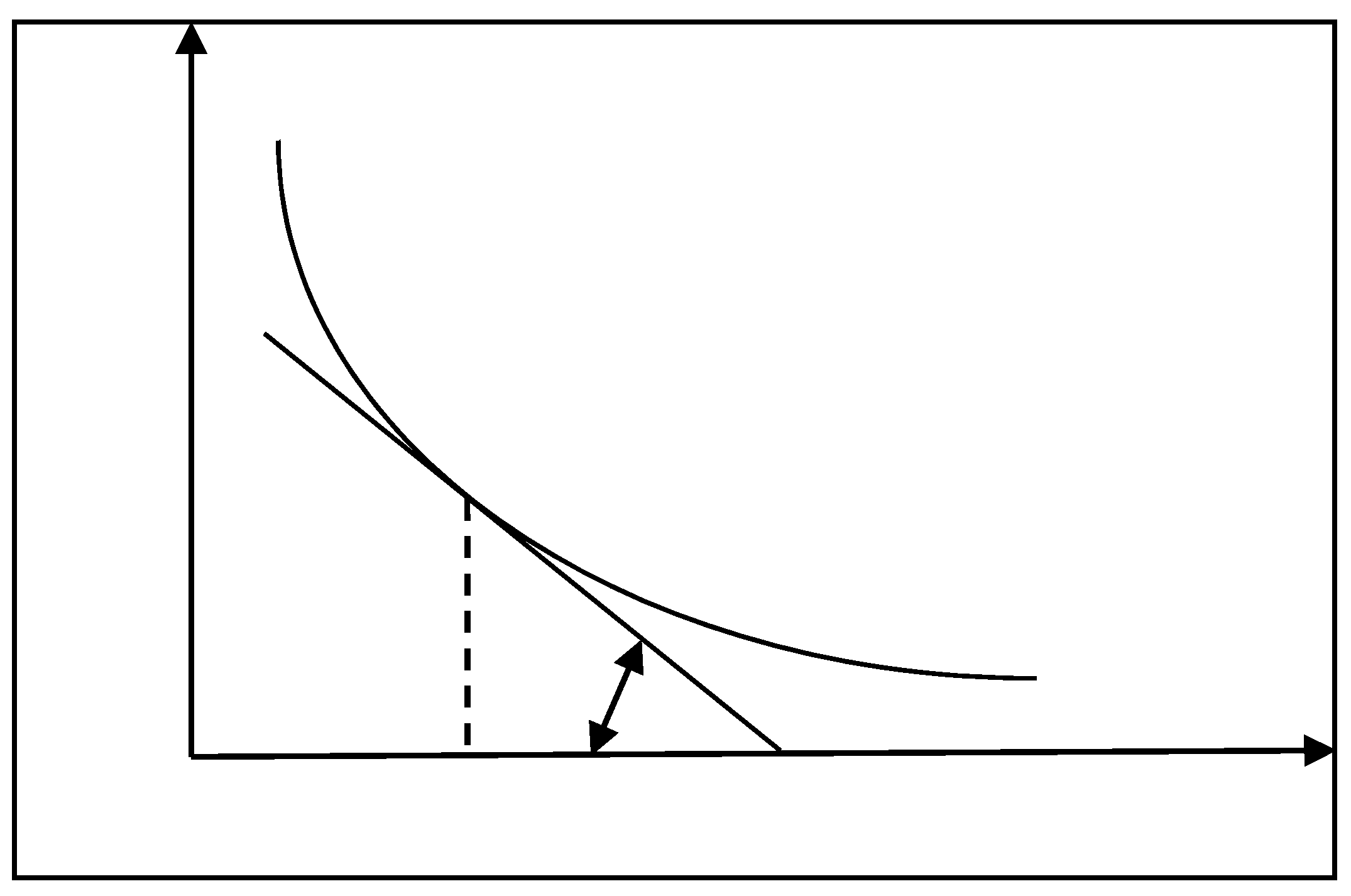
2.2.4. Definition of Slag Free-Running Temperature
3. Results and Discussion
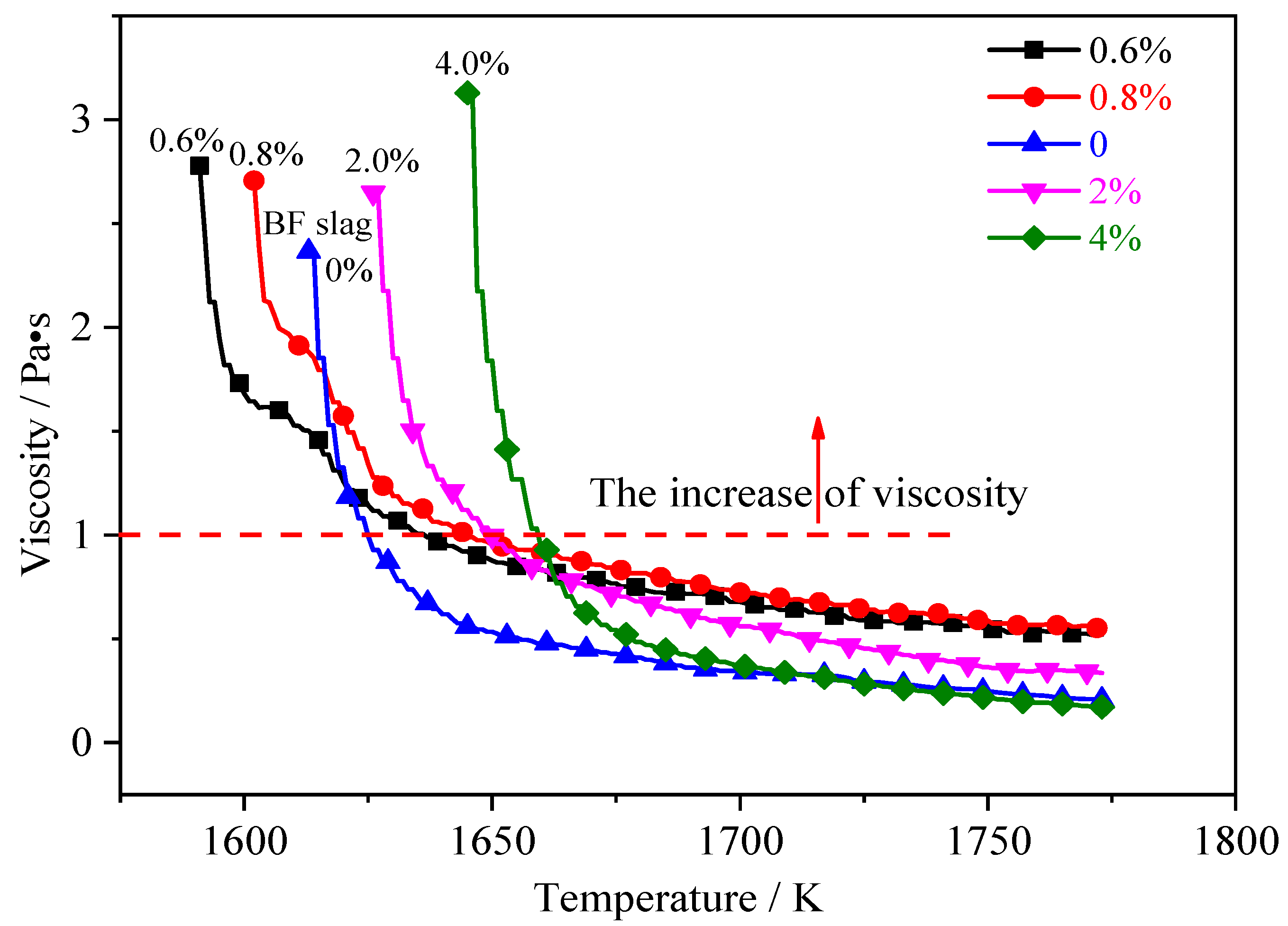
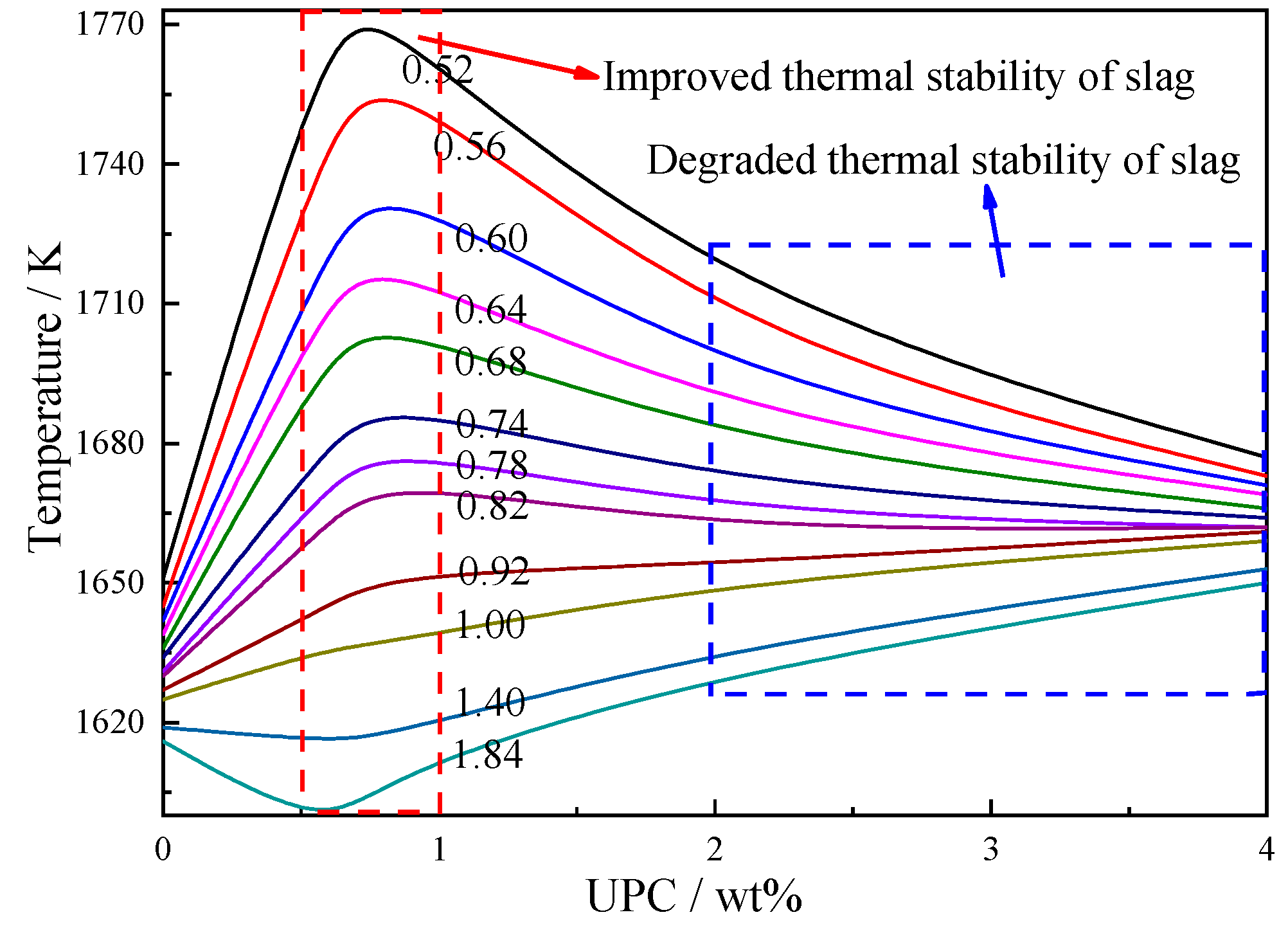
3.1. Effect of the UPC on the Slag Viscosity
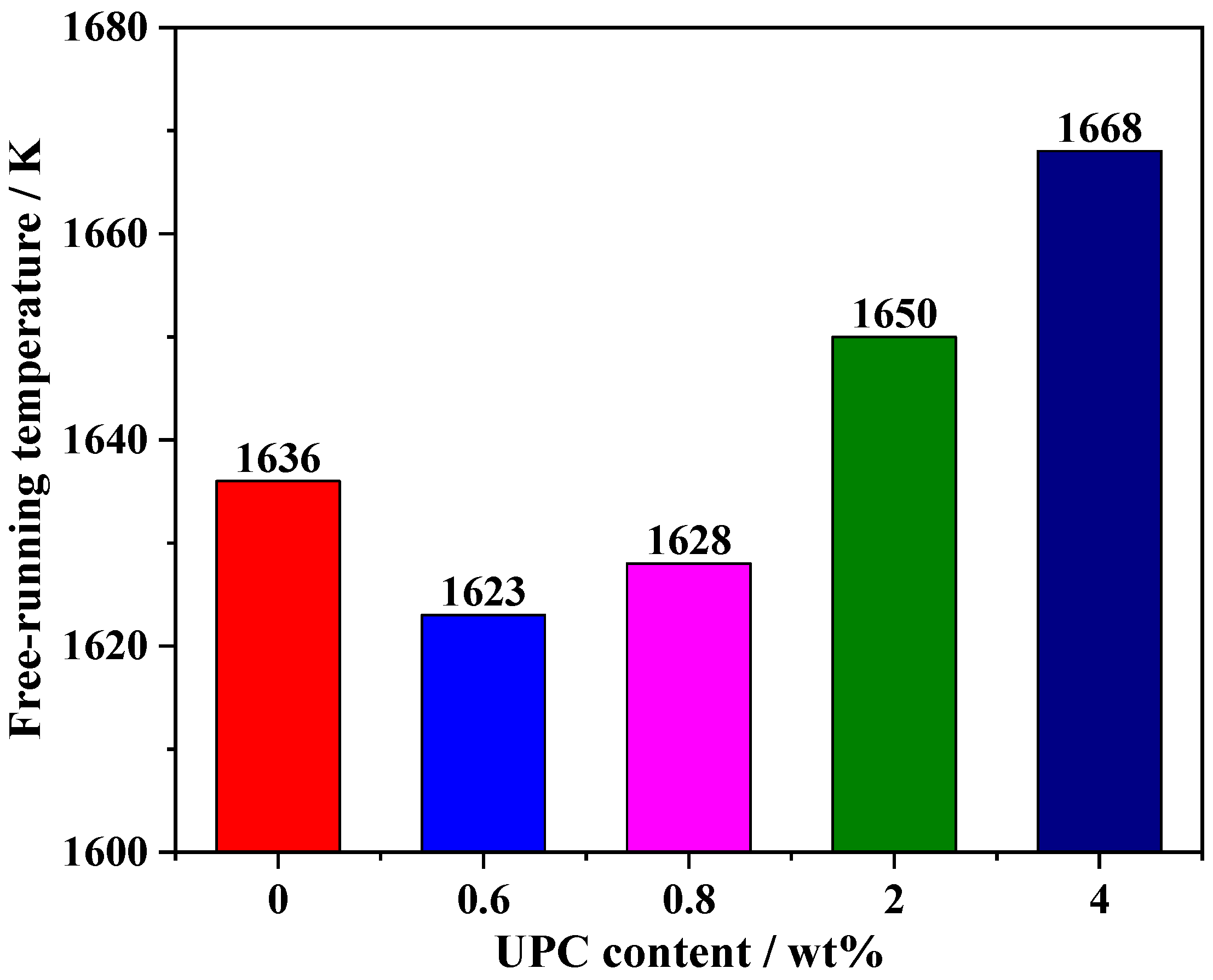
3.2. Effect of the UPC on the Free-Running Temperature of the Slag
3.3. Viscous Flow Activation Energy of the Slag
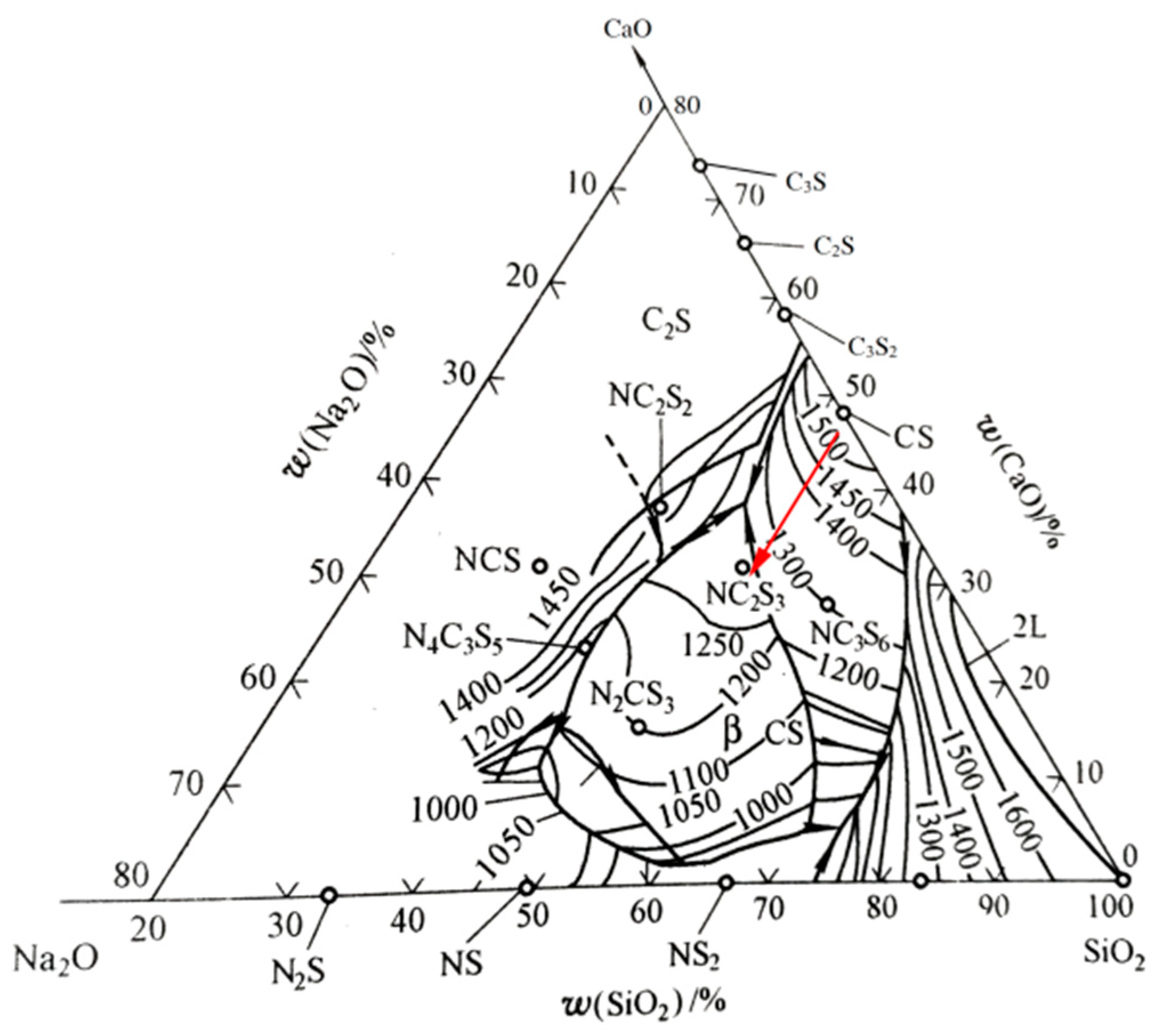
3.4. Theoretical Analysis
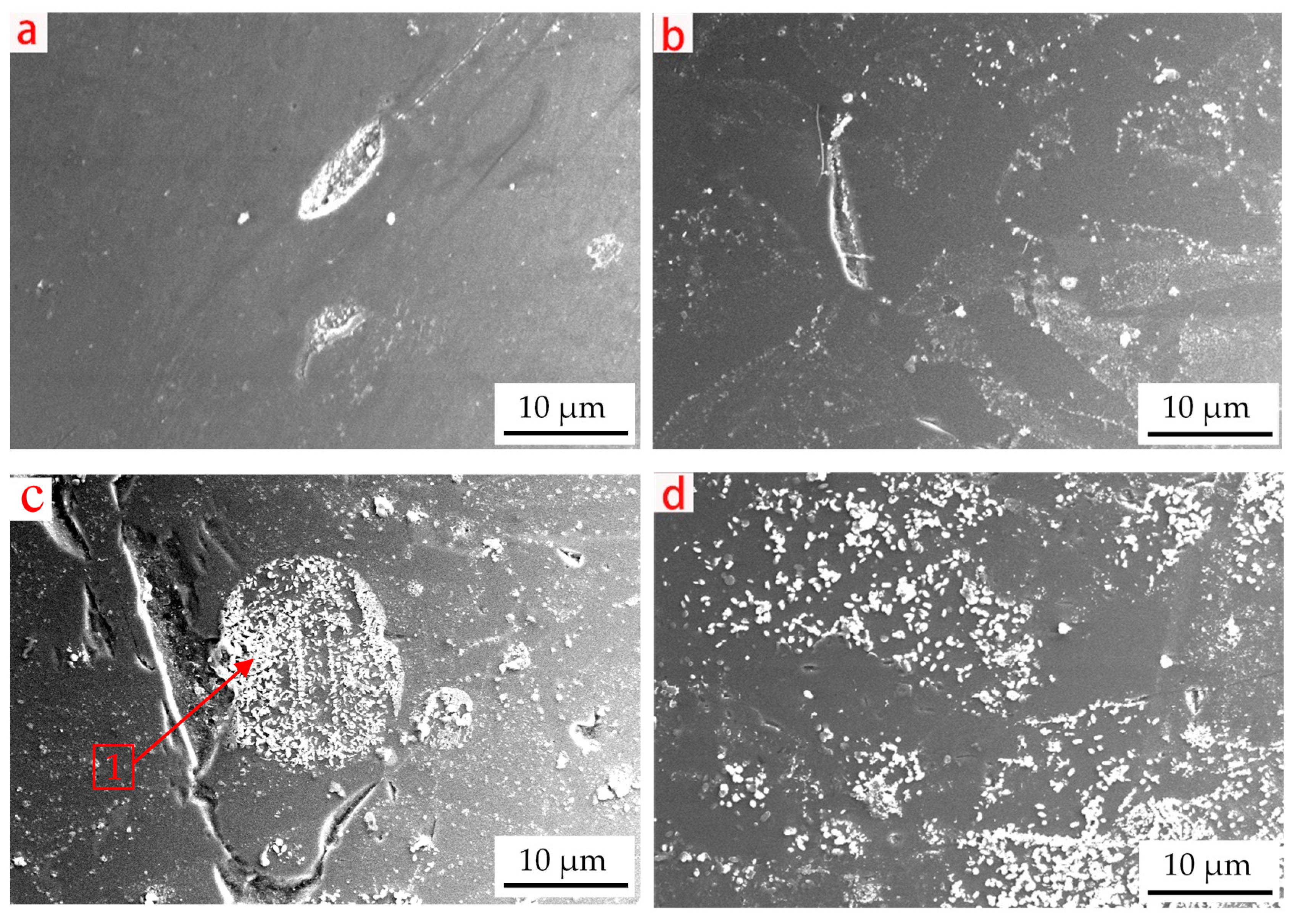
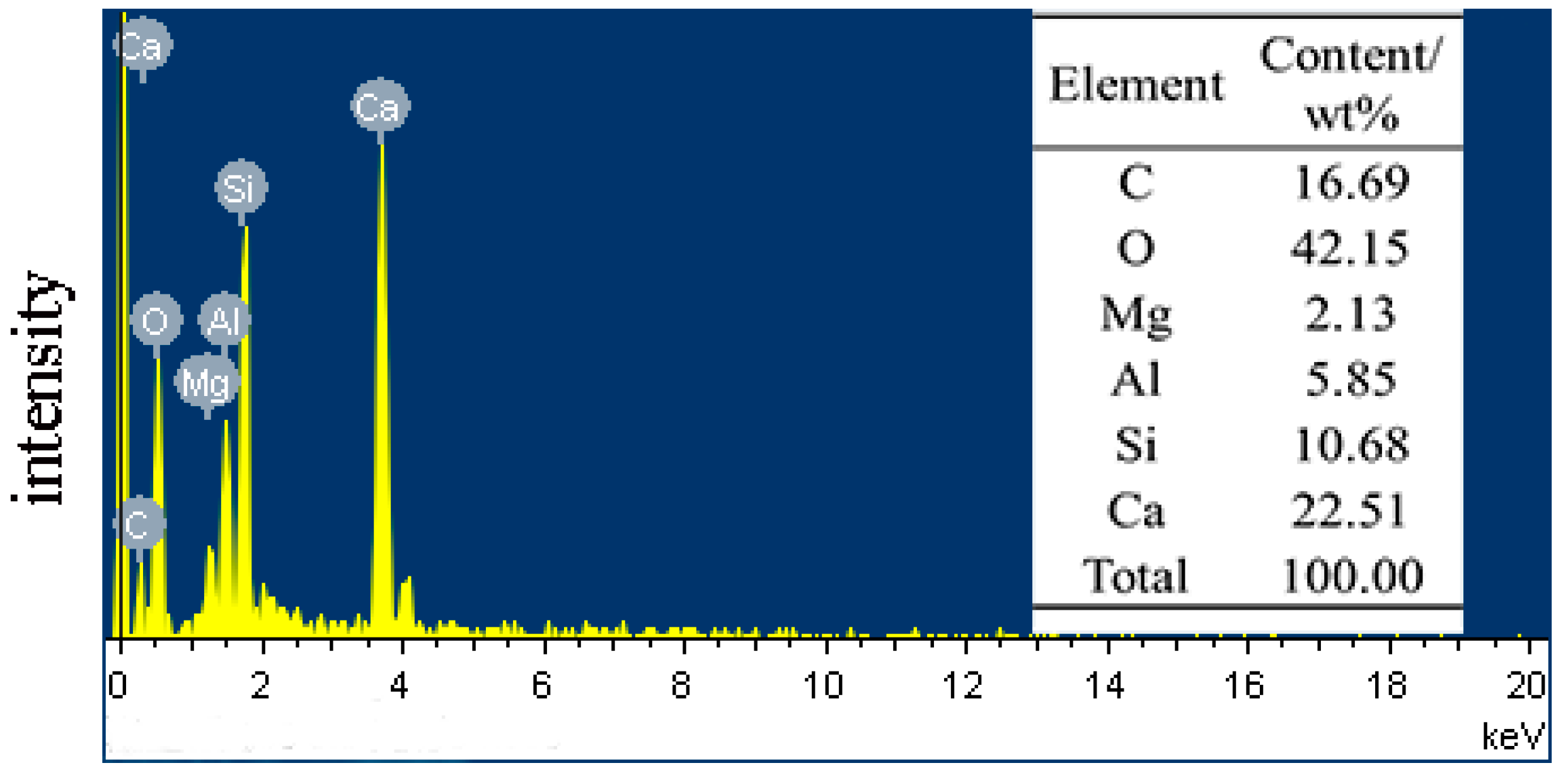
3.5. Analysis of the Slag Microstructure
4. Conclusions
- When the content of UPC in the slag is less than 0.8 wt%, the slag viscosity, free-running temperature and viscous flow activation energy decrease. This is due to the breakdown of the complex spatial network structure of SixOz− y in the slag and the formation of a series of complex compounds with low melting points. Thus, the low content of UPC has the function of diluting the slag to some extent.
- When the content of UPC is in the range of approximately 0.5–1 wt%, the UPC reduces the effect of the temperature on the slag viscosity. When the content of UPC is in the range of approximately 2–4 wt%, the thermal stability of the slag begins to deteriorate, and the UPC has a negative effect on the slag viscosity. To decrease the content of UPC in BF slag to 2 wt%, it is necessary to increase the burnout rate of pulverized coal to 85%.
- The free-running temperature and viscous flow activation energy of slag without UPC are 1626 K and 187.115 kJ/mol, respectively. When the content of UPC in the slag is 0.6 wt%, the free-running temperature and viscous flow activation energy decrease to 1623 K and 120.969 kJ/mol, respectively. However, when the content of UPC increases to 4 wt%, the free-running temperature and viscous flow activation energy increase to 1668 K and 286.625 kJ/mol, respectively.
- When the content of UPC in the slag is high, the main factor affecting the change in the slag viscosity, free-running temperature and viscous flow activation energy is that the UPC entering the slag forms a large number of white particles that are composed of deposited carbon and a high melting point solid solution. These white particles form heterogeneous phases during slag cooling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Guo, B.; Yu, A.; Austin, P.; Zulli, P. Three-dimensional modelling of in-furnace coal/coke combustion in a blast furnace. Fuel 2011, 90, 728–738. [Google Scholar] [CrossRef]
- Ning, X.; Peng, Z.; Wang, G.; Zhang, J.; Song, T. Experimental study on gasification mechanism of unburned pulverized coal catalyzed by alkali metal vapor. J. Energy Inst. 2020, 93, 679–694. [Google Scholar] [CrossRef]
- Hur, N.S.; Cho, B.R.; Choi, J.S.; Hanand, K.W.; Seo, K.Y. High Coal Injection into the Blast Furnace under Productivity. Rev. Metal. CIT 1996, 93, 367–377. [Google Scholar] [CrossRef]
- Steeghs, A.; Schoone, E.; Toxopeus, H. High injection rates of coal into the blast furnace. MPT Metal. Plant. Technol. Int. 1994, 17, 58–65. [Google Scholar]
- Kinura, K.; Kishimoto, S.; Sakai, A.; Triyama, T.; Sato, M. Challenge to the highest PC rate operating at Fu-kuyama NO.4 BF. Rev. Metal. CIT 1996, 93, 575–580. [Google Scholar] [CrossRef]
- Takahashi, K.; Kawai, H.; Suzuki, Y. analysis of stress and buoyancy for solids flow in the low part of a blast furnace. Chem. Eng. Sci. 2002, 57, 215–226. [Google Scholar] [CrossRef]
- Yu, X.; Shen, Y. Modelling of blast furnace with respective chemical reaction in coke and ore burden layers. Metall. Mater. Trans. B 2018, 49, 2370–2388. [Google Scholar] [CrossRef]
- Iwanaga, Y. Investigation on behavior of unburnt pulverized coal in blast furnace. ISIJ Int. 1991, 31, 494–499. [Google Scholar] [CrossRef]
- Su, B.; Zhang, J.; Yan, B.; Che, X.; Wang, G.; Hu, Z. Study on the microstructure and behaviour of unburnt pulverized coal in BF. Adv. Mater. Res. 2011, 236–238, 858–863. [Google Scholar] [CrossRef]
- Diao, R.S.; Hu, B.S. Behavior of unburned particle in blast furnace pulverized coal injection. Iron Steel Vanadium Titan. 2003, 24, 18–21. [Google Scholar]
- Li, Y.; Zhang, J.; Wang, G.; Liang, W.; Zhang, N.; Guo, P. Assessment on the effect of unburned pulverized coal on the properties of coke in blast furnace. Ironmak. Steelmak. 2020, 47, 228–237. [Google Scholar] [CrossRef]
- Xiang, D.; Shen, F.; Jiang, X.; Yang, J.; Li, J.; Gao, Q. protective mechanism of unburned pulverized coal to coke in blast furnac. J. Min. Metal. Sect. B Metal. 2019, 55, 371–380. [Google Scholar] [CrossRef]
- Chai, Y.; Luo, G.; An, S.; Peng, J.; Wang, Y. Influence of unburned pulverized coal on gasification reaction of coke in blast furnace. High Temp. Mater. Proc. 2019, 38, 733–738. [Google Scholar] [CrossRef]
- Zan, R.; Wang, W.; Xu, R.; Schenk, J.; Zheng, H.; Wang, X. Gasification Characteristics and Kinetics of Unburned Pulverized Coal in Blast Furnaces. Energies 2019, 12, 4324. [Google Scholar] [CrossRef]
- Xiang, D.; Shen, F.; Yang, J.; Jiang, X.; Zheng, H.; Gao, Q.; Li, J. Combustion characteristics of unburned pulverized coal and its reaction kinetics with CO2. Int. J. Miner. Metal. Mater. 2019, 26, 811–821. [Google Scholar] [CrossRef]
- Diao, R.S.; Hu, B.S. Influence of unburned PCI on the blast furnace slag viscosity in Panzhihua Steel Co. Iron Steel 2004, 39, 14–16. [Google Scholar]
- Zhou, L.; Zhao, J. Effects on slag viscosity caused by UPC. J. Anhui Univ. Technol. 2004, 21, 1–3. [Google Scholar]
- Liang, Z.; Shen, S. Effect of unburned pulverized coal on slag viscosity. Metall. Sichuan 1996, 3, 20–21. [Google Scholar]
- Kim, J.; Lee, Y.; Min, D.; Jung, S.; Yi, S. Influence of MgO and Al2O3 contents on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1291–1297. [Google Scholar] [CrossRef]
- Gupta, V.; Seshadri, V. Studies on viscosity of high alumina blast furnace slags. Trans. Indian Inst. Met. 1973, 26, 55–64. [Google Scholar]
- Singh, R. Viscosity measurements of high alumina blast furnace slags. Steel India 1984, 7, 73–83. [Google Scholar]
- SUN, C.; Liu, X.; Li, J.; Yin, X.; Song, S.; Wang, Q. Influence of Al2O3 and MgO on the viscosity and stability of CaO-MgO-SiO2-Al2O3 Slags with CaO/SiO2 = 1.0. ISIJ Int. 2017, 57, 978–982. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, D.; Kou, M.; Zhao, B. Viscosities of Iron Blast Furnace Slags. ISIJ Int. 2014, 54, 2025–2030. [Google Scholar] [CrossRef]
- Yan, Z.; Lv, X.; Zhang, J.; Qin, Y.; Bai, C. Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5 wt% TiO2. Can. Metal. Q. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, T.; Xue, X.; Hu, B. Influence of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Slag with High Al2O3 Content. Steel Res. Int. 2016, 87, 87–94. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y. Three-dimensional modelling of charcoal combustion in an industrial scale blast furnace. Fuel 2019, 258, 116088. [Google Scholar] [CrossRef]
- Du, H.; Nie, D. the study on behavior of unburned pulverized coal in blast furnace. Ironmaking 1988, 4, 1–7. [Google Scholar]
- Zhao, Z. Behavior of unburnt pulverized coal in blast furnace. Henan Met. 1988, 2, 10–42. [Google Scholar]
- Hu, K.; Lv, X.; Li, S.; Song, B.; Han, K. Viscosity of TiO2-FeO-Ti2O3-SiO2-MgO-CaO-Al2O3 for high-titania slag smelting process. Metal. Mater. Trans. B 2018, 49, 1963–1973. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; Yao, T.; He, S. Molecular dynamics simulations of the structural properties of Al2O3-based binary systems. J. Non Cryst. Solids 2016, 435, 17–26. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Liang, Q.; Guo, Q.; Yu, G.; Yu, Z. Analysis and prediction of the viscosity of the SiO2-Al2O3-CaO ternary system in completely molten state. Proc. Chin. Soc. Electr. Eng. 2008, 28, 39–43. [Google Scholar]
- Tiwary, J.; Sarkar, S.; Mishra, B.; Mohanty, U. Structural aspects of blast furnace slag. ICE Publ. 2013, 2, 152–162. [Google Scholar] [CrossRef]
- Mills, K. The influence of structure on the physic-chemical properties of slags. ISIJ Int. 1993, 33, 148–155. [Google Scholar] [CrossRef]
- Park, J.; Min, D.; Song, H. Amphoteric behavior of alumina in viscous flow and structure of CaO-SiO2(-MgO)-Al2O3 slags. Metal. Mater. Trans. B 2004, 35, 269–275. [Google Scholar] [CrossRef]
- Henserson, G.; Calas, G.; Stebbins, J. The structure of silicate glasses and melts. Elements 2006, 2, 269–273. [Google Scholar] [CrossRef]
- Xing, X.; Du, Y.; Zheng, J.; Wang, S.; Ren, S.; Ju, J. Isothermal Carbothermal Reduction of FeTiO3 Doped with MgO. JOM 2021, 73, 1328–1336. [Google Scholar] [CrossRef]
- Sohn, I.; Min, D. A review of the relationship between viscosity and the structure of calcium-silicate-based slags in ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]





| Proximate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Carbon | Moisture | Ash | Volatile Matters | |||||
| 79.88 | 0.88 | 11.48 | 7.76 | |||||
| Analysis of ash | ||||||||
| SiO2 | CaO | Al2O3 | MgO | TiO2 | Fe2O3 | K2O | Na2O | Others |
| 47.12 | 4.92 | 35.55 | 1.04 | 1.99 | 6.11 | 0.72 | 0.71 | 1.84 |
| SiO2 | CaO | Al2O3 | MgO | TiO2 | FeO | MnO | S | Others |
|---|---|---|---|---|---|---|---|---|
| 33.34 | 38.94 | 15.59 | 7.79 | 0.59 | 0.36 | 0.22 | 1.10 | 2.07 |
| UPC Contents | Fitting Line | Eη (kJ/mol) | B0/(N·s·m−2) | R2 |
|---|---|---|---|---|
| 0.6 | y = 14,550 x − 8.926 | 120.969 | 1.33 × 10−4 | 0.97 |
| 0.8 | y = 14,965 x − 9.094 | 124.419 | 1.12 × 10−4 | 0.98 |
| 0 | y = 22,506 x − 14.274 | 187.115 | 6.32 × 10−7 | 0.99 |
| 2 | y = 25,513 x − 15.564 | 212.115 | 1.74 × 10−7 | 0.99 |
| 4 | y = 34,475 x − 21.237 | 286.625 | 5.98 × 10−10 | 0.99 |
| Si/O | CaO/SiO2 | Percentage of Molar Oxide | Empirical Formula | |
|---|---|---|---|---|
| 0.250 | 2 CaO·SiO2 | 66 | 1 | |
| 0.286 | 3 CaO·2SiO2 | 60 | 2 | |
| 0.300 | 4CaO·3SiO2 | 57 | 3 | |
| 0.307 | 5CaO·4SiO2 | 55 | 4 | |
| 0.312 | 6CaO·6SiO2 | 54 | 6 | |
| 0.322 | 11CaO·10SiO2 | 52 | 10 | |
| 0.333 | CaO·SiO2 | 50 | ∞ | Sin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.; Shen, F.; Jiang, X.; Gao, Q.; Zheng, H. Effect of Unburned Pulverized Coal on the Melting Characteristics and Fluidity of Blast Furnace Slag. Crystals 2021, 11, 579. https://doi.org/10.3390/cryst11060579
Xiang D, Shen F, Jiang X, Gao Q, Zheng H. Effect of Unburned Pulverized Coal on the Melting Characteristics and Fluidity of Blast Furnace Slag. Crystals. 2021; 11(6):579. https://doi.org/10.3390/cryst11060579
Chicago/Turabian StyleXiang, Dongwen, Fengman Shen, Xin Jiang, Qiangjian Gao, and Haiyan Zheng. 2021. "Effect of Unburned Pulverized Coal on the Melting Characteristics and Fluidity of Blast Furnace Slag" Crystals 11, no. 6: 579. https://doi.org/10.3390/cryst11060579
APA StyleXiang, D., Shen, F., Jiang, X., Gao, Q., & Zheng, H. (2021). Effect of Unburned Pulverized Coal on the Melting Characteristics and Fluidity of Blast Furnace Slag. Crystals, 11(6), 579. https://doi.org/10.3390/cryst11060579






